Abstract
Drug treatment of severe malaria must be rapidly effective. Suppositories may be valuable for childhood malaria when circumstances prevent oral or parenteral therapy. We compared artesunate suppositories (n = 41; 8 to 16 mg/kg of body weight at 0 and 12 h and then daily) with intramuscular (i.m.) artemether (n = 38; 3.2 mg/kg at 0 h and then 1.6 mg/kg daily) in an open-label, randomized trial with children with severe Plasmodium falciparum malaria in Papua New Guinea (PNG). Parasite density and temperature were measured every 6 h for ≥72 h. Primary endpoints included times to 50% and 90% parasite clearance (PCT50 and PCT90) and the time to per os status. In a subset of 29 patients, plasma levels of artemether, artesunate, and their common active metabolite dihydroartemisinin were measured during the first 12 h. One suppository-treated patient with multiple complications died within 2 h of admission, but the remaining 78 recovered uneventfully. Compared to the artemether-treated children, those receiving artesunate suppositories had a significantly earlier mean PCT50 (9.1 versus 13.8 h; P = 0.008) and PCT90 (15.6 versus 20.4 h; P = 0.011). Mean time to per os status was similar for each group. Plasma concentrations of primary drug plus active metabolite were significantly higher in the artesunate suppository group at 2 h postdose. The earlier initial fall in parasitemia with artesunate is clinically advantageous and mirrors higher initial plasma concentrations of active drug/metabolite. In severely ill children with malaria in PNG, artesunate suppositories were at least as effective as i.m. artemether and may, therefore, be useful in settings where parenteral therapy cannot be given.
Most deaths from falciparum malaria occur in children living in remote tropical areas that have limited health care facilities (7). A rapidly acting antimalarial suppository would be valuable in this setting. In contrast to oral therapy, it could be given to severely ill children with vomiting, prostration, and/or impaired consciousness, features associated with a poor prognosis (33). The alternative to rectal drug administration in this situation is intravenous (i.v.) or intramuscular (i.m.) injection, but this requires equipment and trained personnel that are often unavailable in the rural tropics.
The artemisinin derivatives are potent, safe, and well-tolerated antimalarial drugs (13). Artesunate is the most versatile, with its high water solubility facilitating the development of oral, i.v./i.m., and rectal formulations. Artesunate suppositories have been identified by the World Health Organization (WHO) as having great promise, especially for treatment of childhood malaria (34). They have proved safe and efficacious for adults (3, 20, 22) and in small-scale studies of children with nonsevere infections from Africa (11, 19), Southeast Asia (31), South America (10), and Papua New Guinea (PNG) (17). Their pharmacokinetic properties have been characterized previously (5, 11, 16, 19), and maximal plasma drug concentrations, achieved within 1 to 2 h of administration of 10 to 20 mg/kg of body weight, are comparable to those when artesunate at 2 to 4 mg/kg is given by the oral, i.m., or i.v route (16, 19). Although interindividual variability in rectal artesunate bioavailability is high, clinical response and tolerability are maintained over a wide plasma concentration range, consistent with the high therapeutic index of artemisinin drugs (16, 19).
There are few data on the disposition and effectiveness of rectal artesunate in children with severe malaria. In addition, comparative studies are needed to document therapeutic equivalence with established treatments. In one study comparing artesunate suppositories with parenteral quinine in African children and adults with severe malaria (4), there was a significantly greater reduction in parasitemia at 12 and 24 h with the suppository-treated patients. Quinine has, however, been superseded by injectable artemisinin derivatives in many parts of the tropics. Intramuscular artemether clears fever and parasitemia more rapidly than i.m. quinine, with fewer deaths and permanent neurological sequelae (2). Artemether is also less toxic than quinine and can be given once rather than twice daily. Nonetheless, concerns regarding its unpredictable absorption after i.m. injection, particularly with the most severely ill children, exist (12, 25, 27, 32).
In PNG, i.m. artemether has replaced quinine in national treatment guidelines as the first-line treatment for severe malaria. Plasmodium falciparum is highly endemic in coastal and island regions of PNG where most of the population lives, but access to medical care is limited by poor health care infrastructure and rugged terrain. Artesunate suppositories could have advantages over i.m. artemether for severe malaria in this setting. As an extension to a preliminary safety, efficacy, and pharmacokinetic evaluation (16, 17), we have compared the efficacy of artesunate suppositories with that of i.m. artemether for treatment of severe pediatric malaria in PNG.
MATERIALS AND METHODS
Study timelines, site, and approvals.
The present study was conducted over a 9-month period between August 2003 and March 2004 at the provincial hospital in Madang Province on the north coast of PNG. This area has hyperendemic, year-round transmission of P. falciparum (26). P. vivax is also endemic, although most clinical malaria is due to P. falciparum. Ethical approval for the study was obtained from the Medical Research Advisory Committee of PNG. Written informed consent was obtained from parents/guardians of all children before recruitment.
Patients.
