Abstract
A homologous series of mono- and bis-acyl polyamines with varying acyl chain lengths originally synthesized for the purpose of sequestering lipopolysaccharide were evaluated for antimicrobial activity to test the hypothesis that these bis-cationic amphipathic compounds may also bind to and permeabilize intact gram-negative bacterial membranes. Some compounds were found to possess significant antimicrobial activity, mediated via permeabilization of bacterial membranes. Structure-activity relationship studies revealed a strong dependence of the acyl chain length on antimicrobial potency and permeabilization activity. Homologated spermine, bis-acylated with C8 or C9 chains, was found to profoundly sensitize Escherichia coli to hydrophobic antibiotics such as rifampin. Nonspecific cytotoxicity is a potential drawback of these membranophilic compounds. However, the surface activity of these cationic amphipaths is strongly attenuated under physiological conditions via binding to serum albumin. Significant antibacterial activity is still retained in the presence of physiological concentrations of human serum albumin, suggesting that these compounds may serve as leads in the development of novel adjuncts to conventional antimicrobial chemotherapy.
The accelerated emergence of many strains of multidrug-resistant bacteria as a result of widespread use and misuse of antibiotics has mandated the urgent need for a renewed search for novel antibacterial agents. The presence of an outer membrane (OM) in gram-negative bacteria provides an effective protective barrier in these organisms (33, 34) to antimicrobial agents that may otherwise be active. For instance, it has been reported that in antibiotics of natural origin that are active against gram-positive bacteria, more than 90% lacked activity at a useful level against Escherichia coli (55). The barrier, formed by a divalent cation-cross-linked matrix (36, 40) of lipopolysaccharide (LPS) molecules on the outer leaflet of the OM (17, 48), can be breached by metal-chelating agents such as EDTA or via displacement of LPS-bound metals by polycations of diverse structural classes (20, 36, 40, 54, 57).
Polymyxin B (PMB), a cyclic, pentacationic, amphipathic peptide antibiotic isolated from Bacillus polymyxa (49) is a prototype membrane-perturbing agent whose antibacterial action is manifested via its binding to the lipid A moiety of LPS. Perturbation of the OM alone has been thought to result in bacterial killing, since immobilized PMB can disrupt the OM (41); however, alternate hypotheses concerning “self-promoted” uptake of the antibiotic and subsequent perturbation of the inner membrane (IM), culminating in bacterial lysis, have also been suggested (11, 64). The recognition that membrane-active antimicrobials have not yet been exploited adequately in the clinic has spurred the search for noncytolytic, selective bacterial membrane-permeabilizing agents, notable examples of which include cationic peptides (18, 19) and small-molecule PMB mimics (12, 13, 27, 44). The use of cationic peptides as therapeutic agents is fraught with several potential problems, including large therapeutic doses (a consequence of high molecular weight), parenteral administration because of the lack of oral bioavailability, immunogenicity, and nonspecific cytotoxicity (60).
The general pharmacophore determining antibacterial activity in cationic peptides appears to be simply the presence of protonatable positive charges and hydrophobic groups, i.e., cationic amphipathicity (50). We have, for a number of years, been interested in evaluating cationic, amphipathic small molecules as specific lipopolysaccharide sequestrants (for a recent review, see reference 7). Of particular interest are compounds belonging to the lipopolyamine class, characterized by the presence of long-chain acyl or alkyl substituents on polyamine scaffolds; members of the lipopolyamine class bind LPS, are effective in preventing endotoxic shock in animal models, and yet appear to be nontoxic both in vitro and in vivo (10).
We had found that dioleoyl-substituted spermine, although capable of forming stable complexes with LPS with consequent neutralization of endotoxicity, is bereft of antibacterial activity at concentrations up to 75 μg/ml (10). This may have been attributable to the extremely labile ester-linked acyl moieties in dioleoyl-substituted spermine. Indeed, recent reports indicate that, both in lipoamines with stable, amide-linked acyl groups (65) as well as in cationic amphipathic peptides (28, 46), a correlation between antiendotoxic and antibacterial activity does exist. Therapeutic agents with combined antibacterial and endotoxin-sequestering activities may offer significant advantages in addressing the problem of antibiotic-induced endotoxin release (22-24), a contributory factor in the development of endotoxic shock in gram-negative sepsis (38, 39). We recently reported the interactions of a series of N-acylated homologated spermine compounds with LPS (30). These lipopolyamine compounds possess potent endotoxin-sequestering activity in vitro and afford protection in animal models of gram-negative sepsis. Not only are they synthetically easily accessible but, importantly, they are also nontoxic due to their degradation to physiological substituents (polyamine and fatty acid) (4, 10).
In this paper, we present the results of studies on intrinsic antibacterial activity, outer- and inner-membrane-permeabilizing properties, and sensitization of bacteria to hydrophobic antibiotics. Some compounds were found to possess significant antimicrobial activity, which appears to be mediated primarily via permeabilization of bacterial membranes. Structure-activity relationship studies revealed a strong dependence of the acyl chain length on antimicrobial potency and permeabilization activity. Homologated spermine, bis-acylated with C8 or C9 chains, was found to profoundly sensitize E. coli to hydrophobic antibiotics such as rifampin. Nonspecific cytotoxicity is a potential drawback of these membranophilic compounds. However, the surface activity of these cationic amphipaths is strongly attenuated under physiological conditions via binding to serum albumin. Significant antibacterial activity is still retained in the presence of physiological concentrations of human serum albumin, suggesting that these compounds may serve as leads in the development of novel adjuncts to conventional antimicrobial chemotherapy.
MATERIALS AND METHODS
Synthesis.
