Abstract
The fluorinated guanosine analog 2′,3′-dideoxy-3′-fluoroguanosine (FLG) was shown to inhibit wild-type (wt) hepatitis B virus (HBV) replication in a human hepatoma cell line permanently expressing HBV. Experiments performed in the duck model of HBV infection also showed its in vivo antiviral activity. In this study, we investigated the mechanism of inhibition of FLG on HBV replication and its profile of antiviral activity against different HBV or duck hepatitis B virus (DHBV) drug-resistant mutants. We found that FLG-triphosphate inhibits weakly the priming of the reverse transcription compared to adefovir-diphosphate in a cell-free system assay allowing the expression of an enzymatically active DHBV reverse transcriptase. It inhibits more potently wt DHBV minus-strand DNA synthesis compared to lamivudine-triphosphate and shows a similar activity compared to adefovir-diphosphate. FLG-triphosphate was most likely a competitive inhibitor of dGTP incorporation and a DNA chain terminator. In Huh7 cells transiently transfected with different HBV constructs, FLG inhibited similarly the replication of wt, lamivudine-resistant, adefovir-resistant, and lamivudine-plus-adefovir-resistant HBV mutants. These results were consistent with those obtained in the DHBV polymerase assay using the same drug-resistant polymerase mutants. In conclusion, our data provide new insights in the mechanism of action of FLG-triphosphate on HBV replication and demonstrate its inhibitory activity on drug-resistant mutant reverse transcriptases in vitro. Furthermore, our results provide the rationale for further clinical evaluation of FLG in the treatment of drug-resistant virus infection and in the setting of combination therapy to prevent or delay drug resistance.
The development of nucleotide and nucleoside analogs that inhibit the hepatitis B virus (HBV) reverse transcriptase (RT) activity has provided new hope in the treatment of chronic hepatitis B. Lamivudine [(−)-β-l-2′,3′-dideoxy-3′ thiacytidine (3TC)], adefovir [9-(2-phosphonylmethoxyethyl)adenine (PMEA)], and entecavir [2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate (ETV)] are currently the three nucleotide and nucleoside analogs approved for the treatment of HBV infection. However, the slow kinetics of viral clearance during 3TC therapy and the spontaneous viral genome variability lead to the selection of 3TC-resistant mutants. Up to 66% of the patients develop viral resistance after 4 years of 3TC therapy (12). The rtM204V/I mutations frequently associated with 3TC breakthrough are located in the tyrosine-methionine-aspartate-asparate (YMDD) motif within the C domain of the RT and confer 3TC resistance (1). They are often associated with compensatory mutations (rtL180M or rtV173L) in the B domain of the RT (5, 20). ETV has been very recently approved in the United States. It remains efficacious against the 3TC-resistant mutants in vitro and in vivo (17, 20, 28). However, in clinical trials for patients infected with 3TC-resistant HBV strains, prolonged ETV treatment has led to the selection of complex mutants harboring specific polymerase mutations in addition to 3TC-resistant ones associated with a decreased ETV susceptibility (28). 3TC-resistant mutants remain sensitive to PMEA in vitro (25, 32) and in vivo (21). However, recent reports showed that, after 2 years of therapy, a novel rtN236T mutation located in the D domain of the HBV polymerase could be selected, although at a lower rate than the 3TC resistance mutations. This rtN236T mutation confers a 4- to 23-fold reduced susceptibility to PMEA but remains sensitive to 3TC (2, 29).
Other nucleoside analogs are currently in phase III clinical trials, i.e., emtricitabine [(−)-β-2′,3′-dideoxy-5-fluoro-3′-thiacytidine (−FTC)], a close analog of 3TC, and telbivudine [1-(2-deoxy-β-l-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H, 3H)-dione (LdT)] (7, 13). However, 3TC resistance mutations confer cross-resistance to −FTC and LdT (33). Thus, new HBV inhibitors are still needed to rescue drug resistance or to design new combination strategies to either delay or prevent drug resistance. Several anti-HBV compounds are currently under evaluation. One of these, 2′,3′-dideoxy-3′-fluoroguanosine (FLG), is a synthetic deoxyguanosine analog originally developed to inhibit human immunodeficiency virus (HIV) replication (10). The functional similarity of the HBV and HIV DNA polymerases has led to the examination of FLG as an inhibitor of human and duck HBV (DHBV) polymerase activity. The antiviral activity of FLG was investigated in vitro and in vivo on both HBV and DHBV replication (8, 18, 22). In vitro, FLG was shown to be an efficient inhibitor of both DHBV and HBV replication (22). In vivo, FLG showed a favorable safety profile and inhibited DHBV replication in DHBV-infected ducks with a significant reduction of serum DHBV DNA levels (18).
Therefore, the aim of our study was to get some insight on the mechanism of action of FLG on HBV replication and to determine its anti-HBV activity profile against lamivudine- and adefovir-resistant mutants that are commonly observed in clinical practice. To characterize the mechanism of action of FLG-triphosphate (FLG-TP), we used an in vitro assay that enables the expression of the DHBV RT (33, 34). The inhibitory effect of FLG-TP was also assessed on DHBV RT harboring mutations conferring resistance to 3TC, PMEA, or to both 3TC and PMEA. Furthermore, the inhibitory effect of FLG was evaluated in transiently transfected Huh7 cells on the replication of wild-type (wt) and drug-resistant mutant HBV laboratory strains.
MATERIALS AND METHODS
Antiviral drugs.
FLG and its triphosphate form, FLG-TP, 3TC and 3TC-TP, and PMEA and PMEA-diphosphate (PMEA-DP) were provided by Medivir (Huddinge, Sweden).
Expression vectors for the study of wild-type and drug-resistant mutants of DHBV and HBV.