Children aged 1 to 10 years with clinical features of severe malaria (33) were eligible for recruitment. Screening was based on the WHO Integrated Management of Childhood Illness (IMCI) procedures, and inclusion criteria included drowsiness or unconsciousness (Blantyre coma score of <5, assessed >1 h after a convulsion), respiratory distress (respiratory rate of >40/min and/or chest indrawing), severe anemia (hemoglobin of <5.0 g/dl), prostration (inability to sit unaided in a child usually able to do so), convulsions within the previous 24 h, and nausea and vomiting precluding oral therapy. Children with at least one of these features and a blood slide with P. falciparum parasite density of >500/μl at entry were eligible. Mixed infections with P. vivax, P. ovale, or P. malariae were allowed, provided that the P. falciparum parasitemia was >500/μl. Patients with features of another infection, malnutrition, diarrhea, or other significant comorbidity, those who had treatment with an artemisinin derivative within the previous 24 h, or those whose parent/guardian declined to give consent were excluded and treated according to IMCI-based PNG standard treatment guidelines.
Clinical procedures.
At recruitment, an initial clinical assessment was performed, and blood was taken for full blood count and plasma lactate, bicarbonate, urea, bilirubin, and sodium concentrations. A capillary blood glucose concentration was also measured. A computer-generated randomization schedule was used to assign children to artesunate suppositories or i.m. artemether. The subjects in the artesunate suppository group received a combination of one or two 50 and/or 200 mg thermostable suppositories (Rectocaps; Mepha Pharmaceuticals, Aesch-Basel, Switzerland) to a total dose of 8 to 17 mg/kg. The suppositories were kept at room temperature, removed from their airtight foil packaging immediately before use, and administered base first without lubricant. Children were observed for 1 h after dosing, and if the suppositories were expelled, the same dose was readministered and the observation continued. A second dose was given 12 h later and then daily until the child was able to tolerate oral medication. Children in the i.m. artemether group were administered artemether (Kunming Pharmaceuticals, Kunming, China) at a dose of 3.2 mg/kg as an i.m. injection into the anterior thigh or buttock. A daily i.m. dose of 1.6 mg/kg was given subsequently until the child was able to tolerate oral medication. In both treatment groups, continuation of oral therapy comprised artesunate 2 mg/kg daily to complete 7 days of treatment, and a single dose of sulfadoxine 25 mg/kg plus pyrimethamine 1.0 mg/kg was given by mouth at 72 h.
All children were assessed comprehensively every 6 h for ability to tolerate oral medication; ability to eat, drink, and mobilize normally for age; axillary temperature; pulse and respiratory rate; capillary blood glucose; Blantyre coma score; and parasite density. Those with an axillary temperature of >38.0°C were given paracetamol, and tepid sponging and fanning were started. Due to the possibility of serious bacterial infections in children with severe malaria (6), parenteral antibiotics (i.m. chloramphenicol under national treatment guidelines) were also administered if considered appropriate by the treating physician. Other concomitant treatment, including anticonvulsants, iron, and folate, were recorded, as were blood transfusions. All children were kept in hospital for at least 72 h and were discharged when they had been afebrile for 24 h, they were eating, drinking, and mobilizing normally, and serial blood slides had been negative for >24 h.
Microscopy and other field laboratory tests.
Giemsa-stained, thick blood smears were examined by a skilled microscopist (K.L.) who was blinded to treatment allocation. The microscopist viewed >100 fields at a ×1,000 magnification before a slide was considered negative. For positive slides, parasite density was calculated from the number of asexual forms/1,000 leukocytes and the whole blood leukocyte count. Biochemical measurements were performed using a Kodak Ektachem DT60 autoanalyzer (Eastman Kodak, Rochester, NY). Hemoglobin was estimated using a portable spectrophotometer (HemoCue, Angelholm, Sweden) and bedside blood glucose using a Medisense Optium meter (Abbott Diagnostics Division, Doncaster, Australia).
Drug assays.
A subset of the children aged >2 years who were not anemic and whose parent/guardian provided informed consent had four or five additional blood samples taken during the first 12 h. Blood was taken at predetermined intervals of 1, 2, 4, 6, and 12 h after the first dose of study drug and centrifuged within 30 min, and separated plasma was stored at −20°C prior to transport to Australia on dry ice for drug assay. Artesunate and dihydroartemisinin in suppository-treated patients were measured in plasma by high-performance liquid chromatography-mass spectrometry (16), while artemether and dihydroartemisinin after i.m. artemether were quantified by gas chromatography-mass spectrometry (12). Method performance for both assays, assessed as intra- and interday relative standard deviations over the relevant concentration ranges, was similar to that published previously (12, 16).
Efficacy assessment.
The primary efficacy endpoints were the time for parasite density to fall, as assessed by linear interpolation from the parasitemia-time curve, by 50% and 90% of the baseline value (PCT50 and PCT90, respectively) and the equivalent percentage reduction (relative to baseline) in parasite density at 12 and 24 h (PC%12 and PC%24, respectively). An additional primary efficacy endpoint was the time to return to per os status (from admission to first dose of oral medication). Secondary endpoints included the parasite clearance time (from admission to the first of two consecutive negative thick blood films) (PCT), fever clearance time (time from first dose to the first axillary temperature of a 24-h period of <37.5°C), death, neurological sequelae, suspected adverse drug reactions, time to full consciousness in children admitted with impaired conscious state, and time to normal mobility.
Statistical analysis.