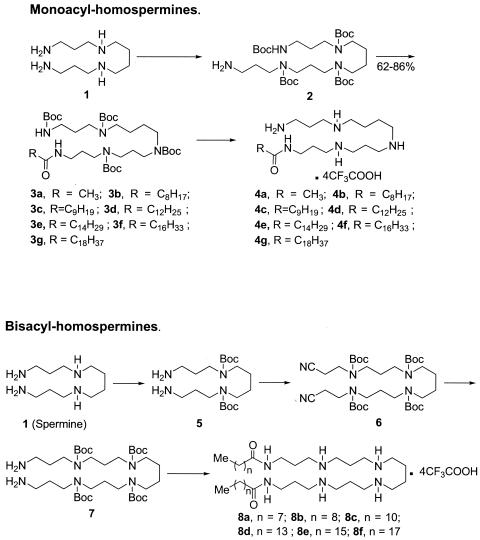
Details of the syntheses of the acylpolyamines (except compound 4g) have recently been published previously (30). A summary of the synthetic strategy and the structures of the mono- and bis-acyl compounds are shown in Fig. 1. Compound 4g was characterized by NMR spectroscopy, and mass spectrometry, and purity was established by elemental analysis.
FIG. 1.
Syntheses of mono- and bis-acyl homospermine compounds. Detailed methods have been published previously (30).
Bacterial strains.
E. coli strain 9637 and Staphylococcus aureus strain 13709 were procured from ATCC (Manassas, VA). For IM permeability assays, E. coli ML-35 (ATCC 43827), a lactose permease-deficient strain with constitutive cytoplasmic β-galactosidase activity, was used (26). Calcium chloride transformation of E. coli ML-35 was performed using the plasmid vector pBR322 (5), encoding tetracycline and ampicillin resistance genes (Promega, Madison, WI). The transformed strain, E. coli ML-35p, selected by ampicillin resistance, was utilized for the OM permeabilization assay. E. coli ML-35p was maintained on Trypticase soy agar plates with 50 μg/ml of ampicillin.
Determination of MIC.
MICs of the acylpolyamines were determined by broth microdilution method (6) per CLSI (formerly NCCLS) guidelines. Mid-log-phase Mueller-Hinton broth (MHB; noncation supplemented) cultures of organisms (40 μl; optical density at 600 nm adjusted to 0.5 AU, and diluted 10-fold) were added to equal volumes of 2-fold serially diluted acylpolyamines in a 384-well microtiter plate with the help of a Biotek Precision 2000 automated microplate pipetting system. The MICs of rifampin, PMB, PMB nonapeptide (PMBN), naphthylacetyl spermine trihydrochloride, and methoctramine tetrahydrochloride (Sigma, St. Louis, MO) were included as reference compounds for comparison of activity. The microtiter plates were sealed and incubated overnight at 37°C. The plates were read at an absorbance of 600 nm. The lowest concentration of an agent inhibiting growth of the organisms was recorded as the MIC.
OM permeability.
A procedure similar to that reported by Lehrer et al. (26), modified for high-throughput readout was used. Nitrocefin (Calbiochem, San Diego, CA) was used for the determination of periplasmic β-lactamase activity since PADAC has been reported to be frequently insensitive to β-lactamase activity in clinically relevant strains of Staphylococcus (1). Harvested mid-log-phase cultures of E. coli ML-35p (optical density at 600 nm adjusted to 0.5 AU) grown in Trypticase soy broth were washed three times with normal saline (0.9%). Nitrocefin was added to a final concentration of 50 μg/ml to the washed bacterial suspension, which was then added to the serially diluted compounds in a 384-well microtiter plate as described earlier. PMB, PMBN, and melittin (Sigma, St. Louis, MO), a potent membrane-active bee venom peptide (16, 26, 37, 45), were used as reference compounds. After various times of incubation at 37°C, β-lactamase activity was measured spectrophotometrically at 486 nm using an automated Spectramax M2 instrument (Molecular Devices, Sunnyvale, CA).
IM permeability.
o-Nitrophenyl-β-d-galactopyranoside (ONPG; Sigma, St. Louis, MO) was used as the substrate to determine β-galactosidase activity (26). Washed cultures of E. coli ML-35 mixed with 1.5 mM ONPG in normal saline (0.9%) were added to serially diluted compounds in a 384-well microtiter plate. PMBN and melittin were used as the controls. The production of o-nitrophenol was quantified absorptiometrically at 420 nm after an incubation period of 1 h at 37°C.
Sensitization to rifampin.
To determine the effect of acylpolyamines on the MIC of the hydrophobic antibiotic rifampin, E. coli strain 9637 was used. Overnight cultures of E. coli grown in MHB preincubated with 10 μM acylpolyamines were added to serially diluted rifampin in a 384-well microtiter plate. After incubation at 37°C overnight, the MIC was determined. Controls included PMBN and melittin (positive) and rifampin alone (negative). All experiments were run in triplicate.
Surface tension measurements.
The surface activity of the test compounds was measured via dynamic bubble pressure and surface age tensiometry (14) using a PocketDyne instrument (Krüss GmbH, Hamburg, Germany) as described earlier (30). Samples were at 500 μM concentration in 50 mM Tris buffer, pH 7.4, containing 5% dimethyl sulfoxide (DMSO). The instrument was calibrated with water at 25°C (72 mN/m), and surface tension values were recorded over a range of bubble surface ages from 100 to 1,500 ms at 25°C.
Hemolytic activity.
To test the hypothesis that the antibacterial activities of the acylpolyamines are a consequence of membrane permeabilization due to their cationic amphipathic nature, we sought to correlate the surface activity of these compounds with hemolytic potency (42). Erythrocyte damage was measured using two different techniques. In the first, hemolysis was quantified using extremely diluted, aged human whole blood such that the effects of the compounds binding to plasma proteins would be negligible and the hemolytic activity would be magnified because of increased osmotic fragility of the erythrocytes as a consequence of depleted Na+ K+ ATPase activity (32). Dilute erythrocyte suspensions were prepared by diluting 1-week-old whole blood obtained by venipuncture from healthy human volunteers 1:1,000 in isotonic (0.9 g/100 ml) saline solution to which was added graded doses of compound. Absorptiometric determinations of hemoglobin released from such dilute erythrocyte suspensions were not reliable. The samples were therefore examined with a Beckman-Coulter Vi-Cell cell viability analyzer (Beckman-Coulter, Hialeah, FL). This instrument implements an automated intravital trypan blue exclusion method using real-time automated video microscopy. Measurement parameters for erythrocytes were gated appropriately on control erythrocytes to specify thresholds of cell recognition and viability. Data on total numbers of cells/ml and viable cells/ml were collected through 50 captured images per sample with a counting accuracy of ±3%. To examine the effect of plasma proteins on the surface activity, some of the experiments were repeated in the presence of near-physiological concentrations of human serum albumin. Because it became apparent that the compounds were binding strongly to albumin, thereby resulting in an almost complete abrogation of hemolytic activity, it was of interest to examine the compounds under physiological conditions. The second method, consequently, was designed to examine the effects of the compounds on whole blood. One hundred microliters of serially diluted compounds was mixed with an equal volume of fresh, undiluted, EDTA-anticoagulated human blood in a 96-well microplate using an automated liquid handler. After incubation at 37°C for 30 min, the plates were centrifuged at 3,000 rpm for 10 min, 80 μl of supernatants was transferred to a fresh plate, and the amount of free hemoglobin released into the supernatant was quantified using absorptiometry at 570 nm. In the latter assay, melittin, a potently hemolytic α-helical bee venom peptide (9), was used as a positive control.