Wild-type (wt) and mutant HBV and DHBV polymerases were used to study their sensitivity to nucleotide and nucleoside analogs. Three mutants were studied: a 3TC-resistant mutant (rtL180M+M204V for HBV, corresponding to rtL489M+M512V for DHBV), a PMEA-resistant mutant (rtN236T for HBV, corresponding to rtN544T for DHBV), and a mutant harboring both 3TC- and PMEA-resistant mutations (rtL180M+M204V+N236T for HBV, corresponding to rtL489M+M512V+N544T for DHBV). The wt and rtL489M+M512V mutant polymerase genes of DHBV were previously cloned into pHP plasmids under the control of an SP6 promoter to study their enzymatic activity in a cell-free system (26). The rtN544T amino acid substitution was introduced by site-directed mutagenesis with the Quickchange kit (Stratagene, La Jolla, CA) into these plasmids to obtain the rtN544T and the rtL489M+M512V+N544T DHBV polymerases. Mutated polymerase gene sequences were checked by sequencing. The plasmid pCMV-HBV, containing 1.1 U of wt or rtL180M+M204V mutant HBV genome length (genotype D, subtype ayw) (3, 26), was modified by site-directed mutagenesis with the Quickchange kit to introduce the rtN236T mutation. The mutated plasmids were then sequenced, and EcoRI-NcoI restriction fragments encompassing the rtL180M+M204V, rtN236T, or rtL180M+M204V+N236T mutations were transferred into the plasmid pTriEXMod-HBV. This latter construct, which contains 1.1 U of HBV genome, enables after cell transfection the production of pregenomic RNA under the control of the chicken beta actin promoter and therefore triggers HBV DNA synthesis (6).
An in vitro assay for the expression of enzymatically active DHBV reverse transcriptase and evaluation of the inhibitory effect of nucleoside triphosphate analogs.
The enzymatically active DHBV polymerase polypeptide was expressed from plasmid pHP. These constructs contain the wt or mutant (rtL489M+M512V, rtN544T, or rtL489M+M512V+N544T) DHBV polymerase gene under the control of the SP6 promoter and the sequence coding for the RNA template of reverse transcription as previously described (4, 30). The DHBV polymerase gene from each construct was transcribed and translated in a coupled transcription-translation rabbit reticulocyte lysate system (TNT SP6 Coupled Reticulocyte Lysate System; Promega, Charbonnières, France) according to the manufacturer's instructions.
The reverse transcription assay was performed as previously described (4, 34, 35). After translation, the viral polymerase was incubated for 30 min at 30°C with an equal volume of a reaction mixture containing 100 mM Tris-HCl, pH 7.5, 30 mM NaCl, 20 mM MgCl2, and a nucleotide mix of which the composition depends on the type of reaction.
The priming reaction corresponds to the synthesis of a short DNA primer, GTAA, for reverse transcription. To study this reaction, the composition of the deoxynucleoside triphosphate (dNTP) mixture depended on the nucleotide or nucleoside analog tested. For FLG-TP, only [α-32P]dGTP (0.165 μM; 3,000 Ci/mm) was used to determine if the drug was able to compete with the incorporation of the first nucleotide (dGTP) in viral minus-strand DNA. For the evaluation of PMEA-DP, the dNTP mixture included cold dGTP and dTTP (100 μM each) with [α-32P]dATP (0.165 μM; 3,000 Ci/mm).
To study the elongation of viral minus-strand DNA, the reaction mixture included the radioactive nucleotide [α-32P]dNTP (0.165 μM; 3,000 Ci/mm), of which the drug tested is the analog, whereas the three other cold dNTPs were used at 100 μM each. Radiolabeled viral DNA covalently attached to polymerase was analyzed through 0.1% sodium dodecyl sulfate-10% polyacrylamide gels as previously published (4, 35), and the dried gels were exposed to X-ray film. A quantitative dot assay was performed after spotting 2 μl of the radiolabeled reverse transcription assay mixture onto DE-81 filters (Whatman) as previously described (34). In each assay, background incorporation was calculated with a control experiment where the reaction was performed without addition of pHP plasmid. All results were expressed in percentages of inhibition of the polymerase activity compared to the reaction where the polymerase was incubated without any inhibitor. Quantification of densitometric scanning of autoradiograms and experiments with DE-81 filters showed similar results.
To determine whether FLG-TP is a competitive inhibitor of the incorporation of the natural nucleotide [α-32P]dGMP into nascent viral DNA, the reverse transcription assay described above was performed with [α-32P]dGTP at a final concentration of 0.165 μM or 0.825 μM together with increasing concentrations of FLG-TP ranging from 0 to 100 μM.
Analysis of the inhibitory activity of FLG on the replication of wild-type and drug-resistant mutants of HBV in Huh7 cells.
The analysis of FLG antiviral activity was evaluated in comparison with that of 3TC and PMEA after transient transfection of Huh7 cells by either wt, 3TC-resistant (rtL180M+M204V), PMEA-resistant (rtN236T), or 3TC- and PMEA-resistant (rtL180M+M204V+N236T) mutant polymerases. Cells seeded in 12-well plates at a density of 7 × 104 cells/cm2 were transfected 1 day postplating with 0.5 μg/well of pTriEXMod-HBV plasmid containing wt or mutant HBV genome, using Fugene 6 transfection reagent (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. Sixty hours posttransfection, treatment with increasing concentrations of drugs was started. Medium was changed daily, and drug administration was performed from day 3 to day 8 posttransfection. Cells were then lysed for analysis of intracellular viral DNA. HBV DNA was purified from intracellular core particles as described earlier (11), separated on agarose gels, analyzed by Southern Blot hybridization, and quantified after phosphorimager scanning (26).
Analysis of cellular toxicity in Huh7 cells.
Cellular viability was assessed by cellular uptake of neutral red dye (9). Cells were seeded in 96-well tissue culture plates and cultured in medium containing increasing concentrations of FLG (from 0 to 1,000 μM), PMEA (from 0 to 500 μM), and 3TC (from 0 to 1,000 μM) with daily change of medium for 5 days. Four wells were analyzed for each drug concentration at the end of the treatment. The CC50 was defined as the drug concentration reducing cell viability by 50%.
