Abstract
The Streptomyces coelicolor partitioning protein ParB binds to numerous parS sites in the oriC-proximal part of the linear chromosome. ParB binding results in the formation of large complexes, which behave differentially during the complex life cycle (D. Jakimowicz, B. Gust, J. Zakrzewska-Czerwinska, and K. F. Chater, J. Bacteriol. 187:3572-3580, 2005). Here we have analyzed the transcriptional regulation that underpins this developmentally specific behavior. Analysis of promoter mutations showed that the irregularly spaced complexes present in vegetative hyphae are dependent on the constitutive parABp1 promoter, while sporulation-specific induction of the promoter parABp2 is required for the assembly of arrays of ParB complexes in aerial hyphae and thus is necessary for efficient chromosome segregation. Expression from parABp2 depended absolutely on two sporulation regulatory genes, whiA and whiB, and partially on two others, whiH and whiI, all four of which are needed for sporulation septation. Because of this pattern of dependence, we investigated the transcription of these four whi genes in whiA and whiB mutants, revealing significant regulatory interplay between whiA and whiB. A strain in which sporulation septation (but not vegetative septation) was blocked by mutation of a sporulation-specific promoter of ftsZ showed close to wild-type induction of parABp2 and formed fairly regular ParB-enhanced green fluorescent protein foci in aerial hyphae, ruling out strong morphological coupling or checkpoint regulation between septation and DNA partitioning during sporulation. A model for developmental regulation of parABp2 expression is presented.
Streptomycetes are gram-positive mycelial soil bacteria with unusual cell division features. In particular, their large linear chromosomes do not show clear-cut partitioning during most of their morphologically complex life cycle (10, 11, 14, 21). The elongated and often branched compartments of vegetative hyphae contain several copies of unsegregated chromosomes. During further development of the Streptomyces coelicolor colony growing on an agar surface (but not in submerged culture), new branches grow into the air for many tens of microns, forming a layer of white aerial mycelium. After cessation of growth, the long tip compartments of aerial hyphae differentiate into chains of exospores. This process starts with the assembly of a regular ladder of FtsZ rings, which are precursors of sporulation septa (46). Formation of sporulation septa is accompanied by chromosome condensation (which is somewhat impaired in ftsZ mutants [16]) and chromosome segregation into unigenomic prespore compartments. During maturation of the spores, the compartments round up and the spore walls thicken and acquire color through synthesis of a polyketide pigment, gray in the case of S. coelicolor and therefore giving rise to gray colonies (9).
White colony (whi) mutants cannot undergo maturation of aerial hyphae. Several whi genes (including whiA, whiB, whiH, whiI, and whiG) are regulators of the early stages of sporulation (1, 2, 8, 11, 13, 15, 44). Mutants of these five genes are defective in sporulation septation (15). whiA encodes a protein of unknown function with orthologues in most other gram-positive bacteria (2). whiB belongs to group of genes found only in actinomycetes, encoding small putative transcription factors containing an Fe-S cluster (13, 25). whiH encodes a member of the GntR family of transcription factors (44), and whiI encodes an atypical member of the response regulator family of proteins but is not adjacent to a potential sensor kinase gene (1). Both whiH and whiI are dependent on the sigma factor encoded by whiG (12).
Both whiA and whiB have two promoters, one low-level constitutive and another strongly transcribed at the time of aerial mycelium growth (2, 49). whiA and whiB deletion mutants have abnormally long coiled aerial hyphae, implying that they are defective in signals for growth cessation (15). In these mutants, chromosomes remain in an uncondensed state with continuous distribution along aerial hyphae. It has been proposed that WhiA/WhiB-dependent growth cessation of aerial hyphae generates signals that are recognized by, and change the behavior of, WhiH and WhiI (11). whiI and whiH transcription is also highly induced at the time of sporulation (1). whiI and whiH mutants both have loosely coiled aerial hyphae, of more or less wild-type length, differing from each other in the extent of chromosome condensation (1, 15). whiI mutants show the same lack of condensation as whiA and whiB mutants, while in the whiH mutant DNA becomes partially condensed, forming irregular patches (1, 15).
