Abstract
The Brucella cell envelope is characterized by the presence of phosphatidylcholine (PC), a common phospholipid in eukaryotes that is rare in prokaryotes. Studies on the composition of Brucella abortus 2308 phospholipids revealed that the synthesis of PC depends on the presence of choline in the culture medium, suggesting that the methylation biosynthetic pathway is not functional. Phospholipid composition of pmtA and pcs mutants indicated that in Brucella, PC synthesis occurs exclusively via the phosphatidylcholine synthase pathway. Transformation of Escherichia coli with an expression vector containing the B. abortus pcs homologue was sufficient for PC synthesis upon induction with IPTG (isopropyl-β-d-thiogalactopyranoside), while no PC formation was detected when bacteria were transformed with a vector containing pmtA. These findings imply that Brucella depends on choline provided by the host cell to form PC. We could not detect any obvious associated phenotype in the PC-deficient strain under vegetative or intracellular growth conditions in macrophages. However, the pcs mutant strain displays a reproducible virulence defect in mice, which suggests that PC is necessary to sustain a chronic infection process.
Phosphatidylcholine (PC) is one of the major structural constituents of the eukaryotic membranes and is the major source of the lipid second messenger involved in signal transduction pathways. PC is also found in an increasing number of prokaryotes, mainly in symbionts, pathogens, and photosynthetic bacteria (18). In eukaryotes, PC is synthesized through two alternative biosynthetic pathways: (i) the CDP-choline pathway (known as the Kennedy pathway) in which CDP-choline condenses with diacylglycerol (DAG) to form PC by the action of the enzyme CDP-choline:1,2-diacylglycerol choline phosphotransferase and (ii) the methylation pathways, which consist of three methylation steps of phosphatidylethanolamine (PE) catalyzed by the enzyme phospholipid N-methyltransferase (Pmt). In this pathway, the methyl donor is S-adenosylmethionine (SAM) (18). It has been assumed that in bacteria, the main pathway for PC synthesis is the methylation pathway. However, a novel pathway was described for the legume symbiont Sinorhizobium meliloti in which choline is directly condensed with CDP-DAG by the action of the enzyme phosphatidylcholine synthase (Pcs) (17). Recently, a CDP-choline pathway for PC formation was also described for Treponema denticola (11). Thus, in prokaryotes, PC is frequently present, and the pathways involved are more complex than originally thought.
Many bacteria, especially those that are plant associated, have both the methylation and the Pcs pathways for PC formation. An S. meliloti mutant deficient in the methylation pathway relies on the supply of choline to form PC through the Pcs pathway (8), thus indicating that Pcs allows the symbiont to produce PC from choline exogenously provided by the plant partner. A double pmtA pcs mutant was unable to form PC and exhibited a severe growth defect, indicating that in S. meliloti, PC is needed for normal growth (7). As PC is present only in a restricted number of bacterial genera, it was speculated that this phospholipid might perform some special function. In fact, a mutant strain of Bradyrhizobium japonicum that forms PC in reduced amounts was incapable of producing efficient symbiosis with its plant host (14).
The membrane of pathogenic bacteria that cause persistent infections, like Pseudomonas aeruginosa, Legionella pneumophila, and Borrelia burgdorferi, contains PC, but the function it plays in the interaction with the corresponding host is completely unknown. Notably, these pathogens have genes coding for Pcs, which may enable them to obtain choline from the host. It was speculated that PC not only might serve as an important structural component of the membranes but also might contribute to pathogenicity (18). This is the case of phosphorylcholine modifications of Haemophilus influenzae lipopolysaccharide (12) or Streptococcus pneumoniae lipoteichoic acid (24), which are only formed by a CDP-choline pathway when choline is supplied externally. Therefore, it is important to understand the role that PC plays in bacterium-host interactions.