RESULTS
FLG-TP inhibits the wild-type DHBV reverse transcriptase activity.
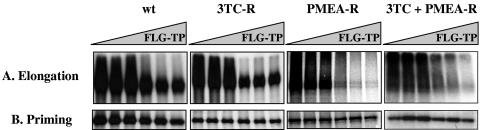
Using an in vitro assay that allows the expression of an enzymatically active DHBV RT, we analyzed the inhibitory effects of the triphosphate form of FLG (FLG-TP) on viral minus-strand DNA synthesis by wt DHBV polymerase in comparison with 3TC-TP and PMEA-diphosphate (DP). The incorporation of [α-32P]dGMP in the presence of increasing concentrations of FLG-TP was reproducibly inhibited in a dose-dependent manner (Fig. 1A). Moreover, FLG-TP inhibited more efficiently the RT activity of the wt viral polymerase than 3TC-TP (50% inhibitory concentration [IC50] = 4 ± 0.9 μM for FLG-TP versus 10.75 ± 4.8 μM for 3TC-TP; P < 0.05) and had inhibitory activity similar to that of PMEA-DP (IC50 = 2.8 ± 0.3 μM; P > 0.05) in our experimental conditions (Table 1).
FIG. 1.
Inhibitory activity of FLG-TP on the activity of wild-type (wt) and different mutant (3TC-R, PMEA-R, and 3TC+PMEA-R) DHBV polymerases. A. Effect of FLG-TP on the elongation of reverse transcription. Assays were performed for 30 min at 30°C with an equal volume of a reaction mixture containing 100 mM Tris-HCl, pH 7.5, 30 mM NaCl, 20 mM MgCl2, dATP, dCTP, dTTP (100 μM each), and [α-32P]dGTP (0.165 μM; 3,000 Ci/mm). The inhibition of [α-32P]dGTP incorporation in the DHBV minus-strand DNA was performed with addition of increasing concentrations of FLG (0, 1, 5, 10, 50, and 100 μM, respectively). Radiolabeled viral DNA covalently attached to polymerase (2 μl out of the 10 μl of the total reaction volume) is analyzed through 0.1% sodium dodecyl sulfate (SDS)-10% polyacrylamide gels, and the dried gels were exposed to X-ray film. B. Effect of FLG-TP on the priming of the reverse transcription. The experimental conditions were the same as those used for theelongation reaction, except that only [α-32P]dGTP (0.165 μM; 3,000 Ci/mm) was added to the reaction mixture. wt, wild-type; 3TC-R, rtL489M+M512V; PMEA-R, rtN544T; 3TC+PMEA-R, rtL489M+M512V+N544T.
TABLE 1.
Inhibitory activity of FLG-TP in comparison with 3TC-TP and PMEA-DP on the priming and elongation activity of wild-type and mutant DHBV reverse transcriptasesa
| wt polymerase
|
3TC-R polymerase
|
PMEA-R polymerase
|
3TC+PMEA-R polymerase
|
|||||
|---|---|---|---|---|---|---|---|---|
| Drug | IC50 (μM) | Pb | IC50 (μM) | Pb | IC50 (μM) | Pb | IC50 (μM) | Pb |
| Priming | ||||||||
| FLG-TP | >100 | >100 | >100 | >100 | ||||
| 3TC-TP | ND | ND | ND | ND | ND | ND | ND | ND |
| PMEA-DP | 4.9 ± 0.4 | <0.05 | 4.6 ± 0.5 | <0.05 | ND | <0.05 | ND | <0.05 |
| Elongation | ||||||||
| FLG-TP | 4 ± 0.9 | 5.43 ± 0.6 | 7.8 ± 1.9 | 6.33 ± 1.3 | ||||
| 3TC-TP | 10.75 ± 4.8 | <0.05 | >100 | <0.05 | 14 ± 5.7 | <0.05 | >100 | <0.05 |
| PMEA-DP | 2.8 ± 0.3 | >0.05 | 0.9 ± 0.1 | <0.05 | 49.5 ± 3.4 | <0.05 | 16.5 ± 7.2 | <0.05 |
For each sample tested, the incorporation of radioactive dGMP in the viral minus strand of DNA was estimated by quantitative dot assay. All results are first expressed in percentage of inhibition of the polymerase activity compared to the reaction performed without any inhibitor. IC50s were then deduced from the inhibition plot. IC50s are mean values obtained from three different experiments. ND, not determined; wt, wild type; 3TC-R, rtL489M+M512V; PMEA-R, rtN544T; 3TC+PMEA-R, rtL489M+M512V+N544T.
The Mann-Whitney test was used to determine whether the activities of FLG-TP, 3TC-TP, and PMEA-DP on priming and elongation were significantly different (P < 0.05).
The inhibitory activities of 3TC-TP, PMEA-DP, and FLG-TP were also tested on the synthesis of short DNA primer. During the priming of reverse transcription, the DHBV polymerase synthesizes a short 4-base DNA oligomer by copying an RNA motif located in the bulge of the epsilon stem-loop (15, 27). The sequence of the DNA primer is GTAA for DHBV. Our results showed that PMEA-DP was a potent inhibitor of the DNA primer synthesis (IC50 = 4.9 ± 0.4 μM), whereas FLG-TP inhibited the priming reaction by only 40% at 100 μM (IC50 > 100 μM) (Fig. 1B, Table 1). 3TC-TP was not tested in this priming reaction, because the short primer for reverse transcription (GTAA) does not include deoxycytidine.