Acute and subacute toxicity studies in mice.
In acute toxicity studies, graded doses of compound 4e (100 μg to 500 μg) in 0.2 ml saline was injected intraperitoneally or subcutaneously in cohorts of 5 CF-1 outbred mice per dose, according to IACUC-approved protocols. Clinical signs of acute toxicity were monitored for 48 h following a single injection. In subacute studies, 100 μg of compound 4e was injected daily in the subcutaneous tissue of the flank region, with the sites alternated every day, for a duration of 15 days. The animals were monitored daily for signs of local irritation, weight loss, and food consumption.
RESULTS AND DISCUSSION
Growth-inhibitory activity against gram-negative and -positive bacteria.
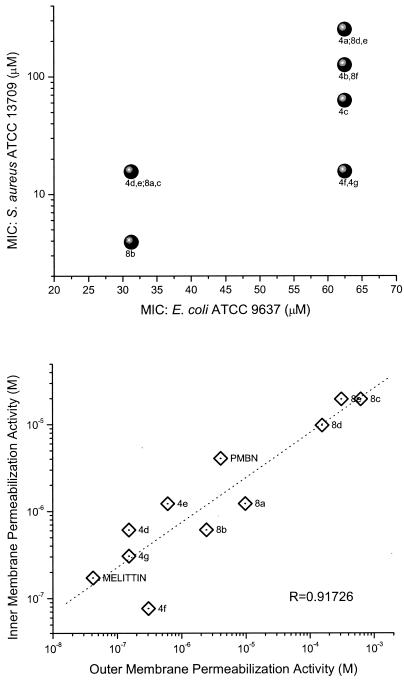
The MICs of the acylpolyamines for E. coli ATCC 9637 and S. aureus ATCC 13709 are summarized in Table 1. Also included in Table 1 are the MICs of naphthylacetylspermine and methoctramine, which are hydrophobically substituted polyamines recently shown to exert membrane-permeabilizing activity (63); PMB, a peptide antibiotic known to disrupt OM integrity (43, 62) by binding to LPS (3, 8, 31, 51); PMBN, a deacylated derivative of PMB known to effectively permeabilize gram-negative OM, but exerting a highly attenuated antimicrobial potency (52-54, 57, 59, 61); and melittin, a cytolytic, highly membrane-active α-helical peptide constituent of bee venom (9, 21, 37, 45). It is noteworthy that the range of MICs of both the mono- and bis-substituted long-chain aliphatic acylpolyamines used in this study against E. coli is rather narrow (31.25 μM to 62.5 μM; two dilutions), while both the mono- (naphthylacetylspermine) and bis-aryl (methoctramine) compounds display significantly lower MICs (1,250 μM and 312.5 μM, respectively) (Table 1). The MICs of these latter two compounds reported by Yasuda et al. (63) for E. coli W3110 were >267 μM (>128 mg/liter) and 22 μM (16 mg/liter), respectively. These discrepancies may be attributable to the differences in the strains used.
TABLE 1.
MICs of lipopolyamines for E. coli and S. aureus
| Compound | MIC [μM (μg/ml)] for:
|
|
|---|---|---|
| E. coli ATCC 9637 | S. aureus ATCC 13709 | |
| 4a | 62.5 (47.3) | 250 (189.3) |
| 4b | 62.5 (53.4) | 125 (106.96) |
| 4c | 62.5 (54.3) | 62.5 (54.3) |
| 4d | 31.25 (28.9) | 15.6 (14.4) |
| 4e | 31.25 (29.3) | 15.6 (14.6) |
| 4f | 62.5 (60.4) | 15.6 (15.1) |
| 4g | 62.5 (62.2) | 15.6 (15.5) |
| 8a | 31.25 (32.9) | 15.6 (16.4) |
| 8b | 31.25 (33.7) | 3.9 (4.2) |
| 8c | 31.25 (35.5) | 15.6 (17.7) |
| 8d | 62.5 (76.3) | 250 (305.3) |
| 8e | 62.5 (79.4) | 250 (319.3) |
| 8f | 62.5 (83.3) | 125 (166.7) |
| PMB | 3.9 (5.4) | 125 (173.2) |
| PMBN | 250 (240.7) | 500 (481.5) |
| Melittin | 175 (498.1) | 5.5 (15.6) |
| Naphthylacetylspermine | 1,250 (599.8) | 1,250 (599.8) |
| Methoctramine | 312.5 (227.7) | 156.25 (113.8) |
A much wider dispersion in intrinsic antibacterial effect against S. aureus is observed, with a range of 4 μM (compound 8b) to 250 μM (compound 4a). A cursory inspection of the data would suggest that the antimicrobial potency against S. aureus could be correlated with the hydrophobicity of the acyl group. For instance, compound 8b, a bis-C8-acyl compound, is expected to be more surface active (see below) than the mono-acetyl compound 4a derivative, which would be consonant with reports in the literature indicating that gram-positive organisms are, in general, more susceptible to cationic amphipathic substances, with susceptibility increasing with amphipathicity (15, 18, 45). However, the MICs for S. aureus of compounds 8a and 8c, both immediate structural neighbors of compound 8b, are considerably higher. Furthermore, we observed a poor correlation between the MICs for E. coli and S. aureus (Fig. 2, top panel). These results point to the potential complexity of physicochemical features that dictate structure-activity relationships, which may not be directly attributable to hydrophobicity. Indeed, measures of hydrophobicity such as C18 reverse-phase high-performance liquid chromatography retention times, computed logP values (data not shown), or surface tension measurements (see below) correlate poorly with the observed antibacterial activities.