Members of the genus Brucella are the etiological agents of brucellosis, a worldwide-distributed zoonosis affecting both wild and domestic mammals as well as humans (6). These are facultative intracellular pathogens capable of invading and proliferating intracellularly in macrophages and nonprofessional phagocytes of its host, establishing long-lasting infections. One of the outstanding characteristics of Brucella is its ability to subvert the macrophage bactericidal defensive mechanisms to create a novel membrane-bound compartment, which supports its intracellular multiplication (4). It was assumed that most of these properties are related to the singular structure and composition of the Brucella cell envelope (CE), which differs from the most-studied gram-negative CE, although the roles that the surface components play in virulence are not completely understood. The Brucella CE is characterized by the presence of a low endotoxic lipopolysaccharide, several porins, and outer membrane proteins covalently bound to the peptidoglycan layer and the presence of PC as one of the main phospholipids (15, 19). Recently, Pcs activity but not PmtA activity was detected in cell extracts of Brucella melitensis, raising the question of whether the Pcs pathway is the only pathway responsible for PC synthesis in this genus (13). In this report, we cloned the pmtA and pcs genes from Brucella abortus 2308 and characterized mutants defective in PC synthesis.
MATERIALS AND METHODS
Media and growth conditions.
Brucella isolates were cultured in rich, complex tryptic soy broth (TSB) or in minimum, defined Gerhardt-Wilson (G-W) medium (10) on a rotary shaker (250 rpm) at 37°C. When needed, choline citrate was added to a final concentration of 10 μM. Media were supplemented with the appropriate antibiotics at the following concentrations: kanamycin, 50 μg/ml; carbenicillin, 50 μg/ml; and gentamicin, 3 μg/ml.
Cloning, gene disruption, and generation of mutant strains.
A DNA fragment of 1,143 bp containing the pmtA gene of B. abortus 2308 (Ba12183) was amplified from genomic DNA using primers pmtAup (5′-ATGGCGCGGACCTTTATTGCA-3) and pmtAdown (5′-AGCGGTTTTGCGTCGGATAAT-3′) and HiFi Platinum Taq DNA polymerase (Invitrogen). The same procedure using primers pcsup (5′-CCGCCACTGATAACAATGTCG-3′) and pcsdown (5′-CCGATTTCCACCTGTTCATAG-3′) was used to amplify a 1,249-bp DNA fragment containing the pcs gene (Ba20693). Both amplicons were ligated into pGemTeasy (Promega Corp.) to generate the intermediate vectors pGemT-pmtA and pGemT-pcs. pGemT-pmtA was subsequently digested with ClaI (NEB, Inc.) and ligated into a kanamycin resistance cassette from pUC4K to generate the plasmid pGemT-pmtA::Kan. pGemT-pcs was digested with HindIII (NEB, Inc.) and ligated into the accI gene conferring resistance to gentamicin to generate pGemT-pcs::Gm. These vectors were introduced into B. abortus 2308 by electroporation to obtain the corresponding knockout mutants. Double recombination events (Kanr Amps or Gmr Amps) were selected, and the corresponding gene knockout was confirmed by genomic PCR. To obtain a pmtA pcs double mutant, pGemT-pmtA::Kan was introduced by electroporation into B. abortus pcs, and the double recombination events (Kanr Amps) were selected and confirmed by genomic PCR. To generate the corresponding complementing plasmids, both amplicons were digested with EcoRI (NEB, Inc.) and ligated into pBBR4 under the lac promoter.
Cell infection assays.
Murine macrophage-like J774 cells were maintained in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum and 2 mM l-glutamine. Cells seeded in 24-well plates (5 × 105 cells/well) were inoculated with 1 ml of cell culture medium (RPMI 1640 medium supplemented with 5% fetal bovine serum and 2 mM l-glutamine without antibiotics) containing bacteria at a multiplicity of infection of 50. Multiwell plates were centrifuged for 10 min at 141 × g at room temperature and placed in a 5% CO2 atmosphere at 37°C. After 30 min, cells were washed four times with phosphate-buffered saline (PBS) (pH 7.4) and incubated with cell culture medium containing gentamicin (100 μg ml−1) and streptomycin (50 μg ml−1) to eliminate extracellular bacteria. At different times, cells were washed four times with PBS and treated for 10 min with 1 ml of 0.1% Triton X-100 in deionized sterile water. The number of intracellular viable bacteria was determined as CFU per milliliter by serial dilution and plating on tryptic soy agar supplemented with antibiotics as needed.
In vivo labeling of B. abortus with [14C]acetate and quantitative analysis of lipid extracts.