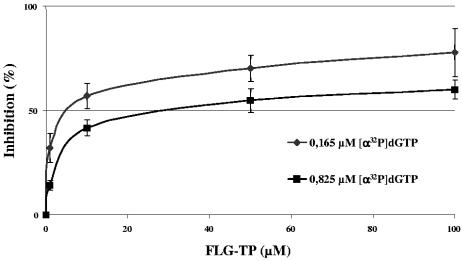
To determine whether FLG-TP may be a competitive inhibitor of dGMP incorporation in DHBV minus-strand DNA, the DHBV cell-free assay was used with radiolabeled [α-32P]dGTP at a final concentration of 0.165 μM or 0.825 μM. When the concentration of [α-32P]dGTP was increased by 5-fold, the IC50 of FLG-TP shifted from 7.5 ± 1.8 μM to 41.0 ± 11.3 μM (5.5-fold increase), suggesting a competitive inhibitory effect of the drug on dGMP incorporation in viral minus-strand DNA (Fig. 2). We also compared the effect of FLG-TP on the termination of viral minus-strand DNA synthesis to that of the corresponding dideoxynucleotide, ddGTP. The DHBV polymerase was incubated in the presence of 0.165 μM of dGTP and [α-32P]TTP with increasing concentrations (0 to 100 μM) of FLG-TP or ddGTP. Increasing concentrations of either FLG-TP or ddGTP inhibited the incorporation of the next radiolabeled TMP, although ddGTP was a more potent inhibitor than FLG-TP (data not shown). Altogether, these results suggest that FLG-TP is likely to be a competitive inhibitor of the incorporation of the natural substrate of the DHBV polymerase, i.e., dGTP, and then inhibits the incorporation of the next nucleotide.
FIG. 2.
FLG-TP is a competitive inhibitor of dGTP, the natural substrate of DHBV polymerase. Competitive inhibition of the incorporation of dGMP by FLG-TP was performed as described in the legend to Fig. 1A with [α-32P]dGTP at a final concentration of 0.165 μM (⧫) or 0.825 μM (▪) with increasing concentrations of FLG-TP (0, 0.5, 1, 10, 50, and 100 μM). Viral nascent minus-strand DNA was analyzed quantitatively by a dot assay, and the results of the inhibition of [α-32P]dGMP incorporation in viral DNA were plotted on the graph to determine the IC50. The graph shown in this figure corresponds to the mean curve of inhibition of [α-32P]dGMP incorporation according to three independent experiments. Error bars indicate standard deviation.
FLG inhibits viral DNA synthesis in Huh7 cells transiently transfected with wild-type HBV genome.
We first determined the cellular viability of Huh7 cells in the presence of increasing concentrations of FLG (ranging from 0 to 1,000 μM) in comparison with increasing concentrations of 3TC (ranging from 0 to 1,000 μM) and PMEA (ranging from 0 to 500 μM) by cellular uptake of neutral red dye. In our experiments, FLG and PMEA displayed a similar cytotoxicity (CC50 = 264 ± 51 μM for FLG, and CC50 = 365 ± 120 μM for PMEA), whereas concentrations of 3TC up to 1,000 μM had no significant toxicity in Huh7 cells.
Huh7 cells were then transiently transfected with pTriEXMod- HBV plasmid containing wt HBV genome and treated for 5 days with increasing concentrations of FLG, 3TC, or PMEA ranging from 0 to 100 μM. Intracellular HBV DNA replicative intermediates were then extracted, submitted to Southern blot analysis, and quantified by phosphorimager scanning densitometry to determine the IC50s of each drug. Results showed that all three nucleotide or nucleoside analogs inhibited wt HBV replication in a dose-dependent manner. Based on the results of three independent experiments, the most potent inhibition of intracellular HBV DNA synthesis was observed with 3TC (IC50 = 0.5 ± 0.4 μM), followed by FLG (IC50 = 9 ± 2.5 μM) and PMEA (IC50 = 15 ± 3.4 μM) (Table 2).
TABLE 2.
Inhibitory activity of FLG, 3TC, and PMEA on the intracellular viral DNA synthesis of wild-type and mutant HBV laboratory strains in transiently transfected Huh7 cellsa
| wt HBV Pol
|
3TC-R HBV Pol
|
PMEA-R HBV Pol
|
3TC+PMEA-R HBV Pol
|
|||||
|---|---|---|---|---|---|---|---|---|
| Drug | IC50 (μM) | RF | IC50 (μM) | RF | IC50 (μM) | RF | IC50 (μM) | RF |
| FLG | 9 ± 2.5 | 1.0 | 8 ± 3.8 | 0.9 | 13 ± 3.4 | 1.4 | 15 ± 6.8 | 1.7 |
| 3TC | 0.5 ± 0.4 | 1.0 | >100 | >200 | 0.8 ± 0.9 | 1.6 | >100 | >200 |
| PMEA | 15 ± 3.4 | 1.0 | 12 ± 4.5 | 0.8 | 35 ± 5.5 | 2.3 | >100 | >6.7 |
RF, resistance factor. Resistance factor is IC50 determined for mutant HBV divided by IC50 determined for wt HBV. wt, wild type; 3TC-R, rtL180M+M204V; PMEA-R, rtN236T; 3TC+PMEA-R, rtL180M+M204V+N236T; Pol, polymerase. IC50s are the means of three independent experiments.
3TC-resistant, PMEA-resistant, and 3TC+PMEA-resistant mutants of DHBV and HBV remain sensitive to FLG.