FIG. 2.
(Top) Correlation of MICs of the acylpolyamines against E. coli (ATCC 9637) and S. aureus (ATCC 13709). MICs were determined by broth microdilution method in Mueller-Hinton broth as per CLSI guidelines. (Bottom) Correlation of OM and IM permeabilizing activity. OM permeabilizing activity was determined using E. coli ML-35p (parent i− z+ y− ATCC 43827 transformed with pBR322 vector encoding periplasmic β-lactamase); the leakage of periplasmic β-lactamase activity was quantified using nitrocefin as a chromogenic substrate. IM permeabilizing activity was determined using E. coli ML-35 using o-nitrophenyl-β-d-galactopyranoside as the substrate to determine the β-galactosidase activity. In all experiments, PMB, PMBN, and melittin were used as reference compounds.
Perturbation of OM and IM.
Our primary interest in examining these compounds, however, lies not so much in evaluating their intrinsic antimicrobial properties but rather in understanding the mechanisms and structure-activity relationships underlying their putative membrane-permeabilizing action and in exploring the possibility of employing such compounds as adjuncts to conventional chemotherapy against resistant organisms for purposes of sequestering endotoxin released as a consequence of gram-negative bacterial lysis. We first investigated whether the acylpolyamines would act on both the IM and OM, presumably as a consequence of nonspecific membranophilic effects, as has been reported for a variety of cationic amphipathic peptides such as melittin (26), defensins (25), and bactenecins (47), or selectively perturb the OM in the manner of PMB (26). OM and IM permeability were determined, respectively, from dose-response curves of nitrocefin and ONPG hydrolysis rates as described in the literature (26). As shown in Fig. 2 (bottom panel), a direct linear relationship was observed between OM- and IM-permeabilizing activities. Furthermore, these two events seem tightly coupled with near-identical kinetics even under conditions of high osmotic strength (data not shown), as has also been observed with antibacterial host defense peptides (25). Although IM damage would necessarily require antecedent OM lysis, the lag times in the hydrolysis of the chromogenic substrates are too short for a clear discrimination to be observed under the experimental conditions employed. As has been hypothesized in the case of antibacterial peptides, the mechanism of bacterial killing likely involves loss of IM integrity.
Sensitization to hydrophobic antibiotics.
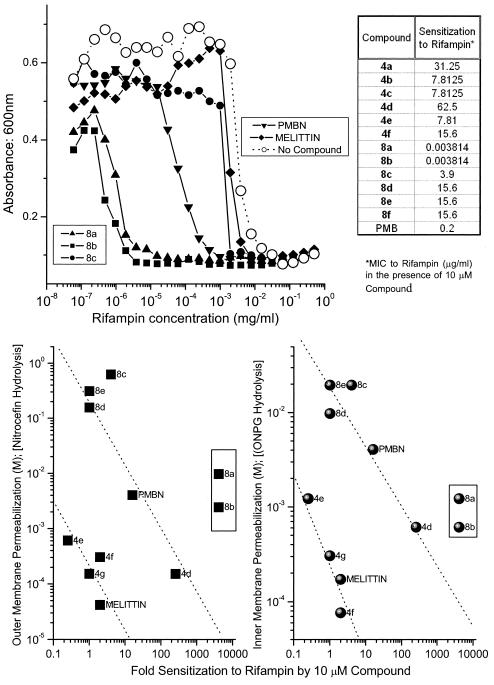
Perturbation of the outer membrane permeability barrier greatly sensitizes gram-negative organisms to otherwise impermeable hydrophobic solutes, with rifampin being a classic example (34, 56, 58). We specifically sought to examine the correlation between OM and IM permeabilization and sensitization to rifampin at a concentration of 10 μM (lower than the MICs) of the acylpolyamines, the results of which are presented in Fig. 3. We had anticipated a simple linear relationship between both OM and IM permeabilization on the one hand (since, as mentioned earlier, we had observed a tightly coupled effect) and sensitization to rifampin on the other. Somewhat to our surprise, we observed a clear demarcation of the compounds into distinct subsets with differential activity (Fig. 3, bottom panel). Compounds 4e to 4g and melittin, all of which are potently membrane permeabilizing, sensitized E. coli ATCC 9637 to an apparently lesser degree than compounds 8c to e, PMBN, and compound 4d. In both of these groups of compounds, there was indeed a demonstrable direct correlation between permeabilizing activity and rifampin sensitization (Fig. 3, bottom panel). Distinct from these two groups, however, compounds 8a and 8b were found to possess extremely high sensitizing activity. A 10 μM concentration of PMBN, a prototype membrane-permeabilizing compound, lowered the MIC of rifampin from 15.625 μg/ml to 0.976 μg/ml (16-fold), while the sensitization activities of compounds 8a and 8b were increased 4,096-fold (Fig. 3, bottom panel). In light of the fact that the nitrocefin and ONPG hydrolysis assays do not discriminate between complete membrane lysis of a small fraction of bacteria from partial lysis of a larger fraction of bacteria, we provisionally interpret these results as follows: long-chain mono-acyl compounds such as compounds 4g and 4f and melittin are highly membrane active (Fig. 2, bottom panel) and are likely to lyse immediately the fraction of the organisms that the compound first comes in contact with, given their higher propensity to self-aggregate in membranes; the remainder of the bacteria are unaffected, continue to proliferate in culture, and thus do not manifest in an apparent enhancement of susceptibility to rifampin. In contrast, the fact that the bis-acyl compounds are less hemolytic than the mono-acyl analogues (see below) suggests that the bis-acyl compounds 8a and 8b may interact with outer membranes more diffusely. We surmise, therefore, that the resultant nonlethal perturbation of a greater fraction of the bacteria is reflected as a profound enhancement in the susceptibility to hydrophobic antibiotics such as rifampin. While the definitive interpretation of these results must await detailed experiments involving measurements with the fraction of bacteria with depolarized membrane potentials using a method such as flow cytometry (35), we wish to point out the significance of the highly pronounced sensitizing activity of compounds such as 8a and 8b relative to PMBN.