Based on parasite clearance data from our previous study (17), a sample size of 35 patients per group was required to detect a 30% difference in PCT50 or PCT90, with a power of ≥80% and level of significance of 5%. Statistical analysis was performed using SPSS version 10 (SPSS Inc., Chicago, IL). Data are presented as means ± standard deviations, mean and 95% confidence intervals, or median and either range or interquartile range. Normally distributed continuous variables were compared by Student's t test and non-normally distributed data by the Mann-Whitney U test. Categorical data were compared using Fisher's exact and chi-square tests.
RESULTS
Patient characteristics.
We identified 124 children with features of severe malaria and an initial blood slide positive for P. falciparum. After excluding ineligible subjects and those found to have a parasite density of <500/μl by microscopic quantification subsequent to randomization, 79 children (41 in the artesunate suppository group and 38 in the i.m. artemether group) were included in the final analysis (Table 1). Features of severity included multiple convulsions (41%), impaired conscious state (28%), respiratory distress (76%), anemia (23%), prostration (34%), frequent vomiting (32%), and inability to tolerate oral medication (35%). There were no significant differences in the prevalences of these features or in the results of baseline laboratory tests by treatment allocation except that the admission parasitemia was lower in the artesunate suppository group than in the i.m. artemether group (Table 1).
TABLE 1.
Characteristics of patients in the artesunate suppository and i.m. artemether groups at the time of admission to the studya
| Measure | Value for group
|
|
|---|---|---|
| Artesunate suppositories | i.m. artemether | |
| No. of patients | 41 | 38 |
| Age (yr) | 3.6 ± 1.6 | 3.8 ± 2.2 |
| Sex (% males) | 20 (50) | 21 (55) |
| Initial drug dose (mg/kg) | 11.2 ± 2.2 | 3.6 ± 0.9 |
| Body wt (kg) | 12.6 ± 3.4 | 13.4 ± 4.8 |
| Axillary temp (°C) | 38.7 ± 1.1 | 38.7 ± 1.5 |
| Respiratory rate (/min) | 43 | 43 |
| Pulse rate (/min) | 122 ± 18 | 122 ± 16 |
| Blantyre coma score | 4.5 ± 1.0 | 4.7 ± 0.7 |
| Parasite density (/μl) | 69,000 (770-458,000)b | 40,100 (775-473,000) |
| Mixed infection with P. vivax (%) | 1 (2) | 3 (8) |
| Hemoglobin (g/dl) | 9.1 ± 2.2 | 8.4 ± 3.0 |
| Blood glucose (mmol/liter) | 7.1 ± 2.7 | 6.6 ± 2.4 |
| Plasma bicarbonate (mmol/liter) | 18.5 ± 5.3 | 17.1 ± 4.8 |
| Plasma lactate (mmol/liter) | 4.4 ± 2.7 | 5.5 ± 4.3 |
| Plasma urea (mmol/liter) | 4.1 ± 1.7 | 3.4 ± 1.2 |
| Plasma bilirubin (μmol/liter) | 28 ± 22 | 28 ± 25 |
Data are expressed as means ± standard deviations or medians (ranges) unless otherwise specified.
Mann-Whitney U test, P < 0.05.
Clinical course.
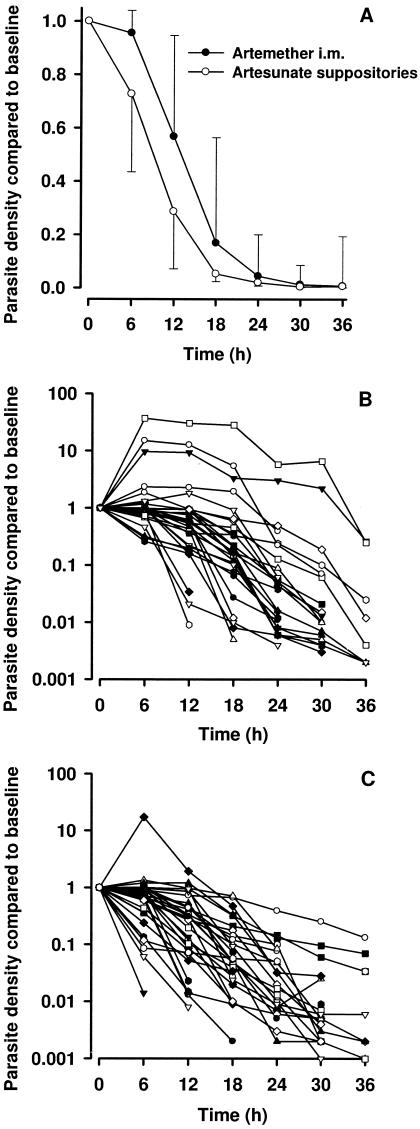
After excluding subjects with missing or unreadable slides, primary parasitological endpoints were evaluable for 37 artesunate suppository and 35 i.m. artemether patients (Table 2). Median parasitemia-time curves for the two treatment groups are shown in Fig. 1A and individual patient plots in Fig. 1B and C. There was a more rapid initial parasite clearance with rectal artesunate. Eight children in the i.m. artemether group and three children in the artesunate suppository group had an increase in parasite density during the first 6 h (odds ratio, 3.8; 95% confidence interval, 0.7 to 17.8; P = 0.08).
TABLE 2.