The enzymatic activity of three DHBV mutant polymerases corresponding to the 3TC-resistant (3TC-R; rtL489M+M512V), PMEA-resistant (PMEA-R; rtN544T), and 3TC+PMEA-resistant (3TC+PMEA-R; rtL489M+M512V+N544T) DHBV mutants was analyzed in the presence of increasing concentrations of FLG-TP, 3TC-TP, and PMEA-DP in comparison to wt DHBV polymerase. 3TC-TP was not active on either 3TC-R or 3TC+PMEA-R DHBV polymerases (IC50 > 100 μM) but had a similar inhibitory activity on both wt (IC50 = 10.75 ± 4.8 μM) and PMEA-R (IC50 = 14.0 ± 5.7 μM) DHBV polymerases (Table 1). PMEA-DP inhibited 3.1-fold more potently the 3TC-R DHBV polymerase (IC50 = 0.9 ± 0.1 μM) than the wt one (IC50 = 2.8 ± 0.3 μM). However, it is less active against the 3TC+PMEA-R (IC50 = 16.5 ± 7.2 μM) and PMEA-R (IC50 = 49.5 ± 3.4 μM) DHBV polymerases (Table 1). Interestingly, FLG-TP inhibited similarly the enzymatic activity of wt (IC50 = 4.0 ± 0.9 μM), 3TC-R (IC50 = 5.43 ± 0.6 μM), PMEA-R (IC50 = 7.8 ± 1.9 μM), or 3TC+PMEA-R (IC50 = 6.33 ± 1.3 μM) mutant DHBV polymerase (Fig. 1A, Table 1).
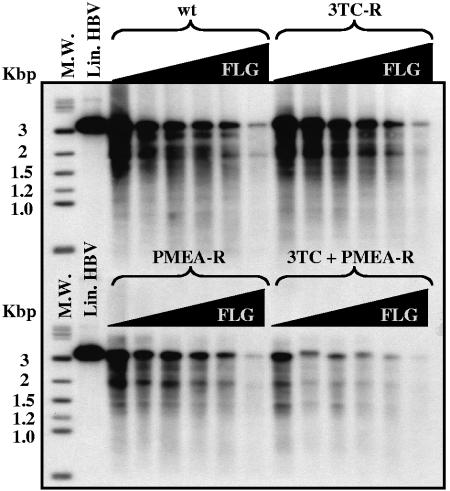
The inhibitory activity of FLG was also evaluated on the replication of 3TC-resistant (3TC-R; rtL180M+M204V), PMEA-resistant (PMEA-R; rtN236T), or 3TC+PMEA-resistant (3TC+PMEA-R; rtL180M+M204V+N236T) HBV mutants in comparison to the wt HBV in transiently transfected Huh7 cells. The same experiments were performed in parallel with 3TC or PMEA as a control. 3TC inhibited similarly the viral genome replication of both wt and PMEA-R HBV strains (IC50s were 0.5 ± 0.4 μM and 0.8 ± 0.9 μM, respectively), whereas 3TC-R and 3TC+PMEA-R HBV mutants were resistant to 3TC (IC50 > 100 μM) (Table 2). PMEA inhibited both wt and 3TC-R HBV strains with comparable IC50s (15 ± 3.4 μM and 12 ± 4.5 μM, respectively). However, the PMEA-R mutant had a 2.3-fold decreased susceptibility to PMEA (IC50 = 35 ± 5.5 μM) compared to wt HBV. The 3TC+PMEA-R mutant was resistant to both 3TC and PMEA (IC50 > 100 μM). Interestingly, all three drug-resistant HBV mutants remained sensitive to FLG with similar IC50s (8 ± 3.8 μM for 3TC-R, 13 ± 3.4 μM for PMEA-R, and 15 ± 6.8 μM for the 3TC+PMEA-R mutant) compared to wt HBV (IC50 = 9 ± 2.5 μM) (Fig. 3, Table 2).
FIG. 3.
Activity of FLG on the replication of wild-type and drug-resistant mutants of HBV in transiently transfected Huh7 cells. The antiviral activity of FLG was analyzed after transient transfection of Huh7 cells with either wild-type HBV genome (wt) or with 3TC-resistant (3TC-R; rtL180M+M204V), PMEA-resistant (PMEA-R; rtN236T), or 3TC+PMEA-resistant (3TC+PMEA-R; rtL180M+M204V+N236T) HBV genome cloned in pTriEXMod-HBV (6). Sixty hours posttransfection, treatment with increasing concentrations of FLG (0, 1, 5, 10, 25, and 100 μM) was started. Media were changed daily, and drug administration was performed from day 3 to day 8 posttransfection. Cells were then lysed, and HBV DNA was purified from intracellular core particles, separated on agarose gels, and submitted to Southern blot analysis. M.W., molecular weight; Lin. HBV, linear HBV DNA.
DISCUSSION
In this study, we investigated the mechanism of action and the antiviral activity profile of FLG on the replication of wt and drug-resistant mutants of DHBV and HBV polymerases in vitro.
To gain new insight about the mechanism of inhibition of viral DNA synthesis by FLG, we assessed in vitro the effect of FLG-TP on the RT activity of an enzymatically active wt DHBV polymerase expressed in a cell-free system. First, we showed that FLG-TP was a weak inhibitor of the priming of the reverse transcription by the DHBV polymerase compared to PMEA-DP (Table 1). This contrasts with other guanosine analogs, such as 2′ carboxydeoxyguanosine, entecavir, and lobucavir, that were shown to inhibit the priming of hepadnavirus reverse transcription (4, 24). However, FLG-TP inhibited significantly the elongation of DHBV minus-strand DNA in a dose-dependent manner. Compared to other nucleoside triphosphate analogs, FLG-TP was as active as PMEA-DP and 2.7-fold more potent than 3TC-TP in inhibiting this reaction. Altogether, these results suggest that FLG-TP might reach the catalytic site of the viral polymerase mainly once viral minus-strand DNA synthesis has been primed, as the viral polymerase undergoes conformational changes (23) that may allow FLG-TP to enter into the catalytic site of the RT.