The lipid compositions of B. abortus 2308 and mutant strains were determined following labeling with [14C]acetate. Cultures of wild-type and mutant strains were grown overnight in TSB medium, and the cultures were washed with G-W minimal medium and used to inoculate 3 ml of G-W minimal medium (with or without choline) at an optical density at 600 nm (OD600) of 0.1. After the addition of 3 μCi of [14C]acetate (56.50 mCi/mmol; New England Nuclear), cultures were incubated to an OD600 of 0.6. The cells were harvested by centrifugation. Lipids were extracted according to the method described previously by Bligh and Dyer (1) and separated onto thin-layer silica gel plates (Kieselgel 60; Merck) in two dimensions (two-dimensional [2D] thin-layer chromatography [TLC]) using chloroform-methanol-water (14:6:1, vol/vol/vol) in the first phase followed by separation in a second phase with chloroform-methanol-acetic acid (13:5:2, vol/vol/vol). After exposure to Biomax Kodak films, lipids were visualized by iodine staining. Spots corresponding to phospholipids were scraped from the plate, and the radioactivity was quantified in a scintillation counter with 2 ml of scintillation liquid.
Expression of the recombinant proteins in Escherichia coli.
The pcs gene from B. abortus 2308 was amplified with primers pcsEN (5′-GGAATTCCATATGAAAACCAAACTGACCGGA-3′) and pcsBS (5′-CGGGATCCTCATGGTGCTTCTCCGCTCTT-3′), digested with NdeI and BamHI, ligated into the pET22 (Novagen) expression vector, and introduced in E. coli BL21 by the CaCl2 method. The same procedure using primers pmtAKN (5′-GGGTACCCATATGGCAGGTCAGCTTGGCAGG-3′) and pmtAHS (5′-CCGAAGCTTCTATACAAGCGGACTGCGATA-3′) was employed to express the recombinant PmtA.
For phospholipid analysis, when 5-ml cultures in LB medium reached an OD600 of 0.2, they were split in two, and IPTG (isopropyl-β-d-thiogalactopyranoside) was added to one of the tubes to a final concentration of 0.5 mM. After 3 h of induction, cells were harvested, and lipids were extracted by the Bligh and Dyer method. Lipids were separated by one-dimensional TLC on high-performance TLC silica gel 60 F254 plates (Merck), and the plates were developed with n-propyl alcohol-propionic acid-chloroform-water (3:2:2:1, vol/vol/vol/vol) (9). The phospholipids were visualized after application of CuSO4/H3PO4 spray reagent and subsequent charring (22).
Mouse infection assays.
Virulence was determined by quantitating the survival of the strains in mouse spleens at different times postinfection as described previously (5). Groups of 9-week-old female BALB/c mice (20 mice per group) were injected intraperitoneally with 105 CFU of B. abortus 2308 or the isogenic mutant B. abortus pcs in 0.2 ml of sterile PBS. At 2, 3, 6, and 10 weeks postinfection, animals were sacrificed, and the spleens were removed, weighed, homogenized in sterile 150 mM NaCl solution, serially diluted, and plated onto TSB agar with the appropriate antibiotics to determine the number of CFU per spleen.
RESULTS
Identification of homologues of pmtA and pcs in Brucella.
The presence of PC in the genus Brucella was first described in 1969 (20). However, the mechanism and genes involved in PC biosynthesis have not been elucidated. To address this issue, the complete genome sequences of B. melitensis 16 M, B. suis 1330, and B. abortus 2308 were examined for the presence of homologues to PmtA and Pcs, the enzymes responsible for the synthesis of PC through either the methylation of PE or the direct condensation of choline with CDP-diacylglycerol, respectively. Chromosome I of the three species contains an open reading frame (ORF) predicted to encode a 22-kDa protein with 60% similarity to PmtA of S. meliloti (B. melitensis BMEI2000, B. suis BR2127, and B. abortus Ba12183). However, a close inspection of the sequences reveals different amino acid substitutions among the three species spanning the conserved consensus motif for SAM-utilizing methyltransferases (VLELGXGXG) (Fig. 1A). This observation raised the question of whether the Brucella pmtA homologue is responsible for synthesis of PC in the genus. A recent work exploring PmtA activity in different bacterial species failed to detect it in cell extracts of B. melitensis 16 M (13).
FIG. 1.