Clinical efficacy endpoints by treatment group
| Measure | Value for groupb
|
Δ (95% CI)c | |
|---|---|---|---|
| Artesunate suppositories | i.m. artemether | ||
| PCT50 (h) | 9.1 ± 4.9 | 13.5 ± 7.9 | −4.3 (−7.5 to −1.2)* |
| PCT90 (h) | 15.6 ± 7.4 | 20.4 ± 8.0 | −4.8 (−8.5 to −1.1)** |
| PC%12 | 17 (0-190)* | 56 (0-3,020) | |
| PC%24 | 0.5 (0-40) | 1.2 (0-580) | |
| Time to per os status (days) | 1.35 ± 0.76 | 1.69 ± 0.82 | −0.25 (−0.62 to +0.11) |
| PCT (h) | 30.3 ± 14.2 | 32.8 ± 12.9 | −2.5 (−8.8 to +3.8) |
| FCT (h)a | 18.3 ± 10.7 | 15.6 ± 9.2 | +2.8 (−1.9 to +7.5) |
FCT, fever clearance time.
Data are expressed as means ± standard deviations or medians (ranges).
Differences between groups (Δ) and their 95% confidence intervals (CI) are shown. *, P < 0.01; **, P < 0.02.
FIG. 1.
(A) Median (interquartile range) proportional reduction in parasite density following treatment with rectal artesunate or i.m. artemether. (B) Individual patient parasite density-time data (various symbols) from 41 children treated with rectal artesunate and (C) individual patient parasite density-time data (various symbols) from 38 children treated with i.m. artemether.
One child in the artesunate suppository group, a 5-year-old girl, died during the study. She presented with impaired conscious state (Blantyre score, 3), multiple convulsions, frequent vomiting, hyperpyrexia (axillary temperature, 39.6°C), and borderline hypoglycemia (2.3 mmol/liter). In addition to rectal artesunate (13 mg/kg) at study entry, she received an i.v. bolus of 50% glucose followed by a 5% dextrose infusion and an i.m. dose of chloramphenicol. Shortly after this, the child vomited and aspirated before progressing to respiratory arrest. Resuscitation, including intubation and ventilation, was unsuccessful, and the child died 2 h after the first rectal artesunate dose, which, on postmortem examination, had been retained.
Twenty-two children had impaired consciousness (Blantyre score, <5) at enrollment. The mean time to recovery of normal conscious state (Blantyre score, 5) for the artesunate suppository group (15 ± 13 h; n = 13) was similar to that for the i.m. artemether group (20 ± 12 h; n = 9; P > 0.2). No child had a detectable residual neurological deficit upon clinical examination at discharge.
Side effects and complications.
Five children passed suppositories within 1 h of the first dose. The suppositories were retained following repeat dosing in all of these cases. Five children (two in the artesunate suppository group and three in the i.m. artemether group) developed constipation and/or abdominal distension during early convalescence. The distension could be marked and usually occurred around days 2 to 4. One child in the i.m. artemether group developed abdominal pain, diarrhea, nausea, and anorexia on days 2 to 4, and another artemether-treated child developed late fever on day 3. In all of these cases, symptoms were mild, their onset was after parasite and fever clearance, resolution occurred spontaneously within 1 to 3 days, and oral artesunate therapy did not have to be discontinued.
Plasma drug concentrations.
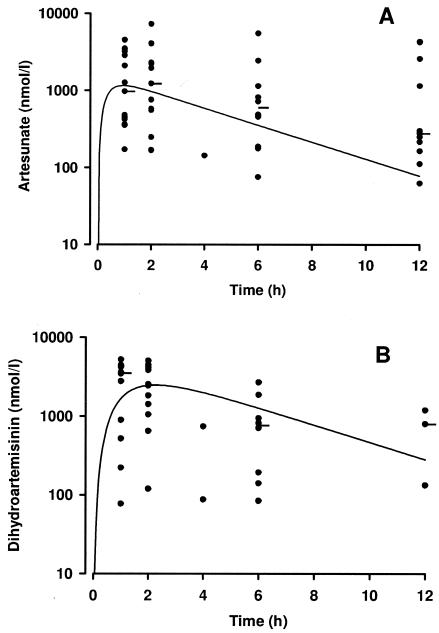
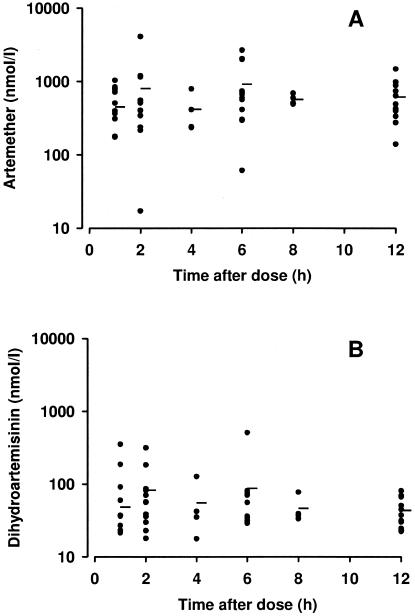
A total of 29 children (15 in the artesunate suppository group and 14 in the i.m. artemether group) had plasma samples taken for drug assay. Data from the artesunate suppository group are shown in Fig. 2A and B, and data for the i.m. artemether group are shown in Fig. 3A and B. Median plasma levels of both artesunate and dihydroartemisinin for this small group of severely ill children were similar to those seen in a larger pediatric sample with uncomplicated malaria from the same geographical area (16). Simulated concentration-time curves based on mean pharmacokinetic parameters from the latter study (16) are shown in Fig. 2A and B for comparison purposes. Because of the small patient numbers, the sparse sampling protocol employed, and the difficulty in generating valid pharmacokinetic data with the artemether-treated patients (12), we did not perform formal pharmacokinetic modeling with either treatment group.
