Abstract
Mefloquine is one of the drugs approved by the FDA for malaria chemoprophylaxis. Mefloquine is also approved for the treatment of malaria and is widely used for this purpose in combination with artesunate. However, the clinical utility of the compound has been compromised by reports of adverse neurological effects in some patients. In the present study, the potential neurological effects of mefloquine were investigated with six 7-week-old female rats given a single oral dose of the compound. Potential mefloquine-induced neurological effects were monitored using a standard functional observational battery, automated open field tests, automated spontaneous activity monitoring, a beam traverse task, and histopathology. Plasma mefloquine concentrations were determined 72 h after dosing by using liquid chromatography-mass spectrometry. Mefloquine induced dose-related changes in endpoints associated with spontaneous activity and impairment of motor function and caused degeneration of specific brain stem nuclei (nucleus gracilis). Increased spontaneous motor activity was observed only during the rats' normal sleeping phase, suggesting a correlate to mefloquine-induced sleep disorders. The threshold dose for many of these effects was 187 mg/kg of body weight. This dose yielded plasma mefloquine concentrations after 72 h that are similar to those observed in humans after the treatment dose. Collectively, these data suggest that there may be a biological basis for some of the clinical neurological effects associated with mefloquine.
Malaria, caused by parasitic protozoa of the genus Plasmodium, is endemic in tropical regions inhabited by approximately 50% of the global population (46). There are estimated to be 350 to 500 million clinical cases and at least a million deaths annually (46). Malaria is also a problem for travelers. In the United States alone, there were 1,337 cases of imported malaria and eight deaths in 2002 (37). In the United States, there are five approved drugs available for malaria chemoprophylaxis. These are mefloquine, doxycycline, atovaquone-proguanil (Malarone), chloroquine, and hydroxychloroquine sulfate (4). Of these, only mefloquine, chloroquine, and hydroxychloroquine sulfate have sufficiently long half-lives to permit weekly dosing (4). Weekly dosing enhances compliance with prophylactic dosing regimens. However, resistance of malaria parasites to chloroquine and hydroxychloroquine sulfate has become extremely widespread (46). Consequently, mefloquine, doxycycline, and atovaquone-proguanil are most commonly used for malaria prophylaxis, although atovaquone-proguanil is most often prescribed for shorter excursions. When given for malaria prophylaxis, mefloquine is given as a 250-mg tablet once weekly (4).
Mefloquine is also U.S. Food and Drug Administration (FDA) approved for the treatment of malaria (34). The product label recommends the administration of 1,250 mg mefloquine hydrochloride (1,140 mg mefloquine base) as a single dose (34). This represents a dose of 12.5 to 25 mg/kg for most adults depending on body weight (50 to 100 kg). However, it is currently more common for the drug to be administered as a split dose (750 and 500 mg 6 hours apart), as recommended by the Centers for Disease Control (CDC) (3). This recommendation is based on the observation that vomiting is seen less frequently with split dosing (32). Outside the United States, mefloquine is most commonly administered in combination with artesunate for treatment of malaria (45). Unfortunately, mefloquine has also been associated with neurological sequelae, including anxiety, panic attacks, suicidal ideation, nightmares, sleep disturbances, dizziness, tremor, headache, mood changes, and fatigue (1, 31). These effects generally occur more frequently at the treatment dose, even in the absence of malaria, than at the prophylaxis dose (31, 33).
These observations suggest that the neurological effects of mefloquine are dose dependent and should have a biological basis. However, for a number of reasons this has never been unequivocally demonstrated. First, there has been only one report, in the patent literature, of mefloquine-attributable neurological effects in an animal model (38). Second, the reported incidence of adverse neurological events after mefloquine administration is variable (5). This is probably due to a combination of factors, including (i) lack of formal diagnostic criteria (case definition), (ii) varying definitions of neurological effects, (iii) varying definitions of mild versus severe effects, and (iv) differences in protocols used for data collection (11, 35). Finally, in vitro studies have shown that mefloquine interacts with a bewildering array of protein targets and cellular signaling pathways (6, 8, 16, 17, 21, 22, 24, 30, 44), any of which may or may not be clinically important. As a first step in the direction of rendering these challenges more tractable, a proof-of-concept in vivo neurological evaluation of the effects of mefloquine would be invaluable. This is the subject of the present study.
We report here the results of an evaluation of the neurological effects of mefloquine in rats. Our objective was to maximize the possibility of detecting any in vivo neurological effects attributable to mefloquine. This was achieved by careful selection of the test system, subject gender, and observation time. At the time this study was conceived, no formal FDA guidelines for neurotoxicity testing existed. In contrast, first-tier neurological screens, such as those recommended by the U.S. Environmental Protection Agency (EPA), are often employed to detect a broad range of possible neurological effects that may be induced by uncharacterized test compounds (43). In the EPA screen, rats are treated with a range of doses of the test compound and potential neurological effects are assessed using a functional observational battery (FOB), automated activity monitoring, and histopathology (43). A modified version of this screen was used here. Female rats were selected since the neurological effects of mefloquine in humans are more often observed in women (18, 19, 36). This may be due to higher plasma concentrations or areas under the curve (19), but a gender bias cannot be ruled out. We chose a 72-h observation window because neurological effects in humans are most often observed during this time frame (18, 36). Finally, in an attempt to put any neurological findings observed into a clinical context, plasma mefloquine concentrations were determined in order to guide dose selection.
MATERIALS AND METHODS
Animals.
Six female 7-week-old Sprague-Dawley rats (Charles River, Wilmington, MA) were used. The rats were acclimatized in animal holding rooms for 7 days before testing began. Food and water were provided ad libitum. Animal holding rooms were on a 12-h light/dark cycle with lights out at 9 p.m. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the NRC Guide for the Care and Use of Laboratory Animals (26).
Chemicals.
Racemic GMP mefloquine hydrochloride was obtained from the Walter Reed Army Institute of Research chemical inventory. All calculations relating to dose and concentrations were made and are expressed in terms of salt weight, since the salt used was identical to the clinical formulation. Corn oil was purchased from Spectrum Organic Products (Petaluma, CA). All perfusion and staining reagents were purchased from FD Neurotechnologies (Baltimore, MD). Ketamine and xylazine were purchased from NSL Animal Health (Baltimore, MD). All other reagents, including liquid chromatography-mass spectrometry supplies and heparin, were purchased from Sigma (St. Louis, MO).