(A) Comparison of pmtA homologues from B. abortus 2308 (B.a.), B. melitensis 16 M (B.m.), B. suis 1330 (B.s.), and Sinorhizobium meliloti (S.m.). Identical residues are marked in black. The consensus motif for SAM-dependent methyltransferases is indicated on the top line. (B) ClustalW alignments of pcs from the selected species mentioned above. The characteristic residues for the CDP-alcohol phosphatidyltransferases are indicated by asterisks.
Recently, Sohlenkamp et al. (17) reported that PC in bacteria could be formed directly by condensation of choline with CDP-DAG via a novel enzymatic activity, the Pcs pathway. Chromosome II of the three species (B. melitensis BMEII0695, B. suis BRA0572, and B. abortus Ba20693) contains an ORF predicted to encode a 29.5-kDa protein with 64% similarity to Pcs from S. meliloti (Fig. 1B). In these ORFs, the consensus motif characteristic of CDP-alcohol phosphatidyltransferases (DGX2ARX12GX3DX3D) is absolutely conserved, suggesting that this gene could be responsible for PC synthesis in the genus.
Synthesis of PC in B. abortus.
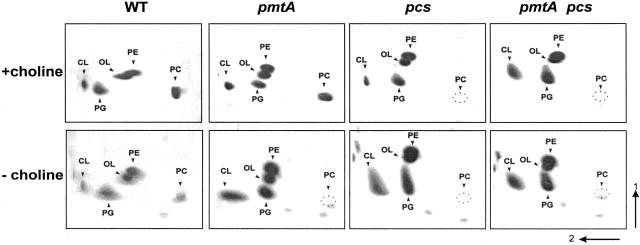
The methylation pathway for PC formation in bacteria uses the endogenous PE as a substrate for three rounds of methylation catalyzed by pmtA, with SAM being the methyl donor. This pathway is completely independent of the presence of choline in the culture medium. In contrast, the novel Pcs pathway directly condenses choline with CDP-DAG to generate PC and CMP. This pathway is dependent on the exogenous supply of choline. To asses whether the synthesis of PC in Brucella is dependent on the presence of choline in the culture medium, the phospholipid composition of B. abortus 2308 grown in the defined G-W medium, with or without the addition of choline, was analyzed by bidimensional TLC. Phospholipid composition of wild-type B. abortus 2308 grown in G-W medium with choline revealed that PC and PE were the most abundant phospholipids (26.78% ± 0.97% and 26.36% ± 5.16%, respectively), and minor spots corresponding to phosphatidylglycerol (PG), cardiolipin (CL), and ornithine lipid (OL) (15.55% ± 0.66%, 4.87% ± 1.59%, and 26.41 ± 0.18%, respectively) were also observed (Table 1). The monomethyl-phosphatidylethanolamine and the dimethyl-phosphatidylethanolamine derivatives were not detected. In contrast, when B. abortus 2308 was grown in G-W medium without choline, the major spots corresponding to PC dramatically decreased (0.37% ± 0.18%) (Table 1). The absence of PC was compensated for by doubling the amount of the other zwitterionic phospholipid, PE (50.13% ± 2.13%), and slightly increasing the content of the anionic phosphatidylglycerol (19.87% ± 1.49%), while the ornithine lipid and cardiolipin remained constant (26.28% ± 0.18% and 3.33% ± 0.27%, respectively). This result indicates that the synthesis of PC in Brucella depends on the presence of choline in the culture medium, suggesting that only the Pcs pathway is functional.
TABLE 1.
Lipid composition of B. abortus 2308, the pcs mutant, and its corresponding complemented strain
| Strain | Lipid composition (% of total 14C ± SD)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC
|
PE
|
OL
|
PG
|
CL
|
||||||
| + | − | + | − | + | − | + | − | + | − | |
| B. abortus 2308 | 26.78 ± 0.97 | 0.37 ± 0.18 | 26.36 ± 5.16 | 50.13 ± 2.13 | 26.41 ± 3.88 | 26.28 ± 0.18 | 15.55 ± 0.66 | 19.87 ± 1.49 | 4.87 ± 1.59 | 3.33 ± 0.27 |
| B. abortus pcs | 0.49 ± 0.70 | 0 | 46.32 ± 1.98 | 55.62 ± 4.33 | 29.92 ± 2.35 | 23.06 ± 6.11 | 20.53 ± 0.39 | 19.18 ± 2.02 | 2.72 ± 0.67 | 2.12 ± 0.24 |
| B. abortus pcs (pBBR-pcs) | 38.77 ± 1.87 | 0.23 ± 0.04 | 22.15 ± 5.93 | 42.94 ± 7.21 | 22.62 ± 3.52 | 33.12 ± 1.90 | 10.13 ± 1.20 | 19.68 ± 4.85 | 6.31 ± 0.66 | 4.00 ± 0.40 |
+ indicates the presence of choline in the culture medium; − indicates the absence of choline in the culture medium.