FIG. 3.
(Top, left) Representative titration experiment showing the sensitization activities of some bis-acyl analogues. E. coli ATCC 9637 was seeded in MHB in checkerboard format in a 384-well plate containing a constant concentration of compound and varying doses of rifampin. Bacterial growth was monitored by turbidimetry at 600 nm. PMBN and melittin were used as positive controls. Wells containing no test compound served as negative controls. (Top, right) Table of values showing sensitization to Rifampin by all compound 4 and 8 series compounds. (Bottom) Plot of OM and IM permeabilization activity against extent of sensitization by the acylpolyamines. Relative sensitization (n-fold) was calculated as the MIC of rifampin alone/MIC of rifampin plus 10 μM Compound.
Structure-activity relationships.
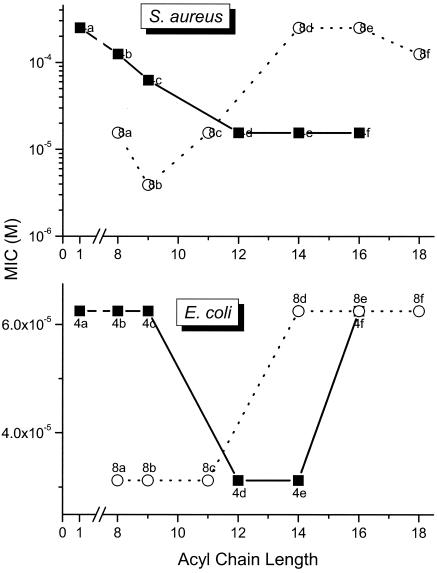
We had reported earlier that the carbon number (hydrophobicity) of the homologous series of mono- and bis-acyl polyamines was a critical structural determinant of LPS-neutralizing potency (30); we had found that for the mono-acyl compound 4 series of compounds, there was a progressive increase in LPS-neutralizing potency, while for the bis-acyl compound 8 series, the activity progressively decreased with acyl chains longer than dodecyl (C12). We were therefore interested in examining possible correlations with MICs for E. coli and to verify if the activity profile would be similar in inhibiting the growth of S. aureus. The results shown in Fig. 4 indicate that against both organisms, a very similar structure-activity correlation is observed. Thus, for the compound 4 series, acyl chain lengths from C12 to C16 result in maximal antimicrobial efficacy against S. aureus. Maximal antibacterial effects are observed between C12 to C14 against E. coli, with the activity falling off at C16, suggesting that the structural requisites for optimal interaction with the gram-negative outer membrane are rather specific. For the compound 8 series, however, the converse is true with the short chain (C8-11) analogues exhibiting maximal antibacterial effect (Fig. 4); the decline in activity in the higher homologues in the compound 8 series is ascribable to progressive loss of aqueous solubility as reported earlier (30). It is of interest that a very similar structure-activity relationship was observed with these compounds in terms of inhibition of LPS-induced tumor necrosis factor alpha and nitric oxide production in murine macrophages (30). The similarities of the compound 4 and 8 series between antimicrobial activity against gram-negative bacteria on the one hand and sequestration of LPS on the other would suggest that the antimicrobial activity may be mediated via the interaction of these compounds with the outer membrane.
FIG. 4.
Correlation of MIC for S. aureus (top) and E. coli (bottom) with length of the acyl group.
Surface activity.
Charged, amphipathic molecules are surface active and can be cytolytic to mammalian cells. In particular, the compound 8 series are analogous to “Gemini surfactants,” so named after their twin-headed structures (29) and could, possibly, display nonspecific cytotoxicity because of membrane-perturbing activity. As expected, the Gemini-like compounds 8a and 8b (measured in 5% DMSO to ensure solubility; the higher homologs were insoluble and could not be tested) are indeed considerably surface active (Fig. 5). For the compound 4 series (all of which were freely soluble in 5% DMSO), there is a distinct correlation between acyl chain length and surface tension-lowering activity, as could be expected, with homologs with longer acyl chains becoming progressively more surface active (Fig. 5, top panel). Contrary to our expectation, we found a lack of correlation between surface activity and MIC against both E. coli and S. aureus, with compound 4d (for both organisms) and compound 8b (for S. aureus) being significant outliers (Fig. 5, bottom panel). These results suggest a specific interaction of these two compounds with bacterial membranes rather than a nonspecific, surface activity-related membrane perturbation. Raman spectroscopic experiments are being planned which may provide a better understanding of the mechanisms of interfacial phenomena at the bacterial cell surface.
FIG. 5.
(Top) Surface tension measurements of compound 4 and 8 series compounds by dynamic pressure tensiometry. The slopes of the lines are directly proportional to the critical micellar concentrations. (Bottom) Correlation of surface activity with antimicrobial activities against E. coli ATCC 9637 and S. aureus ATCC 13709.
Hemolytic activity and in vivo toxicity.
We quantified the hemolysis induced by the compounds using an extremely dilute suspension of washed, aged human erythrocytes under protein-free conditions (isotonic saline). In this assay, erythrocytes become exquisitely susceptible to membrane damage and lysis, not only because of increased osmotic fragility of the erythrocytes due to depleted Na+ K+ ATPase activity (32) but also due to the absence of buffering effects of plasma proteins. As expected (30), increasing acyl chain length is paralleled by higher hemolytic activity, particularly for the compound 4 series (Fig. 6, top left panel). It is to be noted that the hemolytic activity of the bis-acyl compound 8 series is biphasic, increasing substantially from compound 8a (C7) to compound 8c (C10) and then diminishing at higher carbon chain lengths (Fig. 6, top right panel) due to decreasing solubility. Thus, for the compound 8 series, the lack of adequate aqueous solubility may likely account for the progressive decline in antimicrobial activity of the higher homologs as shown in Fig. 4.
FIG. 6.