Experimental design.
This study was conducted in two parts. Phase 1 was a dose-ranging study. In the dose-ranging study, the effects of mefloquine were also assessed on several neurological endpoints (beam traverse and histopathology). In phase 2, larger numbers of animals were treated with a range of doses selected on the basis of the phase 1 study. The potential neurological effects were evaluated using the functional observational battery and automated activity monitoring.
Phase 1.
Groups of four rats were given the drug vehicle (corn oil) alone or mixed with a single dose of orally administered mefloquine at the doses indicated in Table 1. The animals were closely monitored in the posttreatment phase and weighed daily, and any clinical signs indicative of acute toxicity were noted. Euthanasia was considered if clinical signs of toxicity were severe or persistent. For the purposes of determining a 50% lethal dose (LD50), euthanasia was considered equivalent to death. After 72 h postdosing, the animals were anesthetized (ketamine-xylazine 70/6 mg/kg intramuscularly) and whole blood was collected into heparinized syringes via cardiac puncture. Plasma was then separated and mefloquine concentrations were determined as outlined below. During the posttreatment phase, some of the rats were used in a pilot mode to test the effect of mefloquine in a beam traverse test. An additional group of two animals was treated with 765 mg/kg mefloquine to confirm toxicity. Additional animals were dosed with the drug vehicle alone or mefloquine at 187 mg/kg or 765 mg/kg and sacrificed for histopathology as outlined in Table 1.
TABLE 1.
Experimental design
| Factor | Design for:
|
|
|---|---|---|
| Phase 1 | Phase 2 | |
| Purpose | Acute toxicity/PK determination | FOB evaluation (multiple-dose-level study) |
| Dose identification | ||
| Selected neurological endpoints | ||
| Dosing groups | 13.6, 24, 43, 77, 136, 242, 343, 430, 574, and 765 mg/kga | Prophylaxis dose (45 mg/kg) |
| Treatment dose (187 mg/kg) | ||
| Intermediate dose (327 mg/kg) | ||
| MTDb (574 mg/kg) (n = 8 per treatment group) | ||
| Vehicle | Corn oil (3.0 ml/kg, n = 6) | Corn oil (2.25 ml/kg, n = 8) |
Minimum of four rats per group for the toxicity/pharmacokinetics (PK) study, with an additional three rats for the 187-mg/kg group and an additional two rats for the 765-mg/kg group for the histology and confirmatory toxicity experiments.
MTD, maximum tolerated dose.
Determination of phase 2 doses.
Doses of 45, 187, 327, and 574 mg/kg were selected for the phase 2 study. The maximum tolerated dose is represented by 574 mg/kg, and this was calculated as described below. An intermediate dose was 327 mg/kg. Mefloquine doses of 45 and 187 mg/kg were found to generate plasma mefloquine concentrations of the same order of magnitude as those observed after prophylaxis and treatment in humans, respectively. The target mefloquine plasma concentrations were 800 and 2,100 ng/ml. A mefloquine concentration of 800 ng/ml represents the approximate mean steady-state trough plasma level 7 days after weekly doses of mefloquine (18). A mefloquine concentration of 2,100 ng/ml represents the population mean whole-blood level 7 days after administration of a split dose of 25 mg/kg (15 mg/kg followed by 10 mg/kg 24 h later [39]). Whole-blood mefloquine concentrations are generally higher than plasma concentrations, and the dose used is at the higher end of the normal range of mefloquine treatment doses. However, it is important to note that the range of mefloquine concentrations reported in the latter study was very wide (800 to 8,800 ng/ml [39]). In both of the clinical studies, mefloquine levels were determined by high-performance liquid chromatography (18, 39).
Phase 2 experiments.
In this phase, four different dose levels were selected as outlined in Table 1. Experiments were performed with four batches of 10 rats each, with rats randomly allocated to each dosing group (2 rats per dose) in each batch. One day prior to dosing, the animals were subjected to a predose battery of observational and neurological tests as described in the next section. This series of tests was repeated 24 and 48 h after dosing. Rats were sacrificed at 72 h after dosing.
Determination of plasma mefloquine concentrations.
Rat plasma samples were prepared using a plasma crash method in which 50 μl of thawed plasma was added to 150 μl of ice-cold acetonitrile. Samples were vortexed and then centrifuged to precipitate proteins. Triplicate aliquots of the supernatant were then analyzed using liquid chromatography-tandem mass spectrometry. Mefloquine levels were quantified using internal calibration employing plots of the analyte area/internal standard area ratio versus internal standard amount in ng/ml. A test calibration curve prepared in rat plasma was extracted and run using this method the morning of the analysis of submitted samples. Chromatograms were analyzed using the mass spectrometry software Xcalibur Quanbrowser, and peaks were integrated. Peak areas of mefloquine and the internal standard were determined, and their ratios were calculated as analyte area/internal standard area. The amount in ng/ml of mefloquine was determined using the equations derived from the calibration curves. Mefloquine concentrations were determined and are expressed in terms of ng salt/ml.
Histology.
Animals were deeply anesthetized with ketamine-xylazine (100/8 mg/kg) and perfused via the ascending aorta with 100 ml of saline followed by 500 ml of 0.1 M phosphate buffer (pH 7.4) containing 4% paraformaldehyde. The brains were removed and postfixed in the same fixative for 6 h at 4°C. Following immersion in 0.1 M phosphate-buffered saline (pH 7.4) containing 20% sucrose for 48 h (4°C), the brains were rapidly frozen and stored at −75°C.
Rat brains were cut with a cryostat. Serial sections (50 or 30 μm in width) were cut coronally through the whole brain, including the cerebellum and the brain stem, from approximately bregma 3.70 mm to bregma −14.60 mm, as described by Paxinos and Watson (29). For brain regions between bregma 3.70 mm and 8.3 mm, sets of 15 serial sections spanning 510 μm were collected. The first three sections in each set were 50 μm thick, and the final 12 sections were 30 μm in width. For brain regions between −8.3 mm and −14.60 mm, sets of nine serial sections spanning 310 μm were collected. The first two sections in each set were 50 μm in width, and the final seven sections were 30 μm in width.