Segregation of bacterial chromosomes is most extensively studied in single-celled bacteria that divide by binary fission. It is an active process closely coupled to replication (for recent reviews, see references 3, 18, 30, 42, 48, and 52). ParAB homologues were among the earliest identified proteins involved in chromosome segregation (reviewed in references 6 and 17) and have been studied particularly in Bacillus subtilis (22, 39), Caulobacter crescentus (38), Pseudomonas putida (32), and Pseudomonas aeruginosa (4). Their exact functions in chromosome segregation are still not clear. In C. crescentus both genes are essential (37), while in B. subtilis neither is essential, though spo0J (encoding the ParB homologue) is required for formation of endospores and for proper chromosome partitioning during vegetative growth (22, 47). ParB homologues are DNA binding proteins interacting with 14- to 16-bp partitioning sites (parS) (6, 17). In B. subtilis Spo0J binds to eight parS sites in the 20% of the chromosome around the replication origin (31, 33, 34), and Spo0J/ParB colocalizes with the oriC-proximal part of the B. subtilis and C. crescentus chromosomes (19, 34, 38, 51).
Due to the large size and linearity of the Streptomyces chromosome (8.7 Mb for S. coelicolor, 9 Mb for S. avermitilis) (5, 40) and the complexity of growth and morphological development of these organisms, Streptomyces chromosome segregation is expected to be more complex than that of rod-shaped bacteria dividing by binary fission. As in B. subtilis, the Streptomyces parAB genes are arranged in a two-gene operon (29). Disruption of the S. coelicolor parAB operon does not visibly affect colony growth, but chromosome partitioning aberrations are observed in about 13% of spores (29). The S. coelicolor chromosome contains 24 parS sites clustered within a 400-kb region around oriC (5% of the chromosome). Interaction studies, both in vitro and in vivo, indicated that most of the S. coelicolor parS sites are involved in the formation of large nucleoprotein complexes, which also seem to include the segments between parS sites (23). Construction of a strain expressing a ParB-enhanced green fluorescent protein (EGFP) fusion revealed ParB complexes, seen as fluorescent foci, that behaved differently during vegetative growth and in sporulating aerial hyphae (24). In vegetative hyphae, foci formed only transiently during the chromosome replication cycle and were small and irregularly spaced except close to hyphal tips, where complexes appeared to be larger and longer-lived. In contrast, regularly spaced large foci formed shortly before sporulation septation in aerial hyphae, and they disappeared after septation had been completed. Arrays of ParB foci in aerial hyphae were necessary for efficient DNA segregation into spores. Consistent with a role of ParB during sporulation, one of two parAB promoters is strongly expressed at the time immediately preceding sporulation (29). Here, we analyze transcriptional regulation of the parAB operon, its dependence on sporulation signals, and its effect on the formation of the ParB complexes.
MATERIALS AND METHODS
DNA manipulation and bacterial growth conditions.
DNA manipulations were carried out by standard protocols (45). Enzymes were supplied by Roche or New England BioLabs, isotopes were from Amersham-Pharmacia Biotech, and oligonucleotides were from Invitrogen. The S. coelicolor and Escherichia coli strains are listed in Table 1. Culture conditions, antibiotic concentrations, and transformation and conjugation methods followed general procedures for E. coli (45) and Streptomyces (28). S. coelicolor strains were cultivated in tryptone soy broth-yeast extract-malt extract (1:1) complex liquid medium or on mannitol soy flour (MS) agar plates unless otherwise stated.
TABLE 1.
Strains used in this study
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | supE44 ΔlacU169(φ80lacZΔM15)hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Lab stock |
| BW25113/pIJ790 | K-12 derivative: ΔaraBAD ΔrhaBAD/λ-Red(gam bet exo) cat araC rep101(Ts) | 20 |
| ET12567/pUZ8002 | dam-13::Tn9 dcm cat tet hsd zjj-201::Tn10/tra neo RP4 | 41 |
| S. coelicolor strains | ||
| M145 | SCP1− SCP2− | 5 |
| J2538 | M145 parB::apra | 29 |
| J2401 | M145 whiA::hyg | 15 |
| J2402 | M145 whiB::hyg | 15 |
| C70 | A3(2) whiB70 | 8 |
| J2450 | M145 whiI::hyg | 1 |
| J2210 | M145 whiH::hyg | 15 |
| J2418 | M145 ΔftsZ::aphI attBC31::pKF33[ftsZΔp2] | 16 |
| J3310 | M145 parB-egfp | 24 |
| J3311 | M145 whiA::hyg parB-egfp-apra | This study |
| J3312 | M145 whiB::hyg parB-egfp-apra | This study |
| J3313 | M145 whiI::hyg parB-egfp-apra | This study |
| J3314 | M145 whiH::hyg parB-egfp-apra | This study |
| J3315 | M145 ΔftsZ::aphI attBC31::pKF33[ftsZΔp2] parB-egf-apra | This study |
| J3325 | M145 parABΔp1 parB-egfp | This study |
| J3326 | M145 parABΔp2 parB-egfp | This study |
Construction of strains carrying EGFP fusion proteins.