Brucella PC is synthesized by the phosphatidylcholine synthase pathway.
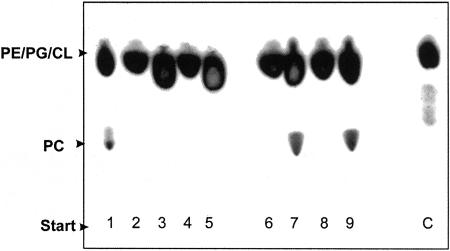
The absence of PC when B. abortus was grown in G-W medium without choline together with the observation that the SAM consensus motif is not present in the Brucella pmtA homologues prompted us to analyze the pathway responsible for its biosynthesis using a genetic approach. The B. abortus pmtA and pcs homologues were disrupted; the corresponding pmtA, pcs, and double pmtA pcs mutants were generated; and the phospholipid profiles were analyzed. When grown in G-W medium with choline, PC was detected in the wild-type and pmtA mutant backgrounds; on the other hand, its synthesis was completely abolished in the pcs and pmtA pcs mutants (Fig. 2). Additionally, when the strains were grown in G-W medium without choline, PC was never detected (Fig. 2). The ability to form PC when cultured in G-W medium with choline was restored in the B. abortus pcs mutant complemented with pBBR4-pcs (Table 1). Taken together, these results suggest that the Pcs pathway is the only way to form PC in Brucella. To further confirm these results, the corresponding pcs and pmtA genes were cloned into the pET22 expression vector under the control of the T7lac promoter and introduced into E. coli BL21, a bacterium that does not contain PC in its membranes. Upon induction with IPTG, PC was formed in E. coli pET-pcs but not in E. coli pET-pmtA (Fig. 3). This finding and the results described above clearly indicate that in Brucella, PC is exclusively formed via the phosphatidylcholine synthase pathway.
FIG. 2.
2D-TLC analysis of total lipids from B. abortus 2308 and its isogenic mutant pmtA, pcs, and pmtA pcs strains. Cells were cultured in G-W medium with or without choline in the presence of [14C]acetate, and the lipids were extracted and separated by 2D-TLC. The lipids PC, PE, OL, CL, and PG are indicated.
FIG. 3.
Lipid analysis after expression of Brucella pmtA and pcs in E. coli. E. coli BL21 transformed with pET-pmtA (lanes 2 to 5) or with pET-pcs (lanes 6 to 9) was grown in LB (lanes 2, 4, 6, and 8) or LB plus IPTG (lanes 3, 5, 7, and 9), and after extraction, lipids were separated by one-dimensional TLC. B. abortus 2308 (lane 1) grown in TSB and phosphatidylethanolamine (lane C) were run as a control. The lipids PC, PE, CL, and PG are indicated.
Characterization of B. abortus pcs mutant.
To determine whether the absence of PC affects the viability of B. abortus, the growth rates in TSB and G-W medium with or without choline were analyzed. In rich complex medium, both wild-type B. abortus 2308 and its derivative pcs mutant displayed similar generation times [G(h)wt (generation time, in hours, of the wild type) = 3.27 ± 0.14 and G(h)pcs = 3.38 ± 0.18, respectively]. A slight increase in the generation time of the pcs mutant was observed when the strains were grown in G-W medium with choline [G(h)wt = 6.76 ± 0.22 and G(h)compl = 6.72 ± 0.17 versus G(h)pcs = 7.52 ± 0.31]. When grown in G-W medium without choline, a condition that precludes the synthesis of PC, the generation times were similar [G(h)wt = 7.30 ± 0.10, G(h)pcs = 7.07 ± 0.12, and G(h)compl = 7.74 ± 0.19]. This result indicates that PC is not essential for B. abortus viability. A change in viability of the pcs mutant following storage at −80°C or 4°C was not observed.