The second slices in each serial set were collected in 0.1 M phosphate buffer containing 4% paraformaldehyde and were processed for the detection of neuronal damage with the silver staining process using FD Neurosilver kits according to the manufacturer's instructions (FD Neurotechnologies, Inc., Baltimore, MD). The principle of the staining is based on the findings that certain components of neurons undergoing degeneration, such as lysosomes, axons, and terminals, become particularly argyrophilic. Under certain conditions, these cellular elements bind silver ions with high affinity. Upon reduction, the silver ions form metallic grains that are visible under light microscopes (12, 13, 27).
Silver-stained sections from control and high-dose (765 mg/kg) rats were examined blindly, and any neuronal degeneration in the cortex, cerebellum, and brain stem was identified. Sections containing any such identified affected regions then underwent detailed examination for all rats, and any neurodegeneration of specific nuclei or regions was rated. This was done via blinded scoring using a relative, semiquantitative scale: no damage (−), borderline damage (+/−), mild damage (+), moderate damage (++), marked damage (+++), or severe damage (++++). Similar semiquantitative scoring approaches are commonly used for a variety of histopathological evaluations (e.g., see references 9 and 28) and have also been used to evaluate the neurotoxic effects of other antimalarials (14).
FOB and neurobehavioral screening battery.
Rats were subjected to a series of individual FOB testing procedures as listed in Table 2 and described by McDaniel and Moser (25). A number of procedures, including grip strength, landing-foot splay, pupil response, and body temperature, were excluded, as resource constraints did not allow data collection. Some testing procedures were optimized for our purposes as follows.
TABLE 2.
Measures of the functional observational and neurobehavioral test battery
| FOB measurea |
|---|
| Autonomic |
| Lacrimation (h) |
| Salivation (h) |
| Urination (o.f.) |
| Defecation (o.f.) |
| Vocalizations (h.c., o.f.) |
| Activity |
| Rearing (o.f.) |
| Motor activity counts (s.m.a.) |
| Home cage posture (h.c.) |
| Speed (o.f.) |
| Path length (o.f.) |
| Resting time (o.f.) |
| Excitability |
| Ease of removal (h) |
| Handling reactivity (h) |
| Arousal (o.f.) |
| Clonic movements (h.c., o.f.) |
| Tonic movements (h.c., o.f.) |
| Palpebral closure (h.c., h) |
| Motor function |
| Gait score (o.f.) |
| Righting reflex (s.r.) |
| Beam balance and traverse (b.t.) |
| Sensorimotor |
| Tail pinch response (s.r.) |
| Click response (s.r.) |
| Touch response (s.r.) |
| Approach response (s.r.) |
| Physiological and other measures |
| Body wt (h) |
| Piloerection (h) |
| Bizarre behavior (h.c., o.f.) |
| Stereotypy (h.c., o.f.) |
Measures are organized according to domains of neurological function and location within testing schedule. Measures shown in boldface were changed by mefloquine treatment. Abbreviations in parentheses refer to the point in the observational battery at which endpoints were measured. h.c., home cage observations; h, handling observations; o.f., open field tests; s.r., stimulus reactivity measurements; b.t., beam testing; s.m.a., spontaneous motor activity measurements.
The open field used was a 16- by 16- by 12-in arena (San Diego Instruments, San Diego, California), and session times were 5 min per animal. These sessions were digitally recorded to allow each animal's path length, rest time, and speed (with and without rests) to be determined using a video tracking system (San Diego Instruments, San Diego, California). In contrast, in the EPA screen, animals are monitored in real time by a single observer. Objective measurements of speed, etc., are not possible using that approach. Nor is it possible to review video footage to more closely examine interesting behavioral changes.
Motor activity as measured by total photo beam breaks was assessed during a 2-h period before and after lights out (7 p.m. to 11 p.m.) by using photo beam racks mounted to the animals' home cages (Hamilton Kinder, Poway, California). This was necessary since we were specifically testing the hypothesis that spontaneous motor activity would increase during the day (sleep phase) but not at night.
The sequence of testing events was conducted as follows: (i) 5 min of home cage observation, (ii) handling/capture response, (iii) open field tests, (iv) stimulus/reactivity monitoring, and (v) spontaneous activity measurements. The FOB was conducted just prior to and 24 h and 48 h after dosing.
Beam training and testing.
In phase 1, selected rats given the drug vehicle or a range of mefloquine doses (14 to 765 mg/kg) were subjected to a beam traverse test. Procedures were performed as described by Lyeth et al. (23) with the following modifications. An elevated 220-cm-long by 2.5-cm-wide beam was utilized. In the week prior to dosing, rats were trained to traverse this beam with the aid of an air gun (rather than white noise) as a nonharmful noxious stimulus to run. On the last of the predose sessions, the time (in seconds) to traverse a 1.5-m section of the beam was recorded for two runs. Beam traverse time was again measured for a single run 24 h and 48 h after dosing. Prior to beam testing rats, a balance test was conducted to ensure that the animals were able to remain balanced on the beam between barriers 30 cm apart for 60 s. No rats tested were unable to perform this task.
Data analysis.
For the phase 2 study, the two lowest doses of mefloquine (45 and 187 mg/kg) were selected such that plasma mefloquine concentrations in rats 72 h after dosing were in the same range as those observed in humans after clinical dosing. The mefloquine doses in rats yielding concentrations of 800 and 2,100 ng/ml 72 h after dosing were estimated by linear regression analysis of mefloquine plasma concentrations versus dose and were found to be 45 mg/kg and 187 mg/kg, respectively.
In the present study, the maximum tolerated dose was defined as the dose resulting in death in approximately 1% of animals (i.e., an LD01). Calculation of an LD01 normally requires formal estimates of the LD50 and variance. Due to humane considerations, data obtained in this study were not sufficient for formal estimates of the LD50 since death was not used as the primary endpoint. An approximate LD50 for female rats was estimated based on clinical observation of the animals and likely clinical outcome in the event of acute toxicity. Variance was estimated using data from a historic acute-toxicity study (20) in which the LD50 of mefloquine in male rats was determined. The LD01 was then estimated using Probit analysis (10).
Changes in FOB endpoints were scored as normal/no change to abnormal/maximum change by using descriptive, quantal, ranked, or continuous scaling systems as described by McDaniel and Moser (25). The exceptions to this were palpebral closure and beam traverse time. All states of palpebral closure were noted for each time point for each rat. For the purposes of analysis, the highest score for each rat was used. For the beam traverse experiments, net beam time was calculated by subtracting the average beam traverse time of two predose runs from the time recorded for a single run at 24 and 48 h. Spontaneous activity data were normalized to predose values prior to further analysis.
