Abstract
Thyroid hormone (TH) is critical for cardiac development and heart function. In heart disease, TH metabolism is abnormal, and many biochemical and functional alterations mirror hypothyroidism. Although TH therapy has been advocated for treating heart disease, a clear benefit of TH has yet to be established, possibly because of peripheral actions of TH. To assess the potential efficacy of TH in treating heart disease, type 2 deiodinase (D2), which converts the prohormone thyroxine to active triiodothyronine (T3), was expressed transiently in mouse hearts by using the tetracycline transactivator system. Increased cardiac D2 activity led to elevated cardiac T3 levels and to enhanced myocardial contractility, accompanied by increased Ca2+ transients and sarcoplasmic reticulum (SR) Ca2+ uptake. These phenotypic changes were associated with up-regulation of sarco(endo)plasmic reticulum calcium ATPase (SERCA) 2a expression as well as decreased Na+/Ca2+ exchanger, β-myosin heavy chain, and sarcolipin (SLN) expression. In pressure overload, targeted increases in D2 activity could not block hypertrophy but could completely prevent impaired contractility and SR Ca2+ cycling as well as altered expression patterns of SERCA2a, SLN, and other markers of pathological hypertrophy. Our results establish that elevated D2 activity in the heart increases T3 levels and enhances cardiac contractile function while preventing deterioration of cardiac function and altered gene expression after pressure overload.
Keywords: calcium, cardiac hypertrophy, sarcoplasmic reticulum, deiodinase, transgenic mice
Thyroid hormone (TH) is essential for normal development in vertebrates (1), with TH levels rising postnatally and peaking in the third week of life (2). This surge is critical for fetal-to-adult switch in the cardiac gene program and is responsible for changes in Ca2+ homeostasis, myosin isozyme content [α-myosin heavy chain (MHC)-to-β-MHC switch], and action potential profile (3, 4). Intriguingly, the genetic and functional changes associated with heart failure, such as reduced Ca2+ transients and α-MHC-to-β-MHC shifts, which recapitulate the fetal gene program, are also observed in hypothyroidism (5, 6). In addition, TH metabolism and signaling are abnormal in heart failure (5, 7, 8). For example, circulating and cardiac triiodothyronine (T3) levels (i.e., active TH) are reduced in advanced heart disease, after acute myocardial infarction, and in patients with cardiopulmonary bypass. These T3 changes occur in association with decreased peripheral conversion of thyroxine (T4) into T3 (5, 8) and elevated cardiac deiodinase type 3 (D3) activity (9). TH receptor expression is also altered in pathological hypertrophy (10), and myocardial contractility is impaired in mice lacking TH receptors (11). Not surprisingly, TH and its analogue 3,5-diiodothyropropionic acid (DITPA) have been advocated for treating heart failure (12, 13) and for reversing cardiac dysfunction in patients with hypothyroidism (5), although TH use has been limited by cardiotoxic effects of TH (14, 15).
Because of similarities between hypothyroidism and heart disease, we hypothesized that cardiac-specific enhancement of TH signaling would prevent the genetic and functional defects in heart failure. Type 2 deiodinase (D2) is a conserved 5′ deiodinase enzyme that is expressed in human myocardium, where it converts prohormone T4 into active T3 (16, 17). We created transgenic mice that conditionally overexpress D2 in the heart. D2 overexpression for 2 weeks caused enhanced contractile function in association with changes in the expression of several calcium handling proteins. After aortic banding (AB), D2 transgenic mice were resistant to pressure overload-induced impairment of calcium cycling and contractility, suggesting that cardiac-specific TH therapy may be useful in treating heart disease patients.
Results
Effects of D2 Expression on Cardiac Function and Ca2+ Cycling.
Doxycycline (DOX) withdrawal for 2 weeks did not affect heart weight-to-body weight ratio or myocardial histology (Fig. 1C and Table 1). Images of frozen heart sections revealed bright but patchy fluorescence in DOX-withdrawn Bin-Off mouse hearts (mouse lines are explained in detail in Methods), consistent with the properties of the α-myosin promoter used to drive tetracycline transactivator (tTA) expression (18), whereas minimal fluorescence was seen, as expected, in Con-Off hearts (Fig. 1C). Cardiac D2 expression (Fig. 1D), D2 activity (Fig. 1E), and T3 levels (Fig. 1F) were elevated (P < 0.05) in Bin-Off compared with Con-Off mice, with no differences in D2 activity of liver, lung, and brain tissues (Fig. 1E) or in cardiac T4 (Fig. 1G), serum T3 (Fig. 1H), or serum T4 levels (Fig. 1I), confirming the cardiac-specific targeting of D2 activity.
Fig. 1.
Cardiac-targeted expression of D2. (A) Results of genotyping by PCR showing a 724-bp product plus a 585-bp product for mice heterozygous for the D2 transgene (tD2) and a 585-bp product for WT mice (see Methods). (B) Schematic representation of the tetracycline-regulated mammalian gene expression system. tTA and EGFP refer to the tetracycline-controlled transactivator fusion protein and the reporter gene EGFP, respectively. (C) Representative gross morphology of whole hearts and confocal microscopy images showing fluorescent signal in Con-Off (Left) and Bin-Off (Right) mice. (D) D2 mRNA levels in Bin-Off mice relative to Con-Off mice. (E) D2 activity in heart, liver, lung, and brain of Bin-Off and Con-Off mice (n = 4). (F–I) Cardiac T3 (F) and T4 (G) levels (n = 10) as well as circulating T3 (H) and T4 (I) levels (n = 6) in Bin-Off and Con-Off mice (#, P < 0.05 in Bin-Off mice compared with all groups; ∗, P < 0.005 in Bin-Off vs. Con-Off mice).
Table 1.