(Top, left) Hemolytic activity of the acylpolyamines in highly dilute, washed, aged, human erythrocytes suspended in isotonic saline quantified by automated video microscopy. (Top, right) Correlation of carbon number of compound 4 and 8 series of compounds with hemolytic activity. IC50, 50% inhibitory concentration. (Bottom, left) Abrogation of hemolysis as described above by representative mono-acyl compounds in the presence of 650 μM human serum albumin. (Bottom, right) Absorptiometric determination of hemolytic activity in fresh, whole human blood by quantifying released hemoglobin. Melittin was used as a positive control.
The pronounced hemolytic activity of long-chain acylated compounds such as compounds 4e and 4f (100% hemolysis at 1 to 5 μM) occasioned concern, and we questioned if the results of this assay employing deliberately exaggerated erythrocytic fragility would be physiologically relevant; that is, if these compounds would likely cause intravascular hemolysis in vivo if administered parenterally. In the course of our investigations, we found that the acylpolyamines bind strongly to albumin. Detailed biophysical studies on the characterization of the binding site on albumin, stoichiometry, and dependence of binding affinity on mono- versus bis-acylation and acyl chain length will be published elsewhere. The hemolytic activity of the acylpolyamines is completely abrogated, even at very high concentrations, in the presence of physiological concentrations (∼650 μM) of human serum albumin as observed with compounds 4f and 4g, shown as representative data (Fig. 6, bottom left panel), indicating that a large fraction of these compounds is bound to albumin and that the protein-bound form would be unlikely to exert toxicity in vivo. The hemolytic activities of these compounds were therefore reexamined using human whole blood and, consistent with our hypothesis, we observed significant hemolysis starting to occur only at millimolar concentrations (Fig. 6, bottom right panel). In these latter experiments, melittin, an α-helical 26-residue hemolytic bee venom peptide (2, 9), caused hemolysis at low micromolar concentrations. Furthermore, preliminary acute (up to 1 mg/mouse; one dose subcutaneously) and subacute (100 μg/mouse, subcutaneously, for 15 days) toxicity studies in CF-1 mice with compounds 4d and 8a have not revealed any detectable toxicity.
Antimicrobial activity of compound 8b in the presence of albumin.
The strong binding of the acylpolyamines to human serum albumin raised the question whether the antimicrobial effects of these compounds would be completely abrogated in the presence of physiological concentrations of albumin. We therefore examined the antimicrobial effect of compound 8b, chosen as a representative compound, in the presence or absence of physiological concentrations of human serum albumin (4.5 g/100 ml; 677 μM). As shown in Fig. 7, there is approximately a fourfold attenuation of MICs (E. coli, 31.25 μM to 125 μM; S. aureus, 3.9 μM to 15.6 μM). These data clearly demonstrate that the acylpolyamines retain significant antibacterial activity in the presence of albumin, suggesting that these compounds may also be active in vivo. Indeed, the LPS sequestration properties of these compounds are also virtually unchanged in the presence of albumin. These results, taken together, would suggest that the Koff rate of the lipopolyamine-albumin complex is rather fast, affording concentrations of free lipopolyamine capable of sequestering monomeric LPS or interacting with bacterial membranes, and yet considerably lower than the carboxymethyl cellulose value, which would account for the lack of hemolytic/membrane-active properties under these conditions. Surface plasmon resonance experiments are currently under way to test this hypothesis.
FIG. 7.
MICs of compound 8b with and without physiological concentration of human serum albumin (HSA). A stock solution of compound 8b was serially diluted in either a 4.5-g/100-ml solution of sterile-filtered HSA or sterile, distilled water. An equal volume of a suspension of either E. coli ATCC 9637 or S. aureus ATCC 13709 in 2× Mueller-Hinton broth was added using an automated liquid dispensing system to a 384-well plate, and bacterial growth was measured by absorptiometry as described in Materials and Methods. Also shown is the MIC of amoxicillin (Amox) with or without HSA, as an internal control.
In conclusion, we have tested the hypothesis that LPS-sequestering bis-cationic amphipathic compounds, typified by the acylpolyamines, may also bind to and permeabilize intact gram-negative bacterial membranes. Although the intrinsic antimicrobial activities of these compounds are modest, bis-acyl analogues such as compounds 8a and 8b display a pronounced sensitizing effect to hydrophobic antibiotics, more than 250 times that of PMBN. Furthermore, compound 8b was found to display significant inhibitory activity against S. aureus. The ease of synthesis will facilitate a more detailed exploration of structure-activity relationships. The lack of in vivo toxicity and the fact that the building blocks (and consequently the products of metabolism) consist of ubiquitous polyamines and fatty acids render this an attractive class of compounds with possible therapeutic value as an adjunct in antimicrobial chemotherapy. In-depth studies on the synergistic activity against the mucoid strain of Pseudomonas and protective effects in animal models of antibiotic-induced LPS release are in progress.
Acknowledgments
This work was supported by NIH grant 1R01 AI50107.
We certify that there are no conflicts of interest. We thank an anonymous reviewer for insightful comments and suggestions.