S. coelicolor mutants expressing ParB-EGFP in different genetic backgrounds were constructed by introducing parB-egfp into the parB chromosomal locus of different strains. The previously described cosmid H24 parB-egfp-apra (24) was used to transform ET12567/pUZ8002, from which it was mobilized into whi mutant derivatives of M145 by conjugation. Cosmid H24 parB-egfp kan::vio-oriT (24) was used to construct parAB promoter mutants. To obtain promoter mutants, first the parAB promoter region was replaced by the apra cassette amplified with oligonucleotides pprom-apra-fw and pprom-apra-rv flanked by unique SwaI restriction sites. Subsequently, H24 parABp::apra parB-egfp kan::vio-oriT was linearized with SwaI and used for coelectroporation of arabinose-induced BW25113/pIJ790 with PCR products encompassing the promoter region containing the desired mutations (obtained using the oligonucleotides for mutation sites, pΔ1p-fw/pΔ1p-rv and pΔ2p-fw/pΔ2p-rv, and outside primers pprom-fw/pprom-rv). Apras transformants were screened for the promoter mutations by restriction digestion of the PCR product, and clones verified by sequencing were used for conjugation into S. coelicolor strain J2538. Chromosomal DNA of all strains constructed was checked by PCR and sequencing. Cell extracts were checked by phosphorimager scanning after sodium dodecyl sulfate-polyacrylamide gel electrophoresis and by Western blotting using polyclonal antibodies against ParB protein, as described previously (24). Promoter mutations were verified by transcriptional analysis of the obtained strains.
Microscopy.
Strains for microscopic observations were inoculated in the acute-angled junction of coverslips inserted at 45° in MM agar containing 1% mannitol (28). Staining procedures were as described previously (46). Briefly, mycelium was fixed for 10 min with paraformaldehyde-glutaraldehyde mixture, digested for 2 min with 1 mg/ml lysozyme, and incubated for 1 h with 10 μg ml−1 wheat germ agglutinin-tetramethylrhodamine (WGA) conjugate (Molecular Probes) for cell wall visualization. After five washes with phosphate-buffered saline, coverslips were mounted in Slow-Fade (Molecular Probes) antifade reagent. Confocal laser scanning microscopy was carried out using a Leica SP2 microscope, equipped with a 63× objective and 488- and 543-nm lasers. Images in TIFF format were analyzed using Leica-Lite software (version 2.0; Heidelberg Microsystems).
RNA preparation and S1 nuclease protection assays.
For total RNA preparation, cultures were grown on cellophane membranes on MM agar containing 1% mannitol and were harvested at different time points as described previously (1). S1 nuclease protection assays were performed using 30 μg of RNA as described by Kelemen et al. (26). Probes were generated by amplification of the promoter region with the pairs of oligonucleotides listed in Table 2.
TABLE 2.