FIG. 2.
(A) Plasma artesunate and (B) dihydroartemisinin concentrations from 15 children following an 11-mg/kg dose of rectal artesunate. The closed circles are individual datum points, while the horizontal bars show median concentrations at the different times. The solid lines show simulated (using mean pharmacokinetic parameters) concentration-time profiles from our previous study of children with uncomplicated malaria (16).
FIG. 3.
(A) Plasma artemether and (B) dihydroartemisinin concentrations from 14 children following a 3.2-mg/kg dose of i.m. artemether. The closed circles are individual datum points, while the horizontal bars show median concentrations at the different times.
The total artemisinin drug exposure in nmol/liter for the two treatments was calculated by adding the concentrations of both artemether and its metabolite dihydroartemisinin for subjects who received artesunate suppositories and both artemether and its metabolite dihydroartemisinin for subjects who received i.m. artemether (Table 3). There was a significantly greater total artemisinin exposure at 1 h and 2 h postdose with the artesunate suppository group (P < 0.02), but exposure levels were similar at 6 and 12 h. The difference in total artemisinin exposure was predominantly due to dihydroartemisinin, concentrations of which were significantly greater for the rectal artesunate group at all time points up to 12 h. Six patients in the artemether group and two patients in the artesunate group had very low total artemisinin exposure (<500 nmol/liter) at 1 and 2 h postdose. Clinical recovery and parasite and fever clearance for these eight patients were similar to those for other patients except for one child from the i.m. artemether group. This child's parasite density rose from 3,655/μl to 46,225/μl during the 12 h after the first injection, fell to below the baseline level by 24 h, and was negative at 36 h.
TABLE 3.
Artemisinin exposure by treatment group at selected times after drug administrationa
| Time after dose (h) | Artesunate suppositoriesb
|
i.m. artemether
|
||
|---|---|---|---|---|
| Dihydroartemisinin (nmol/liter) | Total artemisinin (nmol/liter) | Dihydroartemisinin (nmol/liter) | Total artemisinin (nmol/liter) | |
| 1 | 3,509 (222-5,218)* | 2,690 (172-9,729)* | 69 (21-4,314) | 441 (91-1,192) |
| 2 | 2,437 (120-14,613)* | 3,660 (169-21,871)* | 57 (18-4,186) | 438 (56-1,251) |
| 6 | 819 (84-2,690)* | 661 (75-6,318) | 53 (29-509) | 661 (70-2,307) |
| 12 | 798 (134-1,192)* | 278 (62-5,205) | 31 (22-81) | 514 (67-1,546) |
Total artemisinin concentrations indicate sums of concentrations of plasma artesunate and dihydroartemisinin (for artesunate suppository group) or artemether and dihydroartemisinin (for i.m. artemether group). Data are expressed as medians (ranges).
*, P < 0.02.
DISCUSSION
Our preliminary data (16, 17) suggested that rectal artesunate at 10 to 20 mg/kg was rapidly absorbed in children with uncomplicated malaria in PNG, with plasma concentrations of drug and the active metabolite dihydroartemisinin reaching those achieved after parenteral administration of conventional 2- to 4-mg/kg artesunate doses. At the same time, we were concerned that the current standard therapy for severe malaria in PNG, i.m. artemether, could be suboptimal because of poor absorption (12, 25, 27, 32). Evidence that artesunate suppositories are effective for severe malaria in adults (3, 21, 22) provided further justification for a trial of rectal artesunate with PNG children with complicated P. falciparum infections. The present study demonstrates the benefits of rectally administered artesunate for the initial treatment of severe malaria in children, with a more rapid fall in parasite density than that observed after i.m. artemether. The lower rates of initial parasite clearance in our artemether-treated patients probably reflect the poor absorption of this drug from its i.m. depot, leading to concentrations of artemether plus dihydroartemisinin in a subset of our patients that were six- to eightfold lower 1 to 2 h postdose than the equivalent drug/metabolite levels after rectal artesunate.
The median concentrations of artesunate, artemether, and dihydroartemisinin in the present subjects were in accord with those of previous studies of rectal artesunate (16, 19) or i.m. artemether (12, 25, 27, 32) when administered in equivalent mg/kg doses. The median maximum concentration of drug in serum (Cmax) for 10 Vietnamese adults with severe malaria treated with an initial i.m. dose of 3.2 mg/kg artemether was 574 (range, 67 to 1,641) nmol/liter, and most had peak plasma dihydroartemisinin concentrations of <25 nmol/liter (12). A study of Thai adults with uncomplicated malaria treated with 2 mg/kg i.m. artemether demonstrated a mean Cmax of 121 nmol/liter dihydroartemisinin equivalents by bioassay (32). This was some 16 times less than that seen with equivalent oral dosing, and peak levels occurred at a median of 8 (range, 4 to 24) h after administration (32). Studies of African children with severe malaria have shown a mean Cmax of between 116 to 257 nmol/liter as dihydroartemisinin equivalents (25, 27).