Cardiac morphology and function in mice withdrawn from DOX for 2 (2-wk) or 9 (9-wk) weeks
| Measurement | Con-Off 2-wk (n = 8) | Bin-Off 2-wk (n = 8) | Con-Off 9-wk (n = 8) | Bin-Off 9-wk (n = 8) | Con-Off + PTU* (n = 5) | Bin-Off + PTU* (n = 8) |
|---|---|---|---|---|---|---|
| HR, bpm | 511 ± 8.9 | 513 ± 22.8 | 527 ± 6 | 524 ± 8 | 380 ± 19* | 347 ± 5.8* |
| AWT, mm | 0.66 ± 0.02 | 0.69 ± 0.01 | 0.65 ± 0.01 | 0.66 ± 0.01 | 0.56 ± 0.01 | 0.58 ± 0.01 |
| LVEDD, mm | 4.19 ± 0.09 | 4.27 ± 0.07 | 4.26 ± 0.04 | 4.21 ± 0.03 | 3.89 ± 0.08 | 3.99 ± 0.03 |
| LVESD, mm | 2.21 ± 0.05 | 2.02 ± 0.08 | 2.19 ± 0.07 | 2.01 ± 0.04 | 2.54 ± 0.08* | 2.62 ± 0.04* |
| FS, % | 48.1 ± 1.2 | 55.8 ± 1.3† | 48.6 ± 1.1 | 56.1 ± 1.3† | 35.9 ± 1.1* | 34.3 ± 1* |
| VCFc, circ/s | 9.05 ± 0.2 | 11.7 ± 0.4† | 9.73 ± 0.23 | 11.81 ± 0.23† | 4.4 ± 0.21* | 4.5 ± 0.14* |
| PAVc, cm/s | 95.1 ± 2 | 112.6 ± 3.2† | 93.3 ± 2.5 | 114.7 ± 2.1† | 73.4 ± 4* | 72 ± 2.2* |
| AVA, m/s2 | 57.3 ± 1.9 | 78.8 ± 2.2† | 49.2 ± 2.2 | 73.5 ± 1.9† | 33.2 ± 1.8* | 30.4 ± 2.2* |
| HW/BW, mg/g | 5.2 ± 0.2 | 5.5 ± 0.3 | 4.79 ± 0.2 | 5.4 ± 0.3 |
HR, heart rate; bpm, heart beats per min; AWT, anterior wall thickness; LVEDD, left ventricle end diastolic dimension; LVESD, left ventricle end systolic dimension; FS, fractional shortening; VCFc, velocity of circumferential fiber shortening (circ, circumferences) corrected for HR; PAVc, peak aortic outflow velocity corrected for HR; AVA, aortic velocity acceleration (PAVc/acceleration time); PTU, 5-propyl-2-thiouracil; HW/BW, heart weight/body weight.
*, P < 0.01 compared with corresponding group not treated with PTU;
†, P < 0.01 compared with all groups.
Two weeks after DOX withdrawal, Bin-Off hearts had marked elevations in cardiac contractility as assessed echocardiographically or hemodynamically compared with Con-Off hearts (Table 1 and Fig. 2A and B), consistent with acute effects of exogenous TH (5, 19). No differences in cardiac function were observed between Con-Off, Con-On, and Bin-On mice (data not shown). Enhanced cardiac function in Bin-Off mice resembles that observed in acutely TH-treated mice (19). To examine whether these functional differences were related to TH, mice were made hypothyroid by feeding 5-propyl-2-thiouracil (PTU) (see Methods) before DOX withdrawal. As expected (19), serum T4 levels (≈1/6 of control values) and myocardial contractility (Table 1) were reduced (P < 0.05) in hypothyroid mice. However, DOX withdrawal, despite significant elevations in D2 mRNA levels (data not shown), was not accompanied in hypothyroid Bin-Off mice by increased myocardial contractility (Table 1), suggesting that the cardiac phenotype in euthyroid Bin-Off mice is TH-dependent.
Fig. 2.
Enhancement in cardiac contractility in binary transgenic mice. (A and B) Representative M-mode echocardiograms (A) and aortic velocity profiles (B) in Con-Off and Bin-Off mice. LVEDD, left ventricle end-diastolic dimension; LVESD, left ventricle end-systolic dimension. (C) Representative contractions of cardiomyocytes isolated from Con-Off and Bin-Off mice. (D) Mean percentage of CS, rate of contraction (+dL/dtmax), and rate of relaxation (−dL/dtmax) in field stimulated myocytes. (E) Representative traces of the ratio of Indo-1 fluorescence measured at 405 nm divided by 485 (F405/F485) in Con-Off and Bin-Off mice. (F) Amplitude (A405/485) and time to 50% decay (T50) of Indo-1 fluorescence ratio (F405/F485) to assess Ca2+ transients. (G) Maximal rate of SR Ca2+ uptake (Vmax) in cardiac SR vesicles isolated from Bin-Off relative to Con-Off mice (n = 15; #, P < 0.05).
The cellular mechanisms for elevated contractility in Bin-Off mice was investigated by measuring cell shortening and Ca2+ transients in myocytes isolated 2 weeks after DOX withdrawal. The percentage of cell shortening (CS), +dL/dtmax (rate of contraction), −dL/dtmax (rate of relaxation), and Ca2+ transient amplitudes (Fig. 2 C–F and Table 2) were increased (P < 0.01) along with enhanced (P < 0.05) Ca2+ transient decay kinetics (T50) (Fig. 2F) in Bin-Off mice compared with Con-Off mice.
Table 2.