For each endpoint, descriptive statistics were generated for each treatment group at each dose. The data were examined to identify possible trends across treatment group or dose. Possible changes were evident for palpebral closure and spontaneous motor activity. Where appropriate, data for these potentially affected endpoints were analyzed using two-way analysis of variance (dose versus time). Bonferroni posttests followed to assess individual group differences. Where relevant, differences between two individual groups were tested using a t test with Welch's correction for unequal variance. Differences across treatment groups at a single time point were assessed using one-way analysis of variance with posttesting conducted using Dunnett's multiple-comparison test. This general approach to analysis differs slightly from that used by the EPA in that data for each neurological domain are often pooled for the purposes of analysis.
The beam traverse data were collected from a pilot experiment in phase 1. Consequently, the sample size in each treatment group in this study was insufficient to warrant meaningful statistical analysis of any changes in beam traverse time in any single dose group. Consequently, a post hoc analysis, in which beam traverse data were pooled into three treatment groups and analyzed statistically as described above, was performed.
RESULTS
Mefloquine plasma concentrations, toxicity, and dose selection.
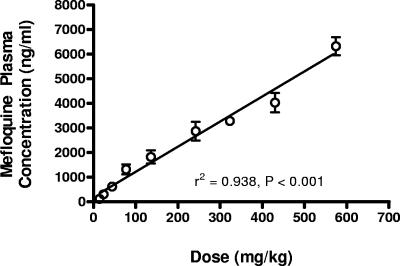
Rat plasma mefloquine concentrations were highly correlated with dose (Fig. 1) (r2 = 0.938, P < 0.001, standard linear regression). The mefloquine dose that generated rat plasma concentrations at 72 h that were comparable to those found in humans after prophylactic dosing was 45 mg/kg. The corresponding dose for treatment was 187 mg/kg. The maximum tolerated dose (i.e., the LD01) was estimated on the basis of the incidence of frank clinical signs of toxicity observed at the various mefloquine doses. These included severe lethargy, weight loss, piloerection, and secretion of porphyrin pigments. At doses less than 765 mg/kg, no acute toxic signs were observed. Acute signs of toxicity were observed with all rats given 765 mg/kg mefloquine. As outlined in Table 3, there was a significant decline in body weight and death would have been the likely outcome in at least three of six rats at the 765-mg/kg dose. Therefore, the LD50 was estimated to be 765 mg/kg and the LD01 was determined to be between 514 and 601 mg/kg by use of a standard Probit analysis (95% confidence intervals). A dose of 574 mg/kg was selected as the maximum tolerated dose. A dose intermediate between the approximate treatment and maximum tolerated doses was also selected for the phase 2 experiments (327 mg/kg).
FIG. 1.
Mefloquine plasma concentrations in juvenile female rats at 72 h at various dose rates. Mefloquine plasma concentrations were highly correlated with dose (r2 = 0.938, P < 0.001, standard linear regression). Doses at which plasma concentrations were estimated to be 800 ng/ml (prophylaxis) and 2,100 ng/ml (treatment) were 45 and 187 mg/kg, respectively.
TABLE 3.
Clinical signs and weight change in six rats administered 765 mg/kg mefloquine
| Time (h) | Wt change for rat no.
|
Decline in relative wt (% predose ± SE) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| 0 | None | None | None | None | None | None | 100 ± 2.0 |
| 24 | None | None | None | None | None | None | 99 ± 2.7 |
| 48 | Mild | Mild | None | None | Mild | Severea | 96 ± 2.2 |
| 72 | Severea | Mild | Severea | Mild | Mild | 91 ± 2.9b | |
Death was the likely outcome for rats with severe clinical symptoms or weight loss. These rats were euthanized.
Significantly different from predose values.
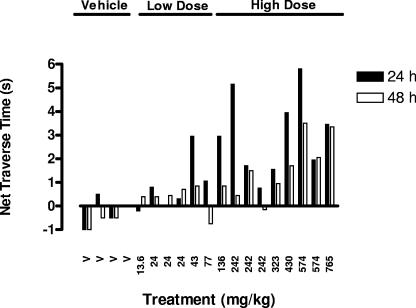
Effects of mefloquine on beam traverse time.
Mefloquine induced a dose-dependent increase in beam traverse time (Fig. 2). This effect was statistically significant if the data are pooled into vehicle alone, low-dose (13.6- to 136-mg/kg), and high-dose (>136-mg/kg) groups (Fig. 2). Further evidence of a dose-related effect is the observation that there was a significant correlation between net beam traverse time and plasma mefloquine concentration at both 24 h (r2 = 0.48, P = 0.006) and 48 h (r2 = 0.67, P = 0.0003) (Fig. 3).
FIG. 2.
Effects of mefloquine on beam traverse time at various doses, as expressed in terms of net change from predose traverse times. Mefloquine induced a general increase in net beam traverse time at 24 and 48 h with increasing dose. This effect was statistically significant (P < 0.01) at the high dose if the data are pooled into three treatment groups (vehicle [V], 13.6 to 136 mg/kg, and >136 mg/kg). Note that the negative change in the vehicle group represents an expected learning effect.
FIG. 3.
Mefloquine induces a delay in beam traverse time that is correlated with plasma concentration. Net beam traverse time was correlated with mefloquine plasma concentrations at 24 h (r2 = 0.48, P = 0.006) and 48 h (r2 = 0.67, P = 0.0003), suggesting a dose-related effect.
Histological changes induced by mefloquine.