Two studies with Thai adults demonstrated higher plasma levels of artemether and a much greater rate of conversion of artemether to dihydroartemisinin (14, 15). The reasons for these apparently discrepant results are unclear but may relate to infection severity (one Thai study was performed with healthy volunteers), ethnic factors, and/or the different age ranges of the subjects. These factors may have impacted hepatic perfusion or hepatic and/or extrahepatic enzymatic metabolism of the parent drug. Alternatively, the dihydroartemisinin assay used for the Thai studies, one of the first such assays developed, may have had a different specificity to other methods.
Based on in vitro parasite culture data, artemether may be intrinsically less potent than either artesunate or dihydroartemisinin (29, 30). This suggests that the total artemisinin concentration derived by adding plasma artemether and dihydroartemisinin concentrations overestimates bioactivity compared to artesunate plus dihydroartemisinin. Dose-response relationships for the artemisinin derivatives remain relatively poorly understood. However, when artesunate is given orally, a positive in vivo relationship between mg/kg dosage and parasite clearance rate appears to exist up to doses of 2 mg/kg, above which a maximal effect is achieved (1). This threshold dose equates with peak plasma concentrations of 1,400 to 2,800 nmol/liter dihydroartemisinin equivalents at 1 to 2 h postdose (28). As an extension of this finding, the total plasma artemisinin concentrations achieved with our artemether-treated patients at 1 and 2 h (medians of 438 and 441 nmol/liter, respectively), while exceeding published 50% inhibitory concentrations for artemether and dihydroartemisinin (8), explain the inferior pharmacodynamic response of this group, particularly with individuals at the lower end of the range of absorption. Indeed, another study has identified a subset of patients with relatively poor outcomes in whom plasma artemether concentrations are undetectable by either bioassay or high-performance liquid chromatography (limits of detection, approximately 35 nmol/liter) (27).
It could be argued that the different pharmacokinetic and pharmacodynamic outcomes in the present study were a function of the total doses administered. The mg/kg dose of artesunate was approximately three times that of artemether. However, it seems unlikely that increasing the dose of artemether would be either practical or effective. An increased dose means an increased injection volume that would be painful and difficult to administer to small children. Furthermore, larger injection volumes may lead to compression of capillary beds surrounding the injection site that could further limit drug absorption (18).
Our study was inadequately powered to detect between-treatment differences in either death or permanent neurological sequelae. However, the mortality and rate of neurological complications in our patients were reassuringly low, with only one such event, a death 2 h after study entry, among 79 severely ill children. Indeed, the range of complications present at study entry for this child and the rapid clinical deterioration suggest strongly that any form of intervention would have had limited benefit. One of our surrogate endpoints (PC%12) was also used in the only other study to evaluate the efficacy of artesunate suppositories for treatment of severe malaria (4). In the latter study, initial parasite clearance was more rapid with rectal artesunate than with i.m. quinine (4). Because of differences in pharmacokinetics and parasite stage specificity, artemisinin drugs clear parasites more rapidly than quinine, regardless of route of administration (23, 24). Our study was concerned with efficacy comparison within the artemisinin group rather than between classes of antimalarial agents.
Since the present study was performed, a large, multicenter, Southeast Asian study has demonstrated that i.v. artesunate is associated with a lower mortality (15%) than i.v. quinine (22%) for severe malaria (9). The benefit of i.v. artesunate was not significant in subjects <15 years of age (9), but this may have been a function of low numbers of patients and a lack of statistical power. These data and the fact that i.v. or i.m. injection of artesunate provides early and reliable therapeutic levels of drug and metabolite (12) without the close observation needed after rectal administration (16) confirm parenteral artesunate as first-line hospital-based treatment for severe malaria, including with children. Where facilities do not exist to safely administer antimalarial drugs by injection, the present and other data (4) indicate that artesunate suppositories are an appropriate alternative.
Both of the drugs used in the present study were well tolerated, although a small number of children in each group (1 in 16 overall) developed generally mild gastrointestinal symptoms after 2 to 4 days of treatment. The time course of these symptoms relative to parasite and fever clearance, their occurrence in both groups of patients, and the fact that they have not been reported in previously published studies could imply that they arose because of a reaction to artemisinin drugs in susceptible Melanesian subjects. However, their spontaneous resolution during oral artesunate therapy suggests that they were a delayed effect of severe malaria on intestinal function.
Rectocaps artesunate suppositories are manufactured to international good manufacturing practice standards and cost approximately $0.38 and $0.68 for 50-mg and 200-mg suppositories, respectively. Based on the number of days of treatment required prior to oral therapy and the dosages administered to the children in the present study, the average cost of rectal treatment was $2.44. The equivalent average cost of i.m. artemether at $1.50/ampoule was $2.49 per child. Because this comparison does not take into account consumables needed to administer injections, rectal artesunate is at least as cost-effective as i.m. artemether for the initial treatment of severe pediatric malaria in PNG. Nevertheless, the close observation necessary to ensure that suppositories are retained may be problematic, even in a hospital setting. Thus, we recommend parenteral artemisinin therapy where drug availability and facilities allow, with suppositories as an excellent alternative where injections cannot be given.