Cardiac morphology and function in mice after pressure overload (and DOX withdrawal) for 9 weeks
| Measurement | Con-Sham (n = 8) | Con-Ban (n = 8) | Bin-Ban (n = 8) |
|---|---|---|---|
| HR, bpm | 527 ± 6 | 536 ± 14 | 526 ± 11 |
| AWT, mm | 0.65 ± 0.01 | 0.85 ± 0.01 | 0.87 ± 0.02 |
| LVEDD, mm | 4.26 ± 0.09 | 4.94 ± 0.09* | 4.52 ± 0.06 |
| LVESD, mm | 2.19 ± 0.07 | 3.62 ± 0.12* | 2.46 ± 0.08 |
| FS, % | 49.2 ± 1.1 | 26.6 ± 2.1* | 46.6 ± 1.3 |
| VCFc, circ/s | 9.74 ± 0.23 | 5.25 ± 0.42* | 9.62 ± 0.26 |
| PAVc, cm/s | 93.3 ± 2.5 | 79.7 ± 2.4* | 107.2 ± 3.4† |
| AVA, m/s2 | 51.6 ± 2.1 | 40.5 ± 1.6* | 64.9 ± 2.4† |
| +dp/dtmax, mmHg/s | 7925 ± 536 | 5359 ± 668* | 10393 ± 517† |
| −dp/dtmax, mmHg/s | 7163 ± 347 | 5540 ± 699* | 10307 ± 373 |
| HW/BW, mg/g | 4.79 ± 0.17 | 8.79 ± 0.78† | 7.51 ± 0.73† |
+dp/dtmax, maximum positive time derivative of the left ventricular pressure; −dp/dtmax, maximum negative time derivative of the left ventricular pressure. Other abbreviations are as in Table 1.
*, P < 0.01 compared with all groups;
†, P < 0.01 compared with Con-Sham.
The cellular results indicate greater sarcoplasmic reticulum (SR) Ca2+ ATPase activity in Bin-Off mice. In agreement with this conclusion, the thapsigargin-sensitive Ca2+ uptake measurements in isolated SR vesicles showed increased (P < 0.005) maximal SR Ca2+ uptake rates (Vmax) and no differences in the [Ca2+] required for half-maximal activity (i.e., EC50) in Bin-Off mice compared with Con-Off mice (Fig. 2G). Enhanced SR Ca2+ uptake in Bin-Off mice suggests increased SR Ca2+ loads. Indeed, after caffeine application, the peak INCX (where NCX is Na+/Ca2+ exchanger) and the time integral of INCX, which reflects the total amount of Ca2+ present in the SR (20), were elevated in Bin-Off myocyte (Fig. 3A and B). The enhanced Ca2+ transients and Ca2+ load in Bin-Off mice occurred without differences in action potential profile (Fig. 3C), L-type Ca2+ current (Fig. 3D), or K+ currents (Fig. 3E), each of which can affect independently Ca2+ transients and myocyte contractility (20).
Fig. 3.
Effects of D2 overexpression on myocyte properties. (A) Bar graphs summarizing studies measuring NCX current (INCX) in myocytes after caffeine application: peak INCX (INCXpeak), time integral of INCX (∫INCX), and INCX decay times (τNCX). (B) Typical INCX traces elicited by application of 20 mmol/liter caffeine to myocytes isolated from Con-Off and Bin-Off hearts. (C–E) Typical action potentials (C), L-type Ca2+current families (D), and transient outward K+ current families (E) recorded in myocytes isolated from Con-Off and Bin-Off mice (n = 15; #, P < 0.05; ∗, P < 0.005 in Bin-Off vs. Con-Off mice).
Effects of D2 on Gene Expression.
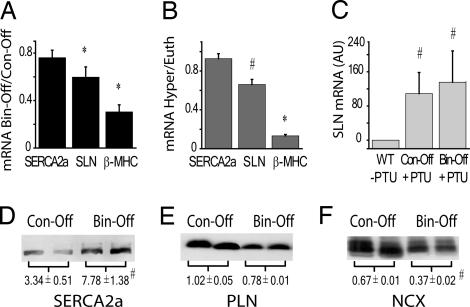
Because TH exerts many of its functional effects through transcriptional regulation (5) and because Ca2+ transients and myocyte contractility were elevated in Bin-Off mice, we measured mRNA levels of selected TH-regulated cardiac genes. Somewhat surprisingly, no differences were observed for sarco(endo)plasmic reticulum calcium ATPase (SERCA)2a (Fig. 4A) or for NCX, phospholamban (PLN), KV1.5, and KV4.2/3 mRNA levels (data not shown) between Bin-Off and Con-Off mice, whereas large reductions (P < 0.05) in β-MHC and sarcolipin (SLN) mRNA were detected 2 weeks (Fig. 4A) after DOX withdrawal. Because SR Ca2+ uptake rates and content were increased in Bin-Off mice and because TH can have nontranscriptional effects (5, 21, 22), we examined protein levels of NCX, SERCA2a, and PLN. Although PLN levels were not different, SERCA2a protein was elevated (P < 0.05), whereas NCX protein was reduced (P < 0.005), in Bin-Off mice compared with Con-Off mice (Fig. 4 D–F), consistent with previous studies in mice acutely treated with TH (23). The reduced NCX protein explains the increased (P < 0.05) time constants for the decay of INCX (i.e., τNCX) after caffeine application (Fig. 3 A and B).
Fig. 4.
Effects of D2 overexpression and TH on mRNA and protein levels. (A) Fractional changes in myocardial mRNA levels of SERCA2a, SLN, and β-MHC. GAPDH levels were used as a loading control (n = 5). (B) Changes in myocardial mRNA levels of SERCA2a, SLN, and β-MHC in ICR WT type mice (n = 4) after i.p. injection with l-thyroxine. (C) SLN mRNA expression relative to GAPDH in hearts from ICR WT mice and Bin-Off and Con-Off mice treated with a PTU-containing diet. (D–F) Western blots and relative quantifications of SERCA2a (D), PLN (E), and NCX (F) in Con-Off and Bin-Off mice. Blots were probed with calsequestrin antibody to check for equality in protein loading (n = 3 for each genotype; #, P < 0.05; ∗, P < 0.005 in Con-Off vs. Bin-Off mice and in euthyroid vs. hypothyroid/hyperthyroid mice).