The FLG inhibitory effect on DHBV reverse transcription is most likely the result of two events: first, a competition with the natural substrate of the polymerase (dGTP) when it is incorporated into DNA, and then the subsequent inhibition of the incorporation of following nucleotides in nascent viral DNA. Indeed, we observed that the IC50 of FLG-TP for the wt DHBV polymerase was increased by 5.47-fold when the concentration of [α-32P]dGTP was increased by 5-fold in the DHBV cell-free assay. Furthermore, FLG-TP inhibited the incorporation of radiolabeled TMP, although with a lower potency than ddGTP (data not shown). This suggests that FLG-TP may be a competitive inhibitor of dGMP incorporation in nascent viral minus-strand DNA and that it may terminate viral DNA chain synthesis, a mechanism previously observed with other nucleoside triphosphate analogs such as 3TC-TP and βLFd4C-TP (16, 26). However, the in vitro HBV priming reaction differs in some features from that of DHBV (14, 15, 23). It will be interesting to see whether the mechanism of action of FLG-TP is similar in an HBV cell-free system, such as the one developed by Seifer et al. (23). Nevertheless, the triphosphate forms of entecavir and lobucavir, two guanosine analogs, were shown to inhibit the in vitro polymerase activity of HBV and DHBV with a similar mechanism (24).
In Huh7 cells transiently transfected with wt HBV genome, FLG inhibited HBV replication slightly more efficiently than PMEA, while 3TC displayed the most potent antiviral activity (Table 2). To summarize, we observed the following order of antiviral activity in Huh7 cells: 3TC ≫ FLG > PMEA. However, FLG-TP, 3TC-TP, and PMEA-DP inhibitory activities on the elongation of viral minus-strand DNA by DHBV polymerase in the cell-free system were ranked in the following order: PMEA-DP ≈ FLG-TP > 3TC-TP. In the latter system, the inhibition of the elongation of the viral minus-strand DNA by a drug corresponds to its cumulative effects on the priming of the reverse transcription and on the termination of the DNA chain. 3TC-TP is not an inhibitor of the priming of the reverse transcription (24). Thus, its impact on the DNA chain elongation relies only on its effect as a DNA chain terminator, which could explain its weakest antiviral activity in the cell-free system compared to PMEA-DP and FLG-TP. Differences in the HBV and DHBV priming reactions (14, 15, 23) may also imply a different mechanism of action of the drugs on these polymerases. This could in turn explain the discrepancy observed between the effects of FLG-TP, PMEA-DP, and 3TC-TP on viral DNA synthesis in the HBV and DHBV in vitro systems of expression. Moreover, previous studies have already evidenced differences between the efficacy of drugs in cultured cells and in cell-free assays (24, 26). This may suggest that the intracellular metabolism of the nucleotide or nucleoside analogs is important to take into consideration for the evaluation of a drug's antiviral activity. The weaker antiviral effects of FLG and PMEA in Huh7 cells compared to 3TC may be explained either by a decreased entry and transport in the cell, by a poorer phosphorylation of these compounds, or by a shorter intracellular half-life of the triphosphate form. These host factors probably also account for the weakest potency of PMEA to inhibit 3TC-R and 3TC+PMEA-R HBV polymerases in cultured cells by comparison with the inhibitory effect of PMEA-DP against the DHBV polymerase mutants in the cell-free system. Nevertheless, our results showed that FLG exhibits an interesting antiviral activity on the replication of wt HBV or DHBV. Moreover, we observed that FLG inhibited the intracellular DNA synthesis of wt HBV in Huh7 cells independently of viral genotypes (data not shown). This is in agreement with clinical studies that showed that antiviral response to other nucleoside analogs is, at least initially, independent of viral genotypes (31).
New findings came from the evaluation of FLG antiviral activity on the replication of HBV and DHBV drug-resistant mutants. The selection of drug-resistant HBV strains during prolonged clinical administration of 3TC or PMEA is becoming a major obstacle for the therapy of chronic HBV infection (2, 12, 29). Currently, the rtL180M+M204V and the rtN236T HBV polymerase mutants are the more frequently observed in patients with 3TC or PMEA treatment failures, respectively. We therefore analyzed the cross-resistance profile of FLG with 3TC-resistant (3TC-R) and PMEA-resistant (PMEA-R) mutants using both DHBV cell-free assay and Huh7 cells transfected with the genomes of these HBV mutants. Our results indicate that there is no significant difference between FLG inhibitory activity on wt HBV or DHBV polymerase and on 3TC-R and PMEA-R mutants in both cell-free assay and Huh7 cells (Tables 1 and 2). We also assessed the antiviral efficiency of FLG against the DNA synthesis by HBV and DHBV polymerases harboring mutations conferring resistance to both 3TC and PMEA (3TC+PMEA-R mutant). Therapy combining 3TC and PMEA to treat patients infected with 3TC-resistant HBV strains (21) may select a multiple-drug-resistant mutant in the long term. In a previous report, we showed that an HBV laboratory strain, harboring the rtL180M+M204V+N236T mutation within the polymerase gene, was not defective for replication and was resistant to both 3TC and PMEA in transiently transfected Huh7 cells (3). Interestingly, we show here that FLG exhibits a similar antiviral activity against the replication of this HBV mutant compared to wt HBV in transiently transfected Huh7 cells. Moreover, these results were consistent with those obtained with the DHBV in vitro expression system.
In this study, we showed that FLG exhibited a similar inhibitory activity on wt and on single- and multiple-drug-resistant HBV and DHBV polymerases in both in vitro systems we used. Moreover, FLG inhibited 3TC+PMEA-R polymerases more efficiently than PMEA, whereas 3TC had no effect on these mutants. Altogether, our results suggest a lack of FLG cross-resistance against single 3TC- and PMEA-resistant mutants as well as against the double 3TC+PMEA-resistant mutant. These findings extend previous studies that showed that only purine analogs are active against the replication of an rtL180M+ M204V HBV mutant resistant to 3TC, whereas the same mutant is resistant to all known pyrimidine analogs (20, 33). Similarly, the susceptibility to FLG of HIV-1 isolates, harboring a mutation complex conferring resistance to thymidine analogues such as zidovudine or stavudine, was weakly decreased in vitro compared to wt HIV-1, and no cross-resistance was observed (H. Zhang, B. Öberg, J. Harmenberg, et al., Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abst r. H-182, 2002). Consequently, FLG appears to be an effective antiviral against both HIV- and HBV-resistant strains and may therefore be evaluated for the treatment of HIV/HBV-coinfected patients.