Acknowledgments
This study was supported by a grant from WHO Roll Back Malaria, the Royal Australasian College of Physicians and National Health, and Medical Research Council grant 353663.
We thank Sister Demok and the nursing staff of the children's outpatient clinic of Modilon Base Hospital in Madang. Particular thanks go to Judy Longo, Barry Kalissa, Cecilia Pakule, and the participating children and their parents. Manasseh Baea, Will Kastens, Ged Casey, and Ivo Mueller of the PNG IMR all provided invaluable assistance. We thank Melba Gomes and Steve Bjorge of WHO/TDR and John Vince of the University of PNG for advice on study design. Mepha Pharmaceuticals provided the artesunate suppositories.
REFERENCES
- 1.Angus, B. J., I. Thaiaporn, K. Chanthapadith, Y. Suputtamongkol, and N. J. White. 2002. Oral artesunate dose-response relationship in acute falciparum malaria. Antimicrob. Agents Chemother. 46:778-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artemether-Quinine Meta-Analysis Study Group. 2001. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 95:637-650. [DOI] [PubMed] [Google Scholar]
- 3.Awad, M. I., A. M. Y. Alkadru, R. H. Behrens, O. Z. Baraka, and I. B. Eltayeb. 2003. Descriptive study on the efficacy and safety of artesunate suppository in combination with other antimalarials in the treatment of severe malaria in Sudan. Am. J. Trop. Med. Hyg. 63:153-158. [PubMed] [Google Scholar]
- 4.Barnes, K. I., J. Mwenechanya, M. Tembo, H. McIlleron, P. I. Folb, I. Ribeiro, F. Little, M. Gomes, and M. E. Molyneux. 2004. Efficacy of rectal artesunate compared with parenteral quinine in initial treatment of moderately severe malaria in African children and adults: a randomised study. Lancet 363:1598-1605. [DOI] [PubMed] [Google Scholar]
- 5.Benakis, A., M. Paris, L. Loutan, C. T. Plessas, and S. T. Plessas. 1996. Pharmacokinetic study of a new pharmaceutical form of artesunate (Plasmotrim-200 Rectocaps) administered in healthy volunteers by the rectal route. Jap. J. Trop. Med. Hyg. 24(Suppl. 1):39-45. [Google Scholar]
- 6.Berkley, J., S. Mwarumba, K. Bramham, B. Lowe, and K. Marsh. 1999. Bacteraemia complicating severe malaria in children. Trans. R. Soc. Trop. Med. Hyg. 93:283-286. [DOI] [PubMed] [Google Scholar]
- 7.Breman, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 64(Suppl. 1-2):1-11. [DOI] [PubMed] [Google Scholar]
- 8.Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the northwestern border of Thailand during five years of extensive ARTS-mefloquine use. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dondorp, A., F. Nosten, K. Stepniewska, N. Day, N. White, et al. 2005. Artesunate versus quinine for treatment of severe malaria: a randomised trial. Lancet 366:717-725. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Landires, E. A. 1996. Efficacy of artesunate suppository followed by oral mefloquine in the treatment of severe falciparum malaria in endemic areas where resistance to chloroquine exists in Ecuador. Jap. J. Trop. Med. Hyg. 24:17-24. [Google Scholar]
- 11.Halpaap, B., M. Ndjave, M. Paris, A. Benakis, and P. G. Kremsner. 1998. Plasma levels of artesunate and dihydroartemisinin in children with Plasmodium falciparum malaria in Gabon after administration of 50-milligram artesunate suppositories. Am. J. Trop. Med. Hyg. 58:365-368. [DOI] [PubMed] [Google Scholar]
- 12.Hien, T. T., T. M. E. Davis, L. V. Chuong, K. F. Ilett, D. X. T. Sinh, N. H. Phu, C. Agus, G. M. Chiswell, N. J. White, and J. Farrar. 2004. Comparative pharmacokinetics of intramuscular artesunate and artemether in severe falciparum malaria. Antimicrob. Agents Chemother. 48:4234-4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hien, T. T., and N. J. White. 1993. Qinghaosu. Lancet 341:603-608. [DOI] [PubMed] [Google Scholar]
- 14.Karbwang, J., K. Na-Bangchang, K. Congpuong, P. Molunto, and A. Thanavibul. 1997. Pharmacokinetics and bioavailability of oral and intramuscular artemether. Eur. J. Clin. Pharmacol. 52:307-310. [DOI] [PubMed] [Google Scholar]
- 15.Karbwang, J., K. Na-Bangchang, T. Tin, K. Sukontason, W. Rimchala, and T. Harinasuta. 1998. Pharmacokinetics of intramuscular artemether in patients with severe falciparum malaria with or without acute renal failure. Br. J. Clin. Pharmacol. 45:597-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karunajeewa, H. A., K. F. Ilett, K. Dufall, A. Kemiki, M. Bockarie, M. P. Alpers, P. H. Barrett, P. Vicini, and T. M. Davis. 2004. Disposition of artesunate and dihydroartemisinin after administration of artesunate suppositories in children from Papua New Guinea with uncomplicated malaria. Antimicrob. Agents Chemother. 48:2966-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karunajeewa, H. A., A. Kemiki, M. P. Alpers, K. Lorry, K. T. Batty, K. F. Ilett, and T. M. Davis. 2003. Safety and therapeutic efficacy of artesunate suppositories for treatment of malaria in children in Papua New Guinea. Pediatr. Infect. Dis. J. 22:251-256. [DOI] [PubMed] [Google Scholar]