Although enhanced Ca2+ handling and contractility in the Bin-Off mice is expected to result from altered SERCA2a and NCX protein expression, reductions in SLN mRNA are intriguing, because SLN is a PLN homologue (24–26); this reduction also implies that SLN is regulated by TH. Indeed, SLN mRNA levels were reduced by ≈40% (P < 0.05) in mice made hyperthyroid by treatment with exogenous TH (Fig. 4B) and were elevated in hypothyroid mice that were fed PTU (Table 1 and Fig. 4C).
Prolonged TH treatment has been shown to impair heart function, possibly because of (peripheral) extracardiac effects (14, 27, 28). As shown in Tables 1 and 2 (see also Table 3, which is published as supporting information on the PNAS web site), not only was deterioration of heart function not observed, but cardiac contractility and Ca2+ transients remained elevated in Bin-Off mice 9 weeks after being withdrawn from DOX compared with Con-Off mice. Moreover, the differences in mRNA and protein expression and SR Ca2+ uptake (data not shown) observed between Bin-Off and Con-Off mice after 2 weeks of DOX withdrawal were identical to those observed after 9 weeks of DOX removal. Therefore, the positive inotropic effects of D2 overexpression persist for prolonged periods.
Prevention of Heart Failure by Cardiac Expression of D2.
Previous studies have suggested that impaired TH signaling and altered TH metabolism occur in heart disease and might contribute to disease pathogenesis (8–10). On the other hand, elevated TH induces heart disease, possibly as a result of peripheral actions (14, 27, 28). To assess whether cardiac-restricted D2 overexpression is beneficial in heart disease, 8-week-old mice were DOX withdrawn and subjected to AB for 9 weeks. Echocardiographic and hemodynamic assessment of myocardial performance showed deterioration of myocardial contractile function accompanied by atrial and ventricular dilation of banded Con-Off (Con-Ban) mice compared with sham-operated Con-Off (Con-Sham) mice, whereas all of the functional alterations seen in Con-Ban mice were prevented in Bin-Off banded (Bin-Ban) mice (Table 2). We found that Con-Sham mice, after 9 weeks of DOX withdrawal, were indistinguishable both functionally (compare Tables 1 and 2) and biochemically (see below) from Con-Off mice 2 weeks or 9 weeks after DOX withdrawal. Therefore, Con-Sham data will not be presented. The differences between Con-Sham and Con-Ban or Bin-Ban mice can also be readily assessed by comparing the results in Figs. 1–4 with those in Fig. 5.
Fig. 5.
D2 expression prevents the development of the molecular and functional changes associated with heart failure. (A and B) Representative gross morphology of whole hearts from Con-Ban and Bin-Ban mice. (C and D) Representative recordings of CS (C) and Ca2+ transients (Indo-1 fluorescence ratio) (D) in field stimulated myocytes isolated from Con-Ban and Bin-Ban mice. (E) Summarized data for percentage of CS and maximal rate of contraction (+dL/dtmax) and relaxation (−dL/dtmax). (F) Summarized results of Indo-1 fluorescence ratio amplitudes (A405/485) measured in myocytes to assess Ca2+ transient amplitudes as well as maximal rate of ventricular SR Ca2+ uptake (Vmax) in isolated SR vesicles. (G and H) Ventricular mRNA levels of SLN, β-MHC, atrial natriuretic factor, and KV4.3 (G) and ventricular protein levels of SERCA2a, PLN, and NCX (H) in Con-Ban and Bin-Ban mice (n = 15; #, P < 0.05 in Con-Ban vs. Bin-Ban mice).
Despite protecting heart function, D2 overexpression had no effect on myocardial hypertrophy (Fig. 5A), establishing that D2 overexpression in the heart allows cardiac hypertrophy to be dissociated from contractile dysfunction (Fig. 5 B–F). Consistent with this, mouse hearts overexpressing D2 were protected against increased expression of pathological hypertrophy markers (KV4.3, atrial natriuretic factor, and β-MHC) in response to AB, as shown in Fig. 5G (6). At the cellular level, it is clear from Fig. 2, that myocyte contractility, Ca2+ transients, and SR Ca2+ uptake rates are reduced in Con-Ban compared with Con-Sham myocytes, whereas Bin-Ban mice were again completely protected against these changes. The cellular changes observed in Con-Ban mice were associated with corresponding reductions (P < 0.05) in SERCA2a, without alterations in PLN and NCX protein levels (Fig. 5H vs. Fig. 4 D–F) compared with Con-Off mice. Remarkably, the changes in SLN (Fig. 5G) and SERCA2a (Fig. 5H) induced by AB were completely prevented in Bin-Ban mice, possibly underlying the increases in Ca2+ transients as well as the increased maximal rate of SR Ca2+ uptake observed in those mice. Taken together, these results establish that D2 overexpression can rescue both functional and pathological genetic remodeling induced by AB.
It could be argued that the preservation of cardiac function occurs in response to D2 overexpression after AB because heart function is elevated by D2 overexpression in the absence of pressure overload. However, careful comparisons of the in vivo functional differences reveal that the relative functional impairment in Bin-Ban mice compared with Bin-Off mice (9 weeks post-DOX) is approximately four-fold less than that observed in Con-Ban compared with Con-Off mice (compare Tables 1 and 2). Similarly, the reduction in CS was much less in Bin-Ban vs. Bin-Off mice (at 9 weeks post-DOX) than that observed between Con-Ban and Con-Off mice. For example, the percentage of CS is reduced by 43 ± 4% in the Con-Ban mice compared with Con-Off (or Con-Sham) mice but is reduced by only 10.3 ± 2.2% in Bin-Ban mice compared with Bin-Off mice (9 weeks). Similar differences in the relative changes between Con-Ban and Con-Off mice vs. Bin-Ban and Bin-Off mice apply to the amplitude of calcium transient and the ±dL/dtmax (Table 3, Fig. 5 C–F, and data not shown). These observations demonstrate that the benefit of D2 overexpression after pressure overload does not simply originate from enhanced contractile function seen with D2 overexpression in nonbanded hearts.