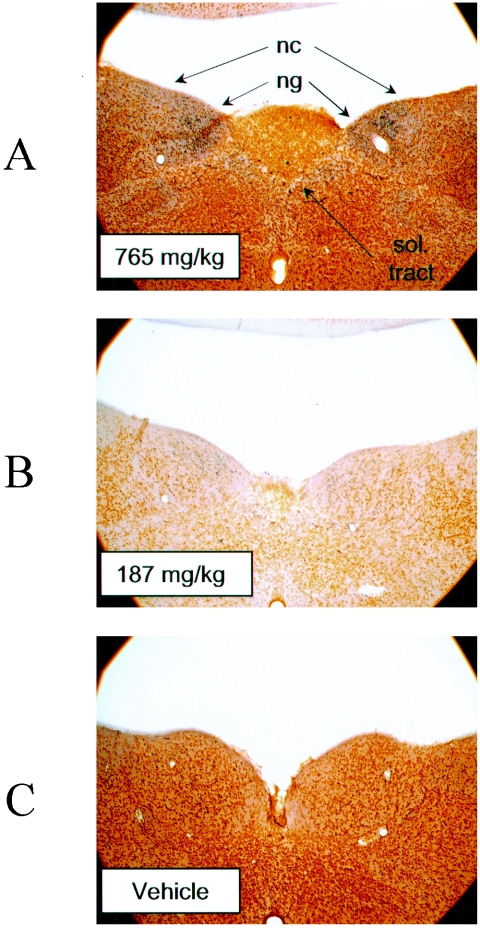
Silver-stained brain sections from rats dosed with the vehicle alone, 187 mg/kg mefloquine, or 765 mg/kg mefloquine were examined to determine whether gross pathological changes were evident. Degenerating fibers were observed in the nucleus gracilis (n. gracilis) and to a lesser extent in the nucleus cuneatus and solitary tract in the high-dose rats. This effect was less pronounced in the 187-mg/kg-dose group and was not present in controls (Fig. 4 and Table 4). Other nuclei of the cortex, cerebellum, and brain stem appeared unaffected by use of this staining procedure.
FIG. 4.
Mefloquine induces neuronal damage in specific brain stem nuclei. Neuronal degeneration, as revealed by the presence of black-stained metallic granules, occurred in the nucleus gracilis (ng) after high-dose mefloquine treatment (765 mg/kg) (A). This effect was less pronounced with the 187-mg/kg dose (B) and was not observed with controls (C). The nucleus cuneatus (nc) and solitary (sol.) tract were affected to a lesser degree. Sections are stained with silver. Magnification, ×4.
TABLE 4.
Histopathological changes observed with mefloquine-treated rats
| Nucleus | Degree of neurodegenerationa for treatment group receiving:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vb | V | V | Mefloquine dose (mg/kg)
|
||||||
| 187 | 187 | 187 | 765 | 765 | 765 | ||||
| Gracilis | − | − | − | + | ++ | +/− | +++ | +++ | ++++ |
| Cuneatus | − | − | − | − | +/− | − | ++ | + | ++ |
| Solitary tract | − | − | − | + | ++ | + | ++ | ++ | ++ |
Neurodegeneration was scored using a relative, semiquantitative scale: no damage (−), borderline damage (+/−), mild damage (+), moderate damage (++), marked damage (+++), or severe damage (++++).
V, vehicle control.
General description of findings in FOB and open field.
In phase 2, functional observational battery, open field, and activity data were examined to assess whether any changes attributable to mefloquine occurred (Table 2). The treatment groups were the drug vehicle and 45, 187, 327, and 574 mg/kg mefloquine. Mefloquine induced dose- and time-dependent changes in motor activity and palpebral closure (home cage), and these observations are discussed in the next section. Mefloquine at a dose of 574 mg/kg induced a significant decline in body weight after 72 h (4.7%, P = 0.0008), whereas no statistically significant change occurred with the other treatment groups. Mefloquine did not alter baseline values for the other parameters evaluated (Table 2). The only other notable change was a tendency towards time-dependent decreased exploratory behavior in open field tests (data not shown).
Mefloquine induced changes in motor activity endpoints.
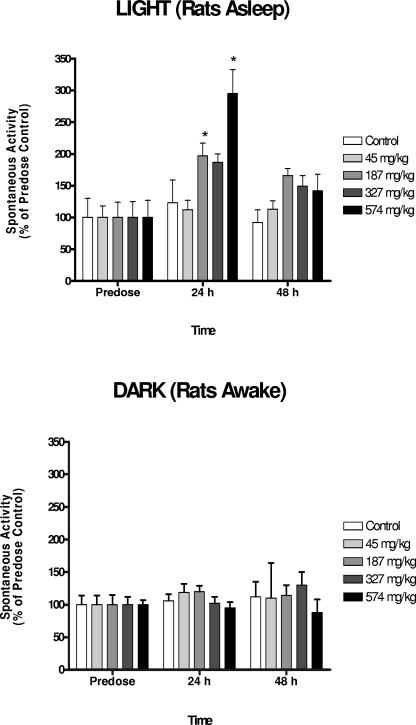
Mefloquine induced a dose- and time-related increase in motor activity during the rats' sleeping phase (i.e., in the light) and a decrease in palpebral closure in the home cage observations (Fig. 5 and 6). Similar changes in motor activity were not observed during the rats' awake phase (i.e., in the dark) (Fig. 5). Together these data suggest that mefloquine disrupts normal sleep patterns in rats. The threshold dose of this effect appears to be the treatment dose (187 mg/kg), with the peak effect being observed at 24 h. In some rats, an unusual pattern of behavior, characterized by excessive grooming, hyperactivity, unusual jumps, and rapid, abrupt, and frequent changes in body position, was observed. We have termed this “anxiousness/hyperactivity”, and its incidence appeared to be time and dose related (Table 5). The effect peaked at 24 h, and the threshold dose was 187 mg/kg. Similar behavior was observed with a single vehicle-dosed rat at all observation times. Consequently, it is possible that mefloquine exacerbates a pattern of behavior that is manifest in a small proportion of the general population (e.g., anxiety).
FIG. 5.
Mefloquine disrupts sleeping patterns of rats in a dose- and time-dependent manner. The effects of dose and time on spontaneous motor activity were evaluated for a 2-h period prior to (light) and after (dark) lights out at night at 9 p.m. Since rats are nocturnal, the “light” testing period corresponds to the animals' sleep phase, while the animals are normally active during the “dark” phase. Mefloquine induced a significant dose-dependent (P = 0.0006) and time-dependent (P < 0.0001) effect on spontaneous activity of rats, and there was a significant interaction between dose and time (P = 0.0055). This effect was not observed during the rats' active phase (P of >0.66 for all factors). Spontaneous activity in the rats' sleep (light) phase peaked at 24 h, and the threshold dose was 187 mg/kg (specific treatment groups which were different from predose values are indicated by an asterisk [P < 0.05]). Mean activity counts in the dark (mean ± standard error of the mean [SEM] of 1,340 ± 74) were significantly higher than those in the light (mean ± SEM of 554 ± 58 [P < 0.0001]), but no differences between groups in predose activity counts were observed in either the light or the dark (P of >0.39 in both cases).
FIG. 6.