- 18.Kokwaro, G. O. 1997. Bioavailability of artemether. Br. J. Clin. Pharmacol. 44:305. [PubMed] [Google Scholar]
- 19.Krishna, S., T. Planche, T. Agbenyega, C. Woodrow, D. Agranoff, G. Bedu-Addo, A. K. Owusu-Ofori, J. A. Appiah, S. Ramanathan, S. M. Mansor, and V. Navaratnam. 2001. Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria. Antimicrob. Agents Chemother. 45:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Looareesuwan, S., C. Viravan, S. Vanijanonta, P. Wilairatana, P. Pitisuttithum, and M. Andrial. 1996. Comparative clinical trial of artesunate followed by mefloquine in the treatment of acute uncomplicated falciparum malaria: two- and three-day regimens. Am. J. Trop. Med. Hyg. 54:210-213. [DOI] [PubMed] [Google Scholar]
- 21.Looareesuwan, S., P. Wilairatana, and M. Andrial. 1996. Artesunate suppository for the treatment of severe falciparum malaria in Thailand. Jpn. J. Trop. Med. Hyg. 24:13-15. [Google Scholar]
- 22.Looareesuwan, S., P. Wilairatana, W. Molunto, K. Chalermrut, P. Olliaro, and M. Andrial. 1997. A comparative clinical trial of sequential treatments of severe malaria with artesunate suppository followed by mefloquine in Thailand. Am. J. Trop. Med. Hyg. 57:348-353. [DOI] [PubMed] [Google Scholar]
- 23.McIntosh, H. M., and P. Olliaro. 1999. Artemisinin derivatives for treating uncomplicated malaria. The Cochrane Database of Systematic Reviews. [Online.] http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD000256/frame.html. [DOI] [PMC free article] [PubMed]
- 24.McIntosh, H. M., and P. Olliaro. 2000. Artemisinin derivatives for treating severe malaria. The Cochrane Database of Systematic Reviews. [Online.] http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD000527/frame.html. [DOI] [PMC free article] [PubMed]
- 25.Mithwani, S., L. Aarons, G. O. Kokwaro, O. Majid, S. Muchohi, G. Edwards, S. Mohamed, K. Marsh, and W. Watkins. 2004. Population pharmacokinetics of artemether and dihydroartemisinin following single intramuscular dosing of artemether in African children with severe falciparum malaria. Br. J. Clin. Pharmacol. 57:146-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moir, J. S., P. A. Garner, P. F. Heywood, and M. P. Alpers. 1989. Mortality in a rural area of Madang Province, Papua New Guinea. Ann. Trop. Med. Parasitol. 83:305-319. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, S. A., E. Mberu, D. Muhia, M. English, J. Crawley, C. Waruiru, B. Lowe, C. R. Newton, P. Winstanley, K. Marsh, and W. M. Watkins. 1997. The disposition of intramuscular artemether in children with cerebral malaria: a preliminary study. Trans. R. Soc. Trop. Med. Hyg. 91:331-334. [DOI] [PubMed] [Google Scholar]
- 28.Newton, P., Y. Suputtamongkol, P. Teja-Isavadharm, S. Pukrittayakamee, V. Navaratnam, I. Bates, and N. White. 2000. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob. Agents Chemother. 44:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ringwald, P., J. Bickii, and L. K. Basco. 1996. In vitro activity of antimalarials against clinical isolates of Plasmodium falciparum in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 55:254-258. [DOI] [PubMed] [Google Scholar]
- 30.Ringwald, P., J. Bickii, and L. K. Basco. 1999. In vitro activity of dihydroartemisinin against clinical isolates of Plasmodium falciparum in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 61:187-192. [DOI] [PubMed] [Google Scholar]
- 31.Sabchareon, A., P. Attanath, P. Chanthavanich, P. Phanuaksook, V. Prarinyanupharb, Y. Poonpanich, D. Mookmanee, P. Teja-Isavadharm, D. G. Heppner, T. G. Brewer, and T. Chongsuphajaisiddhi. 1998. Comparative clinical trial of artesunate suppositories and oral artesunate in combination with mefloquine in the treatment of children with acute falciparum malaria. Am. J. Trop. Med. Hyg. 58:11-16. [DOI] [PubMed] [Google Scholar]
- 32.Silamut, K., P. N. Newton, P. Teja-Isavadharm, Y. Suputtamongkol, D. Siriyanonda, M. Rasameesoraj, S. Pukrittayakamee, and N. J. White. 2003. Artemether bioavailability after oral or intramuscular administration in uncomplicated falciparum malaria. Antimicrob. Agents Chemother. 47:3795-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94(Suppl. 1):S1-S90. [PubMed] [Google Scholar]
- 34.World Health Organization. 2001. Tropical disease research, progress 1999-2000. Special programme for research and training in tropical diseases, 15th programme report. TDR/GEN/01.5. http://www.who.int/tdr/publications/publications/pdf/pr15/pr15.pdf.