Discussion
D2 converts the prohormone TH, T4, to active T3 (16, 17) and is the primary source of T3 in many tissues of the body, such as brain and human heart. Because altered TH signaling or metabolism is postulated to occur in heart disease (5, 7, 8) and because TH has been advocated as cardiac disease treatment (12), we created mice conditionally expressing D2 in the heart (18). Scrupulous feeding of DOX beginning before conception allows the separation of acute actions from chronic actions of TH while also avoiding developmental effects of TH (18). This is critical because developmental maturation of the heart is regulated by TH(1) and the α-MHC promoter used to drive D2 expression is activated during embryogenesis (29).
Consistent with the actions of D2, transient D2 overexpression for 2 weeks led to elevations in cardiac T3 levels without affecting serum T4 and T3 levels, which was associated with increased myocardial contractility and Ca2+ transients, as reported with acute exogenous TH treatment (5, 19). These functional changes appeared to be related to D2-mediated enhancements of T3, because DOX withdrawal of hypothyroid D2 mice did not affect contractility. A recent study suggested that tTA overexpression alone depresses contractility in vivo and elevates it in vitro (30). This was not observed in our DOX-withdrawn tTA-positive mice, consistent with previous studies (31).
Many effects of elevated TH levels occur by means of nuclear TH receptors (i.e., TH receptor α and TH receptor β), leading to transcriptional regulation of many cardiac genes, particularly those involved in Ca2+ cycling (5). Consistent with elevated TH in the hearts of mice overexpressing D2, we observed increases in Ca2+ transients, SR Ca2+ stores, and the maximal rate of SR Ca2+ uptake (Vmax) without alterations in the Ca2+ dependence of SR Ca2+ uptake (i.e., EC50). These changes were associated with increased expression of SERCA2a but not PLN, suggesting that SERCA2a elevations are responsible for the enhanced Ca2+ cycling and contractility in D2-overexpressing mice. However, D2 overexpression also reduced the levels of SLN, which can impair cardiac contractility and depress Ca2+ cycling (26, 32) in mouse myocardium as a result of either direct SERCA inhibition or synergistic interaction with PLN to form highly stable ternary SLN/PLN/SERCA2a complexes (24, 25). Depressed SLN levels in D2-overexpressing hearts appeared to be TH-dependent, because SLN expression was decreased in hyperthyroid hearts and increased in hypothyroid hearts. Thus, although SLN expression in mouse ventricles is low compared with atria (33) (M.G.T., unpublished data), our results support the possibility that SLN changes contribute to changes in Ca2+ cycling and contractility observed in the D2-overexpressing mice. Future studies are clearly warranted to unravel the potential role for SLN in Ca2+ homeostasis.
D2 overexpression also reduced NCX protein expression without changing its mRNA levels. This uncoupling between mRNA and protein could reflect posttranscriptional actions of elevated T3 levels in the D2 mice, as seen for other genes after TH treatment (22). Regardless, reduced NCX protein correlated with slower decay rates of INCX currents after SR Ca2+ release with caffeine (34) in D2-overexpressing mice. The functional consequence of reduced NCX activity/expression is difficult to predict because of complex interactions among NCX, SR, Ca2+ transport, membrane potential, transmembrane gradients of Ca2+ and Na+, and other cellular factors (35). Nevertheless, the conventional view has been that elevated NCX reduces contractility and Ca2+ cycling by depleting the SR of Ca2+ by means of the promotion of Ca2+ extrusion from the cytosol (36), which contributes to the negative force–frequency relationship and diastolic/systolic dysfunction (36, 37). Collectively, these observations suggest that reduced NCX levels in D2-overexpressing mice contribute to elevations of Ca2+ transients and contractility. Further studies are required to validate this conclusion.
D2 expression and elevated T3 levels in the heart were further associated with decreased expression of slow β-MHC levels and reduced β-MHC/α-MHC ratios, a pattern already observed in response to TH treatment in mouse (38). Because small increases in β-MHC/α-MHC ratios correlate with impaired cardiac function (39, 40) and because small elevations of β-MHC disproportionately slow contractile speed (40), it is conceivable that reduced β-MHC expression contributes to the increased myocardial contractility seen in our D2-overexpressing mice.
It is interesting to note that the molecular changes observed in our mice overexpressing D2 also occur in the postnatal period as a result of an endogenous TH surge (2, 4, 23). In contrast, the complex changes observed in heart disease recapitulate the fetal phenotype and mimic hypothyroidism (5, 8). Additionally, abnormal TH signaling/metabolism is reported in both heart disease and after cardiopulmonary bypass surgery (5, 7). Thus, TH therapy has been advocated for treating cardiac disease (12, 13), although clear benefit has been difficult to demonstrate, particularly with chronic use, possibly because of adverse extracardiac effects of TH (5, 27). The response of our D2-overexpressing mice to AB supports the conclusion that cardiac-restricted T3 production by D2 protects against deterioration of heart function induced by pressure overload. This preservation of heart function is undoubtedly complex but is likely related to Ca2+ cycling. Indeed, reduced expression of SERCA2a, along with impaired Ca2+ transients and SR Ca2+ uptake rates induced by pressure overload, were completely ameliorated in D2 transgenic mice, consistent with the beneficial effects of restored SERCA2a/PLN ratios in heart disease (41). Some of the protection could also be related to SLN, consistent with recent studies showing elevated SLN in the diseased heart (42).