Effects of mefloquine on palpebral closure in the home cage during the light phase. Palpebral closure was monitored on a scale from 1 (eyes open) to 4 (eyes closed). Mefloquine induced a dose-dependent (P = 0.0023) and time-dependent (P < 0.0001) decrease in palpebral closure, although there was no significant interaction between dose and time (P = 0.11). There were no significant differences across groups for the predose palpebral closure data (P = 0.27). Significant differences were observed at 24 h for the 187- and 327-mg/kg treatment groups (P < 0.01 [indicated by an asterisk]).
TABLE 5.
Effects of mefloquine on hyperactivity/anxiousness at various time points
| Time | Incidence (%) of hyperactivity/anxiousness in treatment groupa receiving:
|
||||
|---|---|---|---|---|---|
| Vehicle | Mefloquine dose (mg/kg)
|
||||
| 45 | 187 | 327 | 574 | ||
| Predose | 12.5 | 0 | 0 | 0 | 0 |
| 24 h | 12.5 | 0 | 25 | 50 | 50 |
| 48 h | 12.5 | 0 | 0 | 27.5 | 37.5 |
Each treatment group consisted of eight rats.
DISCUSSION
Mefloquine was found to exhibit dose- and concentration-related changes in several important neurological domains, including sleep phase activity (increased photo beam breaks during the sleep phase), excitability (hyperactivity/anxiousness), and motor function (delayed beam traverse). Dose-related brain stem lesions, mainly in the n. gracilis, were also observed. The threshold dose level for these effects was 187 mg/kg. To the best of our knowledge, similar findings have not been reported before, and there has been only one other report of mefloquine-induced neurological effects in animal models in the scientific literature (38). In that study, Shepherd et al. reported clonic convulsions and aggression after sequential daily dosing of mefloquine at 300 mg/kg in mice. While these observations are also noteworthy, the relevance of the dosing regime used to clinical practice is unclear, and plasma mefloquine concentrations were not determined. In contrast, our data indicate that changes in neurological endpoints of clinical relevance occur in rats after administration of mefloquine at doses that yield plasma mefloquine concentrations of the same order of magnitude as the clinical range.
The most frequently observed neurological effects in humans after a single oral dose of mefloquine are nausea, dizziness/vertigo, and sleep disorders (33, 34). The first of these would be difficult to detect in a rat model but the observed weight loss may be a reflection of anorexia, a common effect of nausea in humans. The last two may also have a correlate in our rat model. Sleep disorders induced by mefloquine occur more frequently in humans at the treatment dose than at the prophylaxis dose and decrease in severity with time (33). With rats, we also observed a dose- and time-dependent relative increase in activity during the normal sleeping phase that peaked at 24 h and declined thereafter. The threshold dose was between 45 and 187 mg/kg, respectively. These observations are underscored by the effect of mefloquine on the degree of palpebral closure and hyperactivity observed during home cage monitoring. However, it is interesting that these changes were not reflected in other measures of general activity during the awake phase (for example, in the open field endpoints). Further studies are clearly required to investigate this phenomenon in more depth.
The effect of mefloquine on the sleep phase of rats is probably unrelated to the observed brain stem lesions and may represent a pharmacological as opposed to a permanent toxicological effect of mefloquine. In in vitro studies, mefloquine has been shown to interact with a number of potential neurological targets, including neuronal calcium homeostasis, the endoplasmic reticulum calcium pump, acetylcholinesterase, blood-brain barrier P glycoproteins, glutaminergic synaptic activity, connexins, A2a receptors, and potassium and anion channels (6, 8, 16, 17, 21, 22, 24, 30, 44). The A2a receptor is a strong candidate for potential involvement, given its suspected role in sleep modulation and its potent inhibition by mefloquine (41, 42, 44).
Mefloquine induced delays in beam traverse time that appeared to be correlated with dose and plasma concentration. Impaired motor performance of this nature can occur as a result of anatomical changes, including damage to the vestibular apparatus and/or loss of vestibular or proprioceptive function due to degeneration of the relevant brain stem nuclei (7). For example, ataxia has been associated with the pathological effect of oil-soluble artemisinin antimalarials on vestibular brain stem nuclei (14, 15). The brain stem structure that we observed to be primarily targeted by mefloquine was the n. gracilis. The n. gracilis is a component of the dorsal column system which transfers proprioceptive signals (40). Thus, it is reasonable to suspect that functional deficits in terms of beam traverse may be related to a loss of proprioceptive input. A possible test of this hypothesis would be to compare beam traverse times under normal conditions to those where compensatory visual cues were eliminated by occluding vision. Such an experiment should result in longer beam traverse times if damage to the proprioceptive systems is present. Simple clinical neurological exams of humans might also reveal whether loss of proprioceptive function underpins the vertigo/dizziness seen with some mefloquine-treated patients. It is also important to point out that the mefloquine-induced brain stem injury revealed by silver staining is permanent in nature. What is not clear, however, is whether and at what doses a permanent functional deficit is the result. We hope to address this question in future studies.
This study has demonstrated that mefloquine exhibits dose- and concentration-related endpoints that may be clinically relevant. However, the question of whether the threshold doses for these effects also have clinical relevance is more contentious. The threshold dose for many of the neurological effects in this study was 187 mg/kg. This dose may be clinically relevant since it yielded plasma mefloquine concentrations that are within the same order of magnitude as those observed in humans during treatment of malaria. This dose is 7.2-fold higher in mg/kg terms than that used for malaria treatment of humans (25 mg/kg maximum total dose). This is not surprising given that equivalent doses normally scale on the basis of mg/m2 rather than mg/kg, and the FDA recommends that an allometric scaling factor of 6.2 be applied when extrapolating doses from humans to rats. Nevertheless, it may have been preferable to select a corollary to the human treatment dose on the basis of more-comprehensive information obtained from pharmacokinetic studies with rats (area under the curve and maximum plasma concentrations). Actual mefloquine plasma concentrations prior to 72 h in our model are not known. The equivalence of the dose and the neurological findings observed should be interpreted with this context in mind.
Mefloquine remains a valuable drug for those patients who do not experience adverse effects. However, the continued use of mefloquine will remain controversial given its association with neurological effects in some individuals. Here we have demonstrated that mefloquine induces dose- and concentration-related neurological effects in rats that may have clinical relevance and could result in permanent damage to the central nervous system. Therefore, it is likely that at least some of the clinical neurological effects of mefloquine have a solid biological basis. Further characterization of our animal model and a systematic investigation of the neurological effects of mefloquine are clearly required.