The effect of the pcs mutation on the ability of B. abortus to invade and sustain intracellular replication in murine J774 macrophages was analyzed. The cells were infected with wild-type strain 2308, the pcs mutant, or the pmtA pcs double mutant, and the number of intracellular CFU was determined at different times postinfection. Both the pcs and pmtA pcs mutants were able to replicate intracellularly in a way similar to that of their parental virulent strains, indicating that the absence of PC does not impair the ability of Brucella to invade and replicate intracellularly in murine macrophages (not shown).
Virulence of B. abortus pcs.
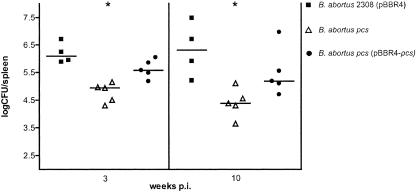
To analyze the virulence of the pcs mutant, an experimental infection of BALB/c mice was performed. Female BALB/c mice were inoculated intraperitoneally with 1 × 105 CFU of wild-type B. abortus 2308 harboring pBBR4, the pcs mutant, and the pcs mutant harboring the complementing pBBR4-pcs plasmid. The number of CFU recovered from the spleen was determined at 3 and 10 weeks postinfection. At all times tested, the mutant strain was significantly attenuated compared with the virulent parental strain (P = 0.0159), displaying a difference in the spleen CFU counts of 2 log units at 10 weeks postinfection (Fig. 4). As expected, the complemented strain displayed not significantly different median CFU counts in comparison with those of the wild-type strain (P = 0.1905). Splenomegaly, a distinctive characteristic of Brucella infection, was also reduced in mice that received the mutant strain (not shown). This result indicates that Brucella abortus requires PC in its cell envelope to cause an efficient infection process in mice.
FIG. 4.
Virulence of B. abortus 2308 pBBR4 (black squares), the pcs mutant (open triangles), and the complemented pcs pBBR-pcs strain in BALB/c mice. Mice were infected by intraperitoneal injection. Individual spleen CFU values were plotted, and the horizontal bars represent the median CFU for each treatment group. *, P < 0.05 (compared to the group that received the wild-type strain). Statistical analysis was performed with a Kruskal-Wallis test. p.i., postinfection.
DISCUSSION
One of the striking peculiarities of the CE of brucellae that distinguish it from other gram-negative bacteria is the presence of PC and PE as the main phospholipids. While PE is a common phospholipid in most gram-negative bacteria, PC is restricted to a limited number of genera, specifically those that establish close interactions with eukaryotes (endosymbionts and intracellular pathogens).
Thiele and Kehr first described the presence of PC in the CE of Brucella in 1969, but until now, the genes and metabolic pathways involved in its synthesis remained unknown (20). The genome of the three main Brucella species harbors two genes coding for homologues to pmtA and pcs of Sinorhizobium meliloti, which suggests that both the methylation of PE and/or the condensation of choline with CDP-DAG could account for PC biosynthesis in this genus.
The phospholipid composition of B. abortus 2308 grown in minimum defined medium indicated that PC biosynthesis was dependent on the presence of choline in the culture medium, suggesting that the methylation pathway is not functional. Generation of mutants in pmtA and pcs genes clearly indicated that PC synthesis in Brucella occurs solely via the phosphatidylcholine synthase pathway. Transformation of E. coli with an expression vector containing the B. abortus pcs homologue was sufficient for PC synthesis upon induction with IPTG, while no PC formation was detected when bacteria were transformed with a vector containing pmtA. These results indicate that the Brucella pmtA homologue is not involved in PC synthesis. In line with our observation, in a recent report by Martinez-Morales et al. (13), PmtA activity could not be detected in cell extracts of B. melitensis 16 M. These findings imply that Brucella depends on choline provided by the host cell to form one of its major phospholipids.