Because T3 modulates the expression of many genes, it is conceivable that other genetic changes help to optimize the beneficial actions of TH on heart function. For example, down-regulation of KV4.3-encoding Ito and increased β-MHC/α-MHC ratio, both of which are prevented in D2-overexpressing mice subjected to pressure overload, have been suggested to contribute to depressed cardiac function in heart disease (39, 40, 43). On the other hand, although defective Ca2+ handling in heart disease has also been linked to elevated NCX expression/activity leading to SR Ca2+ depletion and negative force–frequency relationships (35), no NCX expression changes were observed in mice after pressure overload, as reported previously (36, 37).
As already mentioned, the benefit of D2 overexpression was not simply related to the enhanced baseline contractibility (compare Tables 1 and 2). Rather, protection against pressure overload in mice with increased D2 expression could be related to the reversal of a local cardiac hypothyroidism or correction of the altered TH signaling that is postulated to occur in diseased hearts (5, 7, 9). Consistent with a local hypothyroidism, we find that in response to pressure overload for 9 weeks there is a nearly 5-fold increase in the expression of the type 3 deiodinase (D3), an enzyme that converts T4 and T3 into inactive metabolites, such as reverse-T3 (rT3) (M.G.T., unpublished data). Because D2 can use rT3 as substrate, increased expression of D2 is expected to enhance TH signaling by countering the effects of elevated D3. Alternatively, elevated TH in our D2 mice could simply reverse the changes in gene expression and function induced by the activation of various hypertrophic signaling pathways involved in heart failure, either by directly regulating genes by means of T3 response elements or by interactions with other transcription factors regulating genes with altered expression in heart disease. For example, TH receptors interact with MEF2a (myocyte enhancer factor 2A), a known transcriptional modulator of pathological cardiac growth in myocytes (6, 44). Regardless of the precise mechanism of protection, our results establish that the functional rescue produced by cardiac-specific increases in D2 activity occurs without prevention of increased heart weight-to-body weight ratios. These observations are consistent with the notion that controlled hypertrophy without (excessive) changes in the expression of pathologic hypertrophic markers, such as β-myosin, Kv4.3, and atrial natriuretic factor, as observed in response to exercise (6) and in our D2 mice, is beneficial.
In conclusion, our results demonstrate that cardiac-targeted increases in the D2 activity cause TH-dependent elevations of myocardial contractility and Ca2+ cycling in association with reductions in the levels of SLN, NCX, β-MHC and elevation in SERCA2a levels. Our studies further establish that increased D2 activity can preserve heart function and normalize the expression of several genes involved in pathological remodeling, suggesting that cardiac-specific TH delivery may be an effective treatment in heart disease. However, because our conclusions are based on a rodent model of cardiac disease, it is difficult to predict whether the same approach might be successful in humans. Furthermore, our studies evaluated the effects of D2 overexpression in preventing the development of disease but did not explore the impact that D2 might have at different time points during the course of the disease or when cardiac disease is already established. Future studies are clearly mandatory to validate the therapeutic implications of our results.
Methods
Mouse Maintenance.
Chimeric rat D2 (tgD2) (16) was subcloned in a bidirectional vector [modified pBig (Clontech), with lacZ coding sequence replaced by EGFP] to allow simultaneous expression of D2 and EGFP under the control of the tTA. Two transgenic lines were generated. The results below were obtained in the D2-I line, with similar findings being observed in the second line. Transgenic mice were genotyped by PCR to differentiate between the endogenous D2 (585 bp) and transgenic D2 (724 bp) (Fig. 1 A and B). For most studies, homozygous D2 (tgD2+/+) males were crossed with heterozygous (tTA+/−) females expressing the tTA under the control of the α-myosin promoter (kindly provided by Glen Fishman, New York University School of Medicine, New York), to produce binary mice expressing tgD2 and tTA (tgD2+/−/tTA+/−, Bin) or littermate control mice positive for D2 only (tgD2+/−/tTA−/−, Con). The frequency of various genotypes followed the expected Mendelian pattern. D2 activity was observed in Bin mice but not Con mice (Fig. 1 D and E). Although controls used for the results presented below were tgD2+/−/tTA−/−, identical findings were obtained in tgD2−/−/tTA+/− and WT tgD2−/−/tTA−/− mice. Because TH affects prenatal and postnatal gene expression (1, 5), mothers were administered 0.1% DOX along with 0.5% sucrose in the drinking water for 2–4 weeks before conception until weaning. Offspring were continued on oral DOX until 8–10 weeks of age, at which time DOX was either maintained (Bin-On and Con-On) or removed (Bin-Off and Con-Off). Mice (ICR strain) were made hyperthyroid by daily i.p. injection of l-thyroxine (5 μg/g of body weight) for 8 days and hypothyroid by administering a 0.15% PTU-containing diet (Teklad Premier; Teklad, Madison, WI) for 5 weeks. All studies were performed in accordance with the standards of the Canadian Council on Animal Care.
SERCA Uptake Assay.
SR Ca2+ transport activity was determined as described in ref. 24. The SERCA-specific inhibitor thapsigargin at 1 μM was used to block Ca2+ uptake and to estimate the contribution of other Ca2+ pumps to the measured 45Ca2+ uptake.
Aortic Banding in Mice.
Descending AB was performed in 8-week-old mice, and the mice were assessed 9 weeks later. Administration of DOX to mice was discontinued 1 week before banding surgery.
Data Analysis.
One-way ANOVA combined with the Student–Newman–Keuls test for multiple comparisons between groups was used to test for statistical significance (spss 11; SPSS, Chicago). For the expression studies (mRNA and protein levels), Dunnett’s multiple comparisons test was used. Values are shown as mean ± SEM.