However, the therapeutic potency of a drug does not rely only on its antiviral capacity. Its toxicity also has to be taken into account, since the selectivity index (IC50/CC50) of a drug may prevent the use of optimal dose to reach a more potent antiviral effect, as is the case for adefovir. In in vitro studies, the susceptibilities of PMEA-R and 3TC+PMEA-R HBV strains to PMEA are reduced only by 3.2- to 23-fold and 6.3-fold, respectively (2, 3, 29). Nevertheless, the use of adefovir in the treatment of infection with such HBV-resistant strains is precluded, since increasing the adefovir daily dose from 10 to 30 mg leads to adverse renal effects (19). The comparison of the cytotoxic effects of FLG, 3TC, and PMEA in Huh7 cells indicated that the two purine analogs show similar CC50s but are more cytotoxic than 3TC.
In conclusion, our results obtained in the DHBV cell-free system allowed us to gain new insight on the mechanism of action of FLG. The cross-resistance profile of FLG suggests that this compound could be envisaged as a new alternative drug for the treatment of chronic HBV carriers who have developed resistance to currently approved drug regimens. FLG may also be valuable for the design and evaluation of new combination therapies with other polymerase inhibitors for the treatment of chronic HBV infection to prevent or delay the emergence of drug-resistant mutants.
Acknowledgments
This work was supported by Medivir and by grants from the European Community (HepBVar QLRT-2001-00977 and ViRgil LSHM-CT-2004-503359).
REFERENCES
- 1.Allen, M. I., M. Deslauriers, C. W. Andrews, G. A. Tipples, K. A. Walters, D. L. J. Tyrell, N. Brown, and L. D. Condreay. 1998. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology 27:1670-1677. [DOI] [PubMed] [Google Scholar]
- 2.Angus, P., R. Vaughan, S. Xiong, H. Yang, W. Delaney, C. Gibbs, C. Brosgart, D. Colledge, R. Edwards, A. Ayres, A. Bartholomeusz, and S. Locarnini. 2003. Resistance to adefovir dipivoxil therapy associated with the selection of a novel mutation in the HBV polymerase. Gastroenterology 125:292-297. [DOI] [PubMed] [Google Scholar]
- 3.Brunelle, M. N., A. C. Jacquard, C. Pichoud, D. Durantel, S. Carrouee-Durantel, J. P. Villeneuve, C. Trepo, and F. Zoulim. 2005. Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir. Hepatology 41:1391-1398. [DOI] [PubMed] [Google Scholar]
- 4.Dannaoui, E., C. Trépo, and F. Zoulim. 1997. Inhibitory effect of penciclovir-triphosphate on duck hepatitis B virus reverse transcription. Antivir. Chem. Chemother. 8:38-46. [Google Scholar]
- 5.Delaney, W. E., IV, H. Yang, C. E. Westland, K. Das, E. Arnold, C. S. Gibbs, M. D. Miller, and S. Xiong. 2003. The hepatitis B virus polymerase mutation rtV173L is selected during lamivudine therapy and enhances viral replication in vitro. J. Virol. 77:11833-11841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durantel, D., S. Carrouee-Durantel, B. Werle-Lapostolle, M. N. Brunelle, C. Pichoud, C. Trepo, and F. Zoulim. 2004. A new strategy for studying in vitro the drug susceptibility of clinical isolates of human hepatitis B virus. Hepatology 40:855-864. [DOI] [PubMed] [Google Scholar]
- 7.Gish, R. G., N. W. Leung, T. L. Wright, H. Trinh, W. Lang, H. A. Kessler, L. Fang, L. H. Wang, J. Delehanty, A. Rigney, E. Mondou, A. Snow, and F. Rousseau. 2002. Dose range study of pharmacokinetics, safety, and preliminary antiviral activity of emtricitabine in adults with hepatitis B virus infection. Antimicrob. Agents Chemother. 46:1734-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafkemeyer, P., A. Keppler-Hafkemeyer, M. Haya, M. von Janta-Lipinski, E. Matthes, C. Lehmann, W. Offensperger, S. Offensperger, W. Gerok, and H. Blum. 1996. Inhibition of duck hepatitis B virus replication by 2′,3′ dideoxy 3′ fluoroguanosine in vitro and in vivo. Antimicrob. Agents Chemother. 40:792-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hantz, O., C. Borel, C. Trabaud, F. Zoulim, J. Dessolin, M. Camplo, P. Vlieghe, M. Bouygues, C. Trépo, and J. L. Kraus. 1997. Selective inhibition of the duck hepatitis B virus by a new class of tetraazamacrocycles. Antimicrob. Agents Chemother. 41:2579-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann, H., M. W. Vogt, A. G. Durno, M. S. Hirsch, G. Hunsmann, and F. Eckstein. 1988. Enhanced in vitro inhibition of HIV-1 replication by 3′-fluoro-3′-deoxythymidine compared to several other nucleoside analogs. AIDS Res. Hum. Retrovir. 4:457-466. [DOI] [PubMed] [Google Scholar]
- 11.Horwich, A. L., K. Furtak, J. Pugh, and J. Summers. 1990. Synthesis of hepadnavirus particles that contain replication-defective duck hepatitis B virus genomes in cultured HuH7 cells. J. Virol. 64:642-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai, C. L., J. Dienstag, E. Schiff, N. W. Leung, M. Atkins, C. Hunt, N. Brown, M. Woessner, R. Boehme, and L. Condreay. 2003. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. 36:687-696. [DOI] [PubMed] [Google Scholar]
- 13.Lai, C. L., S. G. Lim, N. A. Brown, X. J. Zhou, D. M. Lloyd, Y. M. Lee, M. F. Yuen, G. C. Chao, and M. W. Myers. 2004. A dose-finding study of once-daily oral telbivudine in HBeAg-positive patients with chronic hepatitis B virus infection. Hepatology 40:719-726. [DOI] [PubMed] [Google Scholar]