Alignment of PmtA homologues from the three Brucella species and S. meliloti indicates that, although highly similar, each of the Brucella homologues had accumulated different amino acid substitutions in a conserved region corresponding to the consensus motif for SAM-dependent methyltransferases, which may explain the lack of PmtA activity (Fig. 1). It could be speculated that for an intracellular pathogen like Brucella, the Pcs pathway for PC formation is selectively advantageous over the methylation pathway, since it is energetically more favorable, whereas the intracellular environment ensures the supply of the precursor choline. The Brucella PmtA homologues could have acquired a novel enzymatic activity not related to PC formation or, alternatively, could reflect an evolutionary remnant. A similar situation was recently described for Pseudomonas aeruginosa, where the synthesis of PC occurs only through the Pcs pathway, whereas the methylation pathway is not functional. Interestingly, the P. aeruginosa PmtA homologue displays differences in the N-terminal region that probably reflect the evolution of a different function for this protein (23).
The role of lipid composition in Brucella remains elusive. We could not detect any obvious phenotype in the PC-deficient strain under vegetative or intracellular growth conditions in macrophages. However, the pcs mutant strain displays a clear virulence defect in mice, indicating that PC is necessary directly or indirectly to sustain a chronic infection. One possible explanation for this phenomenon is that the absence of PC alters the outer membrane composition. Indeed, we detect an altered profile of membrane proteins in the pcs mutant compared with the parental virulent strain by 2D electrophoresis (C. Mujer and D. Comerci, unpublished data). Nevertheless, this membrane alteration affects only the establishment of a chronic infection process in mice while it does not affect some key virulence traits of Brucella, such as invasion, intracellular traffic, brucellosome formation, and intracellular replication (Fig. 4). An alternative explanation is that PC per se could participate in the mouse infection process by modulating the host immune response. Indeed, Brucella PC has been implicated in the inflammatory response of human brucellosis (3). Several studies demonstrated that patients with chronic brucellosis developed an antibody response against Brucella PC that cross-reacted with platelet-activating factor (PAF), a phospholipid-derived second messenger involved in the inflammatory process with an immunosuppressive role (16, 21). Interestingly, those authors found that both the Brucella phospholipid fraction and the patient sera were able to promote platelet aggregation (2, 3). These findings and the structural similarity between PC and PAF led those authors to hypothesize that Brucella PC could mimic PAF exerting an agonistic effect upon binding to the PAF receptor that could modulate the inflammatory response generated during infection. If this is the case, the Brucella pcs mutant described in this work could be a useful tool to define the contribution of PC in the bacterium-host interaction.
Acknowledgments
This work was supported by grant PICT2002 01-11882 to D.J.C. and grant PICT2000 01-09194 to R.A.U. from Agencia Nacional de Promocion Cientifica y Tecnologica, SECyT, Buenos Aires, Argentina, and by grant PID-P5 72-5 to D.J.C. from Comisión Nacional de Energía Atómica, Argentina, and was partially supported by grant PICT2003 05-18393 to D.D.M. D.D.M. is a Career Investigator from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and an International Scholar from the Howard Hughes Medical Institute.
REFERENCES
- 1.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 2.Casao, M. A., R. Diaz, A. Orduna, and C. Gamazo. 2001. Promotion of platelet aggregation by sera from brucellosis patients with antiphosphatidylcholine antibodies. J. Med. Microbiol. 50:965-968. [DOI] [PubMed] [Google Scholar]
- 3.Casao, M. A., J. Leiva, R. Diaz, and C. Gamazo. 1998. Anti-phosphatidylcholine antibodies in patients with brucellosis. J. Med. Microbiol. 47:49-54. [DOI] [PubMed] [Google Scholar]