The remaining methods can be found in Supporting Methods and Table 4, which are published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
This work was supported by an operating grant from the Canadian Institutes for Health Research (to P.H.B., G.Y.O., and D.H.M.), the Heart and Stroke Foundation of Canada (M.G.T., B.-G.K., Y.P., A.O.G., and D.H.M.), the Heart and Stroke/Richard Lewar Centre of Excellence and the University of Toronto (M.G.T, B.-G.K., and Y.P.), a Tailored Advanced Collaborative Training in Cardiovascular Science fellowship and the University of Toronto (M.G.T. and G.Y.O.), and the Thyroid Foundation of Canada Diana Meltzer Abramsky Research Fellowship (to H.S.). W.C. and D.S.G. are supported by National Institutes of Health Grant DK42271. P.H.B. is a Career Investigator with the Heart and Stroke Foundation of Ontario.
Abbreviations
- SERCA
sarco(endo)plasmic reticulum calcium ATPase
- DOX
doxycycline
- TH
thyroid hormone
- SR
sarcoplasmic reticulum
- D2
type 2 deiodinase
- tTA
tetracycline transactivator
- T4
thyroxine
- T3
triiodothyronine
- SLN
sarcolipin
- AB
aortic banding
- PTU
5-propyl-2-thiouracil
- CS
cell shortening
- PLN
phospholamban
- tgD2
chimeric rat D2
- NCX
Na+/Ca2+ exchanger
- MHC
myosin heavy chain
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Bates J. M., St. Germain D. L., Galton V. A. Endocrinology. 1999;140:844–851. doi: 10.1210/endo.140.2.6537. [DOI] [PubMed] [Google Scholar]
- 2.Vigouroux E. Acta Endocrinol. 1976;83:752–762. doi: 10.1530/acta.0.0830752. [DOI] [PubMed] [Google Scholar]
- 3.Wickenden A. D., Kaprielian R., Parker T. G., Jones O. T., Backx P. H. J. Physiol. (London) 1997;504:271–286. doi: 10.1111/j.1469-7793.1997.271be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cernohorsky J., Pelouch V., Korecky B., Vetter R. Am. J. Physiol. 1998;275:H264–H273. doi: 10.1152/ajpheart.1998.275.1.H264. [DOI] [PubMed] [Google Scholar]
- 5.Klein I., Ojamaa K. N. Engl. J. Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 6.McKinsey T. A., Olson E. N. J. Clin. Invest. 2005;115:538–546. doi: 10.1172/JCI24144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinugawa K., Minobe W. A., Wood W. M., Ridgway E. C., Baxter J. D., Ribeiro R. C., Tawadrous M. F., Lowes B. A., Long C. S., Bristow M. R. Circulation. 2001;103:1089–1094. doi: 10.1161/01.cir.103.8.1089. [DOI] [PubMed] [Google Scholar]
- 8.Iervasi G., Pingitore A., Landi P., Raciti M., Ripoli A., Scarlattini M., L’Abbate A., Donato L. Circulation. 2003;107:708–713. doi: 10.1161/01.cir.0000048124.64204.3f. [DOI] [PubMed] [Google Scholar]
- 9.Wassen F. W., Schiel A. E., Kuiper G. G., Kaptein E., Bakker O., Visser T. J., Simonides W. S. Endocrinology. 2002;143:2812–2815. doi: 10.1210/endo.143.7.8985. [DOI] [PubMed] [Google Scholar]
- 10.Kinugawa K., Yonekura K., Ribeiro R. C., Eto Y., Aoyagi T., Baxter J. D., Camacho S. A., Bristow M. R., Long C. S., Simpson P. C. Circ. Res. 2001;89:591–598. doi: 10.1161/hh1901.096706. [DOI] [PubMed] [Google Scholar]
- 11.Gloss B., Trost S. U., Bluhm W. F., Swanson E. A., Clark R., Winkfein R., Janzen K. M., Giles W., Chassande O., Samarut J., Dillmann W. H. Endocrinology. 2001;142:544–550. doi: 10.1210/endo.142.2.7935. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton M. A., Stevenson L. W., Fonarow G. C., Steimle A., Goldhaber J. I., Child J. S., Chopra I. J., Moriguchi J. D., Hage A. Am. J. Cardiol. 1998;81:443–447. doi: 10.1016/s0002-9149(97)00950-8. [DOI] [PubMed] [Google Scholar]
- 13.Morkin E., Pennock G. D., Spooner P. H., Bahl J. J., Goldman S. Thyroid. 2002;12:527–533. doi: 10.1089/105072502760143935. [DOI] [PubMed] [Google Scholar]
- 14.Degens H., Gilde A. J., Lindhout M., Willemsen P. H., Van Der Vusse G. J., Van Bilsen M. Am. J. Physiol. 2003;284:H108–H115. doi: 10.1152/ajpheart.00282.2002. [DOI] [PubMed] [Google Scholar]
- 15.Ladenson P. W. Am. J. Med. 1990;88:638–641. doi: 10.1016/0002-9343(90)90532-i. [DOI] [PubMed] [Google Scholar]
- 16.Croteau W., Davey J. C., Galton V. A., St. Germain D. L. J. Clin. Invest. 1996;98:405–417. doi: 10.1172/JCI118806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dentice M., Morisco C., Vitale M., Rossi G., Fenzi G., Salvatore D. Mol. Endocrinol. 2003;17:1508–1521. doi: 10.1210/me.2002-0348. [DOI] [PubMed] [Google Scholar]