Acknowledgments
The manuscript was reviewed by WRAIR and MRMC, and there is no objection to its publication or dissemination.
The opinions expressed herein are those of the authors and do not reflect the views or opinions of the Department of the Army and the Department of Defense.
This work was conducted under the auspices of the Military Infectious Diseases Research Program (proposal no. A50030_03_WR_OC).
We thank Charles White for statistical advice.
REFERENCES
- 1.Barret, P. J., P. D. Emmins, P. D. Clarke, and D. J. Bradley. 1996. Comparison of adverse events associated with use of mefloquine and combination of chloroquine and proguanil as antimalarial prophylaxis: postal and telephone survey of travelers. BMJ 313:525-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Drug Evaluation and Research. 2005. Estimating the safe starting dose in clinical trials for therapeutics in adult healthy volunteers. [Online.] http://www.fda.gov/cder/guidance/5541fnl.htm. U.S. Food and Drug Administration, Washington, D.C.
- 3.Centers for Disease Control and Prevention. 2005. Treatment of malaria. [Online.] http://www.cdc.gov/malaria/diagnosis_treatment/clinicians2.htm. Centers for Disease Control and Prevention, Atlanta, Ga.
- 4.Centers for Disease Control and Prevention. 2005. Prescription drugs for preventing malaria. [Online.] http://www.cdc.gov/travel/malariadrugs.htm. Centers for Disease Control and Prevention, Atlanta, Ga.
- 5.Croft, A. M., and P. Garner. 2000. Mefloquine for preventing malaria in non-immune adult travelers. Cochrane Database Syst. Rev. 4:CD000138. [DOI] [PubMed]
- 6.Cruikshank, S. J., M. Hopperstad, M. Younger, B. W. Connors, D. C. Spray, and M. Srinivas. 2004. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc. Natl. Acad. Sci. USA 101:12364-12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietrich, M. 2004. Dizziness. Neurologist 10:154-164. [DOI] [PubMed] [Google Scholar]
- 8.Dow, G. S., T. H. Hudson, M. Vahey, and M. L. Koenig. 2003. The acute neurotoxicity of mefloquine may be mediated through a disruption of calcium homeostasis and ER function in vitro. Malar. J. 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernaud-Espinosa, I., M. Nieto-Sampedro, and P. Bovolenta. 1993. Differential activation of microglia and astrocytes in aniso- and isomorphic gliotic tissue. Glia 8:277-291. [DOI] [PubMed] [Google Scholar]
- 10.Finney, M. 1971. Probit analysis, 3rd ed. Cambridge University Press, Cambridge, United Kingdom.
- 11.Frankenfeld, C. 2004. “Serious” and “severe” adverse drug reactions need defining. BMJ 329:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallyas, F., L. Zaborszky, and J. R. Wolff. 1980. Experimental studies of mechanisms involved in methods demonstrating axonal and terminal degeneration. Stain Technol. 55:281-290. [DOI] [PubMed] [Google Scholar]
- 13.Gallyas, F., G. Zoltay, and W. Dames. 1992. Formation of “dark” (argyrophilic) neurons of various origin proceeds with a common mechanism of biophysical nature (a novel hypothesis). Acta Neuropathol. 83:504-509. [DOI] [PubMed] [Google Scholar]
- 14.Genovese, R. F., D. B. Newman, K. A. Gordon, and T. G. Brewer. 1999. Acute high dose arteether toxicity in rats. Neurotoxicology 20:851-860. [PubMed] [Google Scholar]
- 15.Genovese, R. F., D. B. Newman, and T. G. Brewer. 2000. Behavioral and neural toxicity of the artemisinin antimalarial, arteether, but not artesunate and artelinate, in rats. Pharmacol. Biochem. Behav. 67:37-44. [DOI] [PubMed] [Google Scholar]
- 16.Go, M. L., H. S. Lee, and P. Palade. 1995. Effects of mefloquine on Ca2+ uptake by crude microsomes of rabbit skeletal muscle. Arch. Int. Pharmacodyn. Ther. 329:255-271. [PubMed] [Google Scholar]
- 17.Gribble, F. M., T. M. Davis, C. E. Higham, A. Clark, and F. M. Ashcroft. 2000. The antimalarial agent mefloquine inhibits ATP-sensitive K-channels. Br. J. Pharmacol. 131:756-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellgren, U., I. Berggren-Palme, Y. Bervist, and M. Jerling. 1997. Enantioselective pharmacokinetics of mefloquine during long-term intake of the prophylactic dose. Br. J. Clin. Pharmacol. 44:119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kollaritsch, H., J. Karbwang, G. Weiderman, A. Mikolasek, K. NaBangchang, and W. Wernsdorfer. 2000. Mefloquine concentration profiles during prophylactic drug regimens. Wien. Klin. Wochenschr. 112:441-447. [PubMed] [Google Scholar]
- 20.Lee, C., L. D. Kintner, T. R. Castles, A. M. Landes, M. C. Cronin, R. Hutchcraft, and F. Merle. 1972. Acute and subacute toxicities of α-(2piperidyl)-2,8-bis(trifluoromethyl)-4-quinolinemethanol hydrochloride, WR-142490 (AY-65742), in rodents. Interim report for USAMRDC contract no. DA-49-193-MD-2759. (Available upon request.)