- 4.Celli, J., and J. P. Gorvel. 2004. Organelle robbery: Brucella interactions with the endoplasmic reticulum. Curr. Opin. Microbiol. 7:93-97. [DOI] [PubMed] [Google Scholar]
- 5.Comerci, D. J., G. D. Pollevick, A. M. Vigliocco, A. C. Frasch, and R. A. Ugalde. 1998. Vector development for the expression of foreign proteins in the vaccine strain Brucella abortus S19. Infect. Immun. 66:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbel, M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Rudder, K. E., I. M. Lopez-Lara, and O. Geiger. 2000. Inactivation of the gene for phospholipid N-methyltransferase in Sinorhizobium meliloti: phosphatidylcholine is required for normal growth. Mol. Microbiol. 37:763-772. [DOI] [PubMed] [Google Scholar]
- 8.de Rudder, K. E., C. Sohlenkamp, and O. Geiger. 1999. Plant-exuded choline is used for rhizobial membrane lipid biosynthesis by phosphatidylcholine synthase. J. Biol. Chem. 274:20011-20016. [DOI] [PubMed] [Google Scholar]
- 9.de Rudder, K. E., J. E. Thomas-Oates, and O. Geiger. 1997. Rhizobium meliloti mutants deficient in phospholipid N-methyltransferase still contain phosphatidylcholine. J. Bacteriol. 179:6921-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerhardt, P. 1958. The nutrition of brucellae. Bacteriol. Rev. 22:81-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent, C., P. Gee, S. Y. Lee, X. Bian, and J. C. Fenno. 2004. A CDP-choline pathway for phosphatidylcholine biosynthesis in Treponema denticola. Mol. Microbiol. 51:471-481. [DOI] [PubMed] [Google Scholar]
- 12.Lysenko, E., J. C. Richards, A. D. Cox, A. Stewart, A. Martin, M. Kapoor, and J. N. Weiser. 2000. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein-mediated killing. Mol. Microbiol. 35:234-245. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Morales, F., M. Schobert, I. M. Lopez-Lara, and O. Geiger. 2003. Pathways for phosphatidylcholine biosynthesis in bacteria. Microbiology 149:3461-3471. [DOI] [PubMed] [Google Scholar]
- 14.Minder, A. C., K. E. de Rudder, F. Narberhaus, H. M. Fischer, H. Hennecke, and O. Geiger. 2001. Phosphatidylcholine levels in Bradyrhizobium japonicum membranes are critical for an efficient symbiosis with the soybean host plant. Mol. Microbiol. 39:1186-1198. [PubMed] [Google Scholar]
- 15.Moriyon, I., and I. Lopez-Goni. 1998. Structure and properties of the outer membranes of Brucella abortus and Brucella melitensis. Int. Microbiol. 1:19-26. [PubMed] [Google Scholar]
- 16.Prescott, S. M., G. A. Zimmerman, D. M. Stafforini, and T. M. McIntyre. 2000. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69:419-445. [DOI] [PubMed] [Google Scholar]
- 17.Sohlenkamp, C., K. E. de Rudder, V. V. Rohrs, I. M. Lopez-Lara, and O. Geiger. 2000. Cloning and characterization of the gene for phosphatidylcholine synthase. J. Biol. Chem. 275:27500. [DOI] [PubMed] [Google Scholar]
- 18.Sohlenkamp, C., I. M. Lopez-Lara, and O. Geiger. 2003. Biosynthesis of phosphatidylcholine in bacteria. Prog. Lipid Res. 42:115-162. [DOI] [PubMed] [Google Scholar]
- 19.Thiele, O. W., J. Asselineau, and C. Lacave. 1969. On the fatty acids of Brucella abortus and Brucella melitensis. Eur. J. Biochem. 7:393-396. [DOI] [PubMed] [Google Scholar]
- 20.Thiele, O. W., and W. Kehr. 1969. The “free” lipids of Brucella abortus Bang. Concerning the neutral lipids. Eur. J. Biochem. 9:167-175. [In German.] [DOI] [PubMed] [Google Scholar]
- 21.Walterscheid, J. P., S. E. Ullrich, and D. X. Nghiem. 2002. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J. Exp. Med. 195:171-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welsh, C. J., and K. Schmeichel. 1991. Assays for investigations of signal transduction mechanisms involving phospholipase D: mass measurements of phosphatidate, phosphatidylethanol, and diacylglycerol in cultured cells. Anal. Biochem. 192:281-292. [DOI] [PubMed] [Google Scholar]
- 23.Wilderman, P. J., A. I. Vasil, W. E. Martin, R. C. Murphy, and M. L. Vasil. 2002. Pseudomonas aeruginosa synthesizes phosphatidylcholine by use of the phosphatidylcholine synthase pathway. J. Bacteriol. 184:4792-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, J. R., I. Idanpaan-Heikkila, W. Fischer, and E. I. Tuomanen. 1999. Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. Mol. Microbiol. 31:1477-1488. [DOI] [PubMed] [Google Scholar]