- 18.Yu Z., Redfern C. S., Fishman G. I. Circ. Res. 1996;79:691–697. doi: 10.1161/01.res.79.4.691. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz J. N., Robbins J. Am. J. Physiol. 1997;272:H1137–H1146. doi: 10.1152/ajpheart.1997.272.3.H1137. [DOI] [PubMed] [Google Scholar]
- 20.Sah R., Ramirez R. J., Oudit G. Y., Gidrewicz D., Trivieri M. G., Zobel C., Backx P. H. J. Physiol. (London) 2003;546:5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis P. J., Davis F. B. Thyroid. 2002;12:459–466. doi: 10.1089/105072502760143827. [DOI] [PubMed] [Google Scholar]
- 22.Khoury S. F., Hoit B. D., Dave V., Pawloski-Dahm C. M., Shao Y., Gabel M., Periasamy M., Walsh R. A. Circ. Res. 1996;79:727–735. doi: 10.1161/01.res.79.4.727. [DOI] [PubMed] [Google Scholar]
- 23.Reed T. D., Babu G. J., Ji Y., Zilberman A., Ver Heyen M., Wuytack F., Periasamy M. J. Mol. Cell. Cardiol. 2000;32:453–464. doi: 10.1006/jmcc.1999.1095. [DOI] [PubMed] [Google Scholar]
- 24.Asahi M., Kurzydlowski K., Tada M., MacLennan D. H. J. Biol. Chem. 2002;277:26725–26728. doi: 10.1074/jbc.C200269200. [DOI] [PubMed] [Google Scholar]
- 25.Asahi M., Sugita Y., Kurzydlowski K., De Leon S., Tada M., Toyoshima C., MacLennan D. H. Proc. Natl. Acad. Sci. USA. 2003;100:5040–5045. doi: 10.1073/pnas.0330962100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asahi M., Otsu K., Nakayama H., Hikoso S., Takeda T., Gramolini A. O., Trivieri M. G., Oudit G. Y., Morita T., Kusakari Y., et al. Proc. Natl. Acad. Sci. USA. 2004;101:9199–9204. doi: 10.1073/pnas.0402596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu L. W., Benvenuti L. A., Liberti E. A., Carneiro-Ramos M. S., Barreto-Chaves M. L. Am. J. Physiol. 2003;285:R1473–R1480. doi: 10.1152/ajpregu.00269.2003. [DOI] [PubMed] [Google Scholar]
- 28.Klein I. Endocrinology. 1988;123:203–210. doi: 10.1210/endo-123-1-203. [DOI] [PubMed] [Google Scholar]
- 29.Palermo J., Gulick J., Colbert M., Fewell J., Robbins J. Circ. Res. 1996;78:504–509. doi: 10.1161/01.res.78.3.504. [DOI] [PubMed] [Google Scholar]
- 30.McCloskey D. T., Turnbull L., Swigart P. M., Zambon A. C., Turcato S., Joho S., Grossman W., Conklin B. R., Simpson P. C., Baker A. J. Physiol. Genomics. 2005;22:118–126. doi: 10.1152/physiolgenomics.00016.2005. [DOI] [PubMed] [Google Scholar]
- 31.Sanbe A., Gulick J., Hanks M. C., Liang Q., Osinska H., Robbins J. Circ. Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 32.Babu G. J., Zheng Z., Natarajan P., Wheeler D., Janssen P. M., Periasamy M. Cardiovasc. Res. 2005;65:177–186. doi: 10.1016/j.cardiores.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Asahi M., Nakayama H., Tada M., Otsu K. Trends Cardiovasc. Med. 2003;13:152–157. doi: 10.1016/s1050-1738(03)00037-9. [DOI] [PubMed] [Google Scholar]
- 34.Kerfant B.-G., Gidrewicz D., Sun H., Oudit G. Y., Penninger J. M., Backx P. H. Circ. Res. 2005;96:1079–1086. doi: 10.1161/01.RES.0000168066.06333.df. [DOI] [PubMed] [Google Scholar]
- 35.Terracciano C. M. Ann. N.Y. Acad. Sci. 2002;976:520–527. doi: 10.1111/j.1749-6632.2002.tb04786.x. [DOI] [PubMed] [Google Scholar]
- 36.Hasenfuss G., Schillinger W., Lehnart S. E., Preuss M., Pieske B., Maier L. S., Prestle J., Minami K., Just H. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 37.Schillinger W., Fiolet J. W., Schlotthauer K., Hasenfuss G. Cardiovasc. Res. 2003;57:921–933. doi: 10.1016/s0008-6363(02)00826-x. [DOI] [PubMed] [Google Scholar]
- 38.Ojamaa K., Kenessey A., Shenoy R., Klein I. Am. J. Physiol. 2000;279:E1319–E1324. doi: 10.1152/ajpendo.2000.279.6.E1319. [DOI] [PubMed] [Google Scholar]
- 39.Nakao K., Minobe W., Roden R., Bristow M. R., Leinwand L. A. J. Clin. Invest. 1997;100:2362–2370. doi: 10.1172/JCI119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tardiff J. C., Hewett T. E., Factor S. M., Vikstrom K. L., Robbins J., Leinwand L. A. Am. J. Physiol. 2000;278:H412–H419. doi: 10.1152/ajpheart.2000.278.2.H412. [DOI] [PubMed] [Google Scholar]
- 41.MacLennan D. H., Kranias E. G. Nat. Rev. Mol. Cell. Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 42.Pashmforoush M., Lu J. T., Chen H., Amand T. S., Kondo R., Pradervand S., Evans S. M., Clark B., Feramisco J. R., Giles W., et al. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 43.Oudit G. Y., Kassiri Z., Sah R., Ramirez R. J., Zobel C., Backx P. H. J. Mol. Cell. Cardiol. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- 44.Lee Y., Nadal-Ginard B., Mahdavi V., Izumo S. Mol. Cell. Biol. 1997;17:2745–2755. doi: 10.1128/mcb.17.5.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.