REFERENCES
- 1.Anhalt, J. P., and R. Nelson. 1982. Failure of Padac test strips to detect staphylococcal β-lactamase. Antimicrob. Agents Chemother. 21:993-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard, E., J. F. Faucon, and J. Dufourcq. 1982. Phase separation induced by melittin in negatively-charged phospholipid bilayers as detected by fluorescence polarization and differential scanning calorimetry. Biochim. Biophys. Acta 688:152-162. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjya, S., S. A. David, V. I. Mathan, and P. Balaram. 1997. Polymyxin B nonapeptide: conformations in water and in the lipopolysaccharide-bound state determined by two-dimensional NMR and molecular dynamics. Biopolymers 41:251-265. [Google Scholar]
- 4.Blagbrough, I. S., A. J. Geall, and S. A. David. 2000. Lipopolyamines incorporating the tetraamine spermine, bound to an alkyl chain, sequester bacterial lipopolysaccharide. Bioorg. Med. Chem. Lett. 10:1959-1962. [DOI] [PubMed] [Google Scholar]
- 5.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heynacker, H. W. Boyer, J. H. Crosa, and S. Falkow. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 6.Clinical Microbiology and Infection. 2003. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 9:1-7. [DOI] [PubMed] [Google Scholar]
- 7.David, S. A. 2001. Towards a rational development of anti-endotoxin agents: novel approaches to sequestration of bacterial endotoxins with small molecules. J. Mol. Recognit. 14:370-387. [DOI] [PubMed] [Google Scholar]
- 8.David, S. A., K. A. Balasubramanian, V. I. Mathan, and P. Balaram. 1992. Analysis of the binding of polymyxin B to endotoxic lipid A and core glycolipid using a fluorescent displacement probe. Biochim. Biophys. Acta 1165:147-152. [DOI] [PubMed] [Google Scholar]
- 9.David, S. A., V. I. Mathan, and P. Balaram. 1992. Interaction of melittin with endotoxic lipid A. Biochim. Biophys. Acta 1123:269-274. [DOI] [PubMed] [Google Scholar]
- 10.David, S. A., R. Silverstein, C. R. Amura, T. Kielian, and D. C. Morrison. 1999. Lipopolyamines: novel antiendotoxin compounds that reduce mortality in experimental sepsis caused by gram-negative bacteria. Antimicrob. Agents Chemother. 43:912-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devine, D. A., and R. E. Hancock. 2002. Cationic peptides: distribution and mechanisms of resistance. Curr. Pharm. Des. 8:703-714. [DOI] [PubMed] [Google Scholar]
- 12.Ding, B., Q. Guan, J. P. Walsh, J. S. Boswell, T. W. Winter, E. S. Winter, S. S. Boyd, C. Li, and P. B. Savage. 2002. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 45:663-669. [DOI] [PubMed] [Google Scholar]
- 13.Ding, B., N. Yin, J. Cardenas-Garcia, R. Evanson, T. Orsak, M. Fan, G. Turin, and P. B. Savage. 2004. Origins of cell selectivity of cationic steroid antibiotics. J. Am. Chem. Soc. 126:13642-13648. [DOI] [PubMed] [Google Scholar]
- 14.Fainerman, V. B., and R. Miller. 2004. Maximum bubble pressure tensiometry-an analysis of experimental constraints. Adv. Colloid Interface Sci. 108-109:287-301. [DOI] [PubMed] [Google Scholar]
- 15.Fidai, S., S. W. Farmer, and R. E. Hancock. 1997. Interaction of cationic peptides with bacterial membranes. Methods Mol. Biol. 78:187-204. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich, C., M. G. Scott, N. Karunaratne, H. Yan, and R. E. Hancock. 1999. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 43:1542-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funahara, Y., and N. Hiroshi. 1980. Asymmetric localization of lipopolysaccharides on the outer membrane of Salmonella typhymurium. J. Bacteriol. 141/3:1463-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hancock, R. E. 1997. Peptide antibiotics. Lancet 349:418-422. [DOI] [PubMed] [Google Scholar]
- 19.Hancock, R. E., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock, R. E. W., and P. G. W. Wong. 1984. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob. Agents Chemother. 26:48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houston, M. E., Jr., L. H. Kondejewski, D. N. Karunaratne, M. Gough, S. Fidai, R. S. Hodges, and R. E. Hancock. 1998. Influence of preformed alpha-helix and alpha-helix induction on the activity of cationic antimicrobial peptides. J. Pept. Res. 52:81-88. [DOI] [PubMed] [Google Scholar]
- 22.Hurley, J. C. 1992. Antibiotic-induced release of endotoxin: a reappraisal. Clin. Infect. Dis. 15:840-854. [DOI] [PubMed] [Google Scholar]
- 23.Hurley, J. C. 1995. Antibiotic-induced release of endotoxin. A therapeutic paradox. Drug Saf. 12:183-195. [DOI] [PubMed] [Google Scholar]
- 24.Jackson, J. J., H. Kropp, and J. C. Hurley. 1994. Influence of antibiotic class and concentration on the percentage of release of lipopolysaccharide from Escherichia coli. J. Infect. Dis. 169:471-473. [DOI] [PubMed] [Google Scholar]
- 25.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehrer, R. I., A. Barton, and T. Ganz. 1988. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J. Immunol. Methods 108:153-158. [DOI] [PubMed] [Google Scholar]
- 27.Li, C., L. P. Budge, C. D. Driscoll, B. M. Willardson, G. W. Allman, and P. B. Savage. 1999. Incremental conversion of outer-membrane permeabilizers into potent antibiotics for gram-negative bacteria. J. Am. Chem. Soc. 121:931-940. [Google Scholar]
- 28.Mayo, K. H., J. Haseman, H. C. Young, and J. W. Mayo. 2000. Structure-function relationships in novel peptide dodecamers with broad-spectrum bactericidal and endotoxin-neutralizing activities. Biochem. J. 349:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menger, F. M., and J. S. Keiper. 2000. Gemini surfactants. Angew. Chem. Int. Ed. Engl. 39:1906-1920. [DOI] [PubMed] [Google Scholar]
- 30.Miller, K. A., E. V. K. Suresh Kumar, S. J. Wood, J. R. Cromer, A. Datta, and S. A. David. 2005. Lipopolysaccharide sequestrants: structural correlates of activity and toxicity in novel acylhomospermines. J. Med. Chem. 48:2589-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison, D. C., and D. M. Jacobs. 1976. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry 13:813-818. [DOI] [PubMed] [Google Scholar]
- 32.Nagini, S., and S. Selvam. 1997. Biochemical indicators of membrane damage in the plasma and erythrocytes of rats fed the peroxisome proliferator di(2-ethylhexyl)phthalate. Med. Sci. Res. 25:119-121. [Google Scholar]
- 33.Nikaido, H. 1988. Bacterial resistance to antibiotics as a function of outer membrane permeability. J. Antimicrob. Ther. 22:17-22. [DOI] [PubMed] [Google Scholar]
- 34.Nikaido, H., and M. Vaara. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Novo, D. J., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 2000. Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Microccocus luteus. Antimicrob. Agents Chemother. 44:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osborn, M. J. 1979. Biosynthesis and assembly of the lipopolysaccharide of the outer membrane, p. 15-34. In M. Inouye (ed.), Bacterial outer membranes. Biogenesis and functions. John Wiley & Sons, New York, N.Y.