- 14.Lanford, R., L. Notvall, and B. Beames. 1995. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J. Virol. 69:4431-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanford, R., L. Notvall, H. Lee, and B. Beames. 1997. Transcomplementation of nucleotide priming and reverse transcription between independently expressed TP and RT domains of the hepatitis B virus reverse transcriptase. J. Virol. 71:2996-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Guerhier, F., C. Pichoud, S. Guerret, M. Chevallier, C. Jamard, O. Hantz, X. Y. Li, S. H. Chen, I. King, C. Trepo, Y. C. Cheng, and F. Zoulim. 2000. Characterization of the antiviral effect of 2′,3′-dideoxy-2′,3′-didehydro-beta-l-5-fluorocytidine in the duck hepatitis B virus infection model. Antimicrob. Agents Chemother. 44:111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine, S., D. Hernandez, G. Yamanaka, S. Zhang, R. Rose, S. Weinheimer, and R. J. Colonno. 2002. Efficacies of entecavir against lamivudine-resistant hepatitis B virus replication and recombinant polymerases in vitro. Antimicrob. Agents Chemother. 46:2525-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lofgren, B., K. Vickery, Y. Y. Zhang, and E. Nordenfelt. 1996. 2′,3′-Dideoxy-3′-fluoroguanosine inhibits duck hepatitis B virus in vivo. J. Viral Hepat. 3:61-65. [DOI] [PubMed] [Google Scholar]
- 19.Marcellin, P., T. T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 20.Ono, S. K., N. Kato, Y. Shiratori, J. Kato, T. Goto, R. F. Schinazi, F. J. Carrilho, and M. Omata. 2001. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J. Clin. Investig. 107:449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters, M. G., H. Hann, P. Martin, E. J. Heathcote, P. Buggisch, R. Rubin, M. Bourliere, K. Kowdley, C. Trepo, D. Gray, M. Sullivan, K. Kleber, R. Ebrahimi, S. Xiong, and C. L. Brosgart. 2004. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 126:91-101. [DOI] [PubMed] [Google Scholar]
- 22.Schroder, I., B. Holmgren, M. Oberg, and B. Lofgren. 1998. Inhibition of human and duck hepatitis B virus by 2′,3′-dideoxy-3′-fluoroguanosine in vitro. Antivir. Res. 37:57-66. [DOI] [PubMed] [Google Scholar]
- 23.Seifer, M., R. Hamatake, M. Bifano, and D. N. Standring. 1998. Generation of replication-competent hepatitis B virus nucleocapsids in insect cells. J. Virol. 72:2765-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seifer, M., R. K. Hamatake, R. J. Colonno, and D. N. Standring. 1998. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob. Agents Chemother. 42:3200-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seigneres, B., S. Aguesse-Germon, C. Pichoud, I. Vuillermoz, C. Jamard, C. Trepo, and F. Zoulim. 2001. Duck hepatitis B virus polymerase gene mutants associated with resistance to lamivudine have a decreased replication capacity in vitro and in vivo. J. Hepatol. 34:114-122. [DOI] [PubMed] [Google Scholar]
- 26.Seigneres, B., C. Pichoud, P. Martin, P. Furman, C. Trepo, and F. Zoulim. 2002. Inhibitory activity of dioxolane purine analogs on wild-type and lamivudine-resistant mutants of hepadnaviruses. Hepatology 36:710-722. [DOI] [PubMed] [Google Scholar]
- 27.Tavis, J. E., B. Massey, and Y. Gong. 1998. The duck hepatitis B virus polymerase is activated by its RNA packaging signal, epsilon. J. Virol. 72:5789-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenney, D. J., S. M. Levine, R. E. Rose, A. W. Walsh, S. P. Weinheimer, L. Discotto, M. Plym, K. Pokornowski, C. F. Yu, P. Angus, A. Ayres, A. Bartholomeusz, W. Sievert, G. Thompson, N. Warner, S. Locarnini, and R. J. Colonno. 2004. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob. Agents Chemother. 48:3498-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villeneuve, J. P., D. Durantel, S. Durantel, C. Westland, S. Xiong, C. L. Brosgart, C. S. Gibbs, P. Parvaz, B. Werle, C. Trepo, and F. Zoulim. 2003. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J. Hepatol. 39:1085-1089. [DOI] [PubMed] [Google Scholar]
- 30.Wang, G. H., and C. Seeger. 1992. The reverse transcriptase of hepatitis B virus acts a protein primer for viral DNA synthesis. Cell 71:633-670. [DOI] [PubMed] [Google Scholar]
- 31.Westland, C., W. Delaney IV, H. Yang, S. S. Chen, P. Marcellin, S. Hadziyannis, R. Gish, J. Fry, C. Brosgart, C. Gibbs, M. Miller, and S. Xiong. 2003. Hepatitis B virus genotypes and virologic response in 694 patients in phase III studies of adefovir dipivoxil. Gastroenterology 125:107-116. [DOI] [PubMed] [Google Scholar]
- 32.Xiong, X., C. Flores, H. Yang, J. J. Toole, and C. S. Gibbs. 1998. Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology 28:1669-1673. [DOI] [PubMed] [Google Scholar]
- 33.Zoulim, F. 2004. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antivir. Res. 64:1-15. [DOI] [PubMed] [Google Scholar]
- 34.Zoulim, F., E. Dannaoui, C. Borel, O. Hantz, T.-S. Lin, S.-H. Liu, C. Trépo, and Y.-C. Cheng. 1996. 2′,3′-Dideoxy-β-l-5-fluorocytidine inhibits duck hepatitis B virus reverse transcription and suppresses viral DNA synthesis in hepatocytes, both in vitro and in vivo. Antimicrob. Agents Chemother. 40:448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zoulim, F., and C. Seeger. 1994. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J. Virol. 68:6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]