- 21.Lim, L. Y., and M. L. Go. 1985. The anticholinesterase activity of mefloquine. Clin. Exp. Pharmacol. Physiol. 12:527-531. [DOI] [PubMed] [Google Scholar]
- 22.Lu, L., F. Leonessa, M. T. Baynham, R. Clarke, F. Gimenez, Y. T. Pham, F. Roux, and I. W. Wainer. 2001. The enantioselective binding of mefloquine enantiomers to P-glycoprotein determined using an immobilized P-glycoprotein liquid chromatographic stationary phase. Pharm. Res. 18:1327-1330. [DOI] [PubMed] [Google Scholar]
- 23.Lyeth, B. G., J. Y. Jiang, Q. X. Gong, R. J. Hamm, and H. F. Young. 1995. Effects of mu opioid agonist and antagonist on neurological outcome following traumatic brain injury in the rat. Neuropeptides 29:11-19. [DOI] [PubMed] [Google Scholar]
- 24.Maertens, C., L. Wei, G. Droogmans, and B. Nilius. 2000. Inhibition of volume-regulated and calcium-activated chloride channels by the antimalarial mefloquine. J. Pharmacol. Exp. Ther. 295:29-36. [PubMed] [Google Scholar]
- 25.McDaniel, K. L., and V. C. Moser. 1993. Utility of a neurobehavioral screening battery for differentiating the effects of two pyrethroids, permethrin and cypermethrin. Neurotoxicol. Teratol. 15:71-83. [DOI] [PubMed] [Google Scholar]
- 26.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 27.Nauta, W. J. H., and P. A. Gygax. 1954. Silver impregnation of degenerating axons in the central nervous system: a modified technique. Stain Technol. 29:91-93. [DOI] [PubMed] [Google Scholar]
- 28.Ogeng'o, J. A., D. L. Cohen, J. G. Say, W. B. Matuja, H. M. Chande, J. N. Kitinya, J. K. Kimani, R. P. Friedland, H. Mori, and R. N. Kalaria. 1996. Cerebral amyloid beta protein deposits and other Alzheimer lesions in non-demented elderly East Africans. Brain Pathol. 6:101-108. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos, G., and C. Watson. 1986. The rat brain in stereotaxic coordinates. Academic Press, New York, N.Y.
- 30.Pham, Y.-T., A. Régina, R. Farinotti, P.-O. Couraud, I. W. Wainer, F. Roux, and F. Gimenez. 2000. Interactions of racemic mefloquine and its enantiomers with P-glycoprotein in an immortalized rat brain capillary endothelial cell line, GPNT. Biochim. Biophys. Acta 1524:212-219. [DOI] [PubMed] [Google Scholar]
- 31.Phillips-Howard, P. A., and F. O. ter Kuile. 1995. CNS adverse events associated with antimalarial agents: fact or fiction? Drug Saf. 12:370-383. [DOI] [PubMed] [Google Scholar]
- 32.Price, R., J. A. Simpson, P. Teja-Isavatharm, M. M. Than, C. Luxemburger, D. G. Heppner, T. Chongsuphajaisiddhi, F. Nosten, and N. J. White. 1999. Pharmacokinetics of mefloquine combined with artesunate in children with acute falciparum malaria. Antimicrob. Agents Chemother. 43:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rendi-Wagner, P., H. Noedl, W. H. Wernsdorfer, G. Weidermann, A. Mikolasek, and H. Kollaritsch. 2002. Unexpected frequency, duration and spectrum of adverse events after therapeutic dose of mefloquine in healthy adults. Acta Trop. 81:167-173. [DOI] [PubMed] [Google Scholar]
- 34.Roche Pharmaceuticals. 2005. Lariam product information. [Online.] http://www.rocheusa.com/products/lariam/pi.pdf. Roche Pharmaceuticals, Nutley, N.J.
- 35.Ronn, A. M., J. Ronne-Rasmussen, P. C. Gotzsche, and I. C. Bygbjerg. 1998. Neuropsychiatric manifestations after mefloquine therapy for Plasmodium falciparum malaria: comparing a retrospective and prospective study. Trop. Med. Int. Health 3:83-88. [DOI] [PubMed] [Google Scholar]
- 36.Schlagenhauf, P., R. Steffen, H. Lobel, R. Johnson, R. Letz, A. Tschopp, Y. Bergqvist, O. Ericsson, U. Hellgren, L. Rombo, S. Mannino, J. Handschin, and D. Sturchler. 1996. Mefloquine tolerability during chemoprophylaxis: focus on adverse assessments, stereochemistry and compliance. Trop. Med. Int. Health 4:485-494. [DOI] [PubMed] [Google Scholar]
- 37.Shah, S., S. Filler, L. M. Causer, A. K. Rowe, P. B. Bloland, A. M. Barber, J. M. Roberts, M. R. Desai, M. E. Parise, and R. W. Steketee. 2004. Malaria surveillance—United States, 2002. Morb. Mortal. Wkly. Rep. 53:21-36. [PubMed] [Google Scholar]
- 38.Shepherd, J., et al. September 1988. Use of (+) mefloquine for the treatment of malaria. International patent number WO 98/39003.
- 39.Simpson, J. A., R. Porce, F. ter Kuile, P. Teja-Isavatharm, F. Nosten, T. Chongsuphajaisiddhi, S. Looareesuwan, L. Aarons, and N. J. White. 1999. Population pharmacokinetics of mefloquine in patients with acute falciparum malaria. Clin. Pharmacol. Ther. 66:472-484. [DOI] [PubMed] [Google Scholar]
- 40.Tracy, D. J., and P. M. E. White. 1995. The sensosomatory system, p. 689-704. In G. Paxinos (ed.), The rat nervous system. Academic Press, San Diego, Calif.
- 41.Tung, A., S. Herrera, M. J. Szafran, K. Kasza, and W. B. Mendelson. 2005. Effect of sleep deprivation on righting reflex in the rat is partially reversed by administration of adenosine A1 and A2 receptor antagonists. Anesthesiology 102:1158-1164. [DOI] [PubMed] [Google Scholar]
- 42.Urade, Y., N. Eguchi, W. M. Qu, M. Sakata, Z. L. Huang, J. F. Chen, M. A. Schwarzschild, J. S. Fink, and O. Hayaishi. 2003. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology 61:S94-S96. [DOI] [PubMed] [Google Scholar]
- 43.U.S. Environmental Protection Agency. 1998. Health effects test guidelines: OPPTS 870.6200 neurological screening battery. U.S. Environmental Protection Agency, Washington, D.C.
- 44.Weiss, S. M., K. Benwell, I. A. Cliffe, R. J. Gillespie, A. R. Knight, J. Lerpiniere, A. Misra, R. M. Pratt, D. Revell, R. Upton, and C. T. Dourish. 2003. Discovery of nonxanthine A2a receptor antagonists for the treatment of Parkinson's disease. Neurology 61:S101-S106. [DOI] [PubMed] [Google Scholar]
- 45.Wongsrichanalai, C., S. Prajakwong, S. R. Meshnick, G. D. Shanks, and K. Thimasarn. 2004. Mefloquine—its 20 years in the Thai Malaria Control Program. Southeast Asian J. Trop. Med. Public Health 35:300-308. [PubMed] [Google Scholar]
- 46.World Health Organization. 2005. World malaria report 2005. [Online.] http://www.rbm.who.int/wmr2005. World Health Organization, Geneva, Switzerland.