- 37.Piers, K. L., M. H. Brown, and R. E. Hancock. 1994. Improvement of outer membrane-permeabilizing and lipopolysaccharide-binding activities of an antimicrobial cationic peptide by C-terminal modification. Antimicrob. Agents Chemother. 38:2311-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prins, J. M., M. A. van Agtmael, E. J. Kuijper, S. J. van Deventer, and P. Speelman. 1995. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J. Infect. Dis. 172:886-891. [DOI] [PubMed] [Google Scholar]
- 39.Prins, J. M., S. J. H. Van Deventer, E. J. Kuijper, and P. Speelman. 1994. Clinical relevance of antibiotic-induced endotoxin release. Antimicrob. Agents Chemother. 38:1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rietschel, E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zähringer, U. Seydel, F. Di Padova, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217-225. [DOI] [PubMed] [Google Scholar]
- 41.Rosenthal, K. S., and D. R. Storm. 1977. Disruption of the Escherichia coli outer membrane permeability barrier by immobilized polymyxin B. J. Antibiot. 30:1087-1092. [DOI] [PubMed] [Google Scholar]
- 42.Ross, B. P., A. C. Braddy, R. P. McGeary, J. T. Blanchfield, L. Prozkai, and I. Toth. 2004. Micellar aggregation and membrane partitioning of bile salts, fatty acids, sodium dodecyl sulfate, and sugar-conjugated fatty acids: correlation with hemolytic potency and implications for drug delivery. Mol. Pharm. 1:233-245. [DOI] [PubMed] [Google Scholar]
- 43.Schindler, P. R., and M. Teuber. 1975. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob. Agents Chemother. 8:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt, E. J., J. S. Boswell, A. Walsh, M. M. Schellenberg, T. W. Winter, C. Li, G. W. Allman, and P. B. Savage. 2001. Activities of cholic acid-derived antimicrobial agents against multidrug-resistant bacteria. J. Antimicrob. Chemother. 47:671-674. [DOI] [PubMed] [Google Scholar]
- 45.Scott, M. G., M. R. Gold, and R. E. Hancock. 1999. Interaction of cationic peptides with lipoteichoic acid and gram-positive bacteria. Infect. Immun. 67:6445-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott, M. G., A. C. Vreugdenhil, W. A. Buurman, R. E. Hancock, and M. R. Gold. 2000. Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J. Immunol. 164:549-553. [DOI] [PubMed] [Google Scholar]
- 47.Skeriavaj, B., D. Romeo, and R. Gennaro. 1990. Rapid membrane permeabilization and inhibition of vital functions of gram-negative bacteria by bactenecins. Infect. Immun. 58:3724-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder, D. S., and T. J. McIntosh. 2000. The lipopolysaccharide barrier: correlation of antibiotic susceptibility with antibiotic permeability and fluorescent probe binding kinetics. Biochemistry 39:11777-11787. [DOI] [PubMed] [Google Scholar]
- 49.Storm, D. R., and K. Rosenthal. 1977. Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 46:723-763. [DOI] [PubMed] [Google Scholar]
- 50.Strøm, M. B., B. E. Haug, M. L. Skar, W. Stensen, T. Stiberg, and J. S. Svendsen. 2003. The pharmacophore of short cationic antibacterial peptides. J. Med. Chem. 46:1567-1570. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, C. J., N. Surolia, and A. Surolia. 1999. Surface plasmon resonance studies resolve the enigmatic endotoxin neutralizing activity of polymyxin B. J. Biol. Chem. 274:29624-29627. [DOI] [PubMed] [Google Scholar]
- 52.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2000. Structure activity relationship study of polymyxin B nonapeptide. Adv. Exp. Med. Biol. 479:219-222. [DOI] [PubMed] [Google Scholar]
- 53.Tsubery, H., I. Ofek, S. Cohen, and M. Fridkin. 2000. Structure-function studies of polymyxin B nonapeptide: implications to sensitization of gram-negative bacteria. J. Med. Chem. 43:3085-3092. [DOI] [PubMed] [Google Scholar]
- 54.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaara, M. 1993. Antibiotic-supersusceptible mutants of Escherichia coli and Salmonella typhimurium. Antimicrob. Agents Chemother. 37:2255-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaara, M., and M. Porro. 1996. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob. Agents Chemother. 40:1801-1805. (Erratum 41:496, 1997.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaara, M., and T. Vaara. 1983. Polycations as outer membrane disorganizing agents. Antimicrob. Agents Chemother. 24:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaara, M., and T. Vaara. 1983. Polycations sensitize enteric bacteria to antibiotics. Antimicrob. Agents Chemother. 24:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaara, M., and T. Vaara. 1983. Sensitization of gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature 303:526-528. [DOI] [PubMed] [Google Scholar]
- 60.van't Hof, W., E. C. I. Veerman, E. J. Helmerhorst, and A. V. N. Amerongen. 2001. Antimicrobial peptides: properties and applicability. Biol. Chem. 382:597-619. [DOI] [PubMed] [Google Scholar]
- 61.Viljanen, P., H. Matsunaga, Y. Kimura, and M. Vaara. 1991. The outer membrane permeability-increasing action of deacylpolymyxins. J. Antibiot. (Tokyo) 44:517-523. [DOI] [PubMed] [Google Scholar]
- 62.Wiese, A., M. Munstermann, T. Gutsmann, B. Lindner, K. Kawahara, U. Zahringer, and U. Seydel. 1998. Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted transport. J. Membr. Biol. 162:127-138. [DOI] [PubMed] [Google Scholar]
- 63.Yasuda, K., C. Ohmizo, and T. Katsu. 2004. Mode of action of novel polyamines increasing the permeability of bacterial outer membrane. Int. J. Antimicrob. Agents 24:67-71. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, L., P. Dhillon, H. Yan, S. Farmer, and R. E. Hancock. 2000. Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3317-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zorko, M., A. Majerle, D. Sarlah, M. M. Keber, B. Mohar, and R. Jerala. 2005. Combination of antimicrobial and endotoxin-neutralizing activities of novel oleoylamines. Antimicrob. Agents Chemother. 49:2307-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]