Oligonucleotides used in this study
| Namea | Sequenceb | Application |
|---|---|---|
| pprom-apra-fw | CACGCATGCCGGAGTGTCGCGGCAGTTCGGCATCAGCGGCATTTAAAT GGAACTTCATGAGCTCAGCC | Insert SwaI restriction site in parAB promoter |
| pprom-apra-rv | CAGCACGACCGATGCGCGTGTCGTCCATCGGAGGCGGTGTATTTAAAT AGCTCCATCAGCAAAAGGGG | |
| pΔ1p-fw | CCAGAGGCATGGGAGGGGCCGGCCCCTGCGAGCCTGAAGTCG | Mutation of parABp1 |
| pΔ1p-rv | CGACTTCAGGCTCGCAGGGGCCGGCCCCTCCCATGCCTCTGG | |
| pΔ2p-fw | GTTCGGCATCAGCGGCGGCCGGCCCCGTTTCACGTGAAACGTCGC | Mutation of parABp2 |
| pΔ2p-rv | GCGACGTTTCACGTGAAACGGGGCCGGCCGCCGCTGATGCCGAAC | |
| pprom-fw | CGCAAGCTTTCCACACAAGCTGCCCTGCT | Amplification of parAB promoter |
| pprom-rv | CCGGATCCGACCCGGGTCTGCTCGGGTCGC | |
| pparABS1* | CATCGGAGGCGGTGTTTCACG | |
| phrdB1* | GCCATGACAGAGACGGACTCGGCG | Amplification of hrdB probe |
| phrdB2 | CGGCCGCAAGGTACGAGTTGATGA | |
| pFP180 | AATACCGCATCAGGCGCCATTCG | Amplification of whiA probe |
| pOWA7* | GCCAGCAGCTCCGGGTCGTG | (on pIJ6412 [2] template) |
| pwhiB2 | ATGGGCTTGGTTCCGCA | Amplification of whiB probe |
| pwhiB4* | CGAGTTCCTCGTCCGCGTCGTCG | |
| pwhiH7* | ACGGGTAGCGGTCGAGTTCGCCCGGGT | Amplification of whiH probe |
| pwhiH2 | GTCGTCGTACCGCTCGTACAG | |
| pOWI7 | GGGTCCGCACGTCCGGAGGA | Amplification of whiI probe |
| pOWI8* | GACGGTGGAACGGACGCGCG |
*, radiolabeled for S1 nuclease protection assay.
Boldface indicates mutated nucleotides, and italics indicate restriction sites.
RESULTS
Spatial separation of parABp1 and parABp2 promoter expression.
Previous work (29) showed that the two promoters of parAB have distinct temporal patterns of activity. To investigate if the promoters were also expressed in a spatially specific manner, we introduced mutations expected to interrupt the −10 region of parABp1 or parABp2 into strain J3310, previously engineered to produce the ParB-EGFP fusion. The strains obtained, J3325 (parABΔp1) and J3326 (parABΔp2), were subjected to microscopic analysis.
In J3326, ParB-EGFP formed foci in vegetative mycelium with the same distribution and intensity as in the parental J3310 strain (Fig. 1). On the other hand, only faint, diffused fluorescence was visible in the aerial hyphae of J3326, and arrays of bright foci were never observed. In addition, we analyzed sporulation-associated chromosome segregation in J3326. DNA staining of its spore chains showed that 13% of spores were anucleate, just as observed previously for the parB deletion mutant, confirming that the production of elevated levels of ParB-EGFP is vital for proper chromosome partitioning in aerial hyphae.
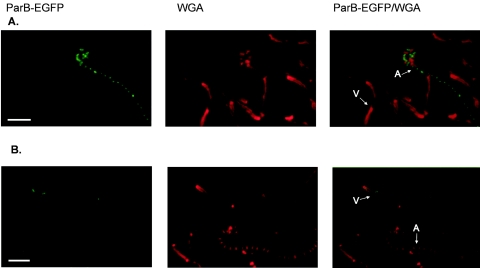
FIG. 1.
ParB-EGFP complex formation in parAB promoter mutant strains (J3325 and J3326). Sample images show typical distributions of ParB-EGFP foci in vegetative and aerial mycelium of the parABp1 (A) and parABp2 (B) mutants, respectively. Images show ParB-EGFP fluorescence, cell walls stained with WGA conjugate, and an overlay of the two fluorescence signals. V, examples of vegetative hyphae; A, examples of aerial hyphae. Scale bars, 5 μm.
J3325 had an exactly complementary phenotype (Fig. 1). Inactivation of parABp1 entirely abolished ParB-EGFP complex formation in vegetative mycelium but did not influence either the fluorescence intensity or the spacing of sporulation-associated complex formation in aerial hyphae (1.3 μm for 91 foci measured). Moreover, J3325 was no more defective in DNA segregation into spores (4% anucleate) than its parental strain, J3310. Thus, our results demonstrated that parABp1 and parABp2 promoter activities are distinct both temporally and spatially.
Sporulation-associated ParB-EGFP foci are absent or markedly reduced in the aerial mycelium of early-sporulation (whi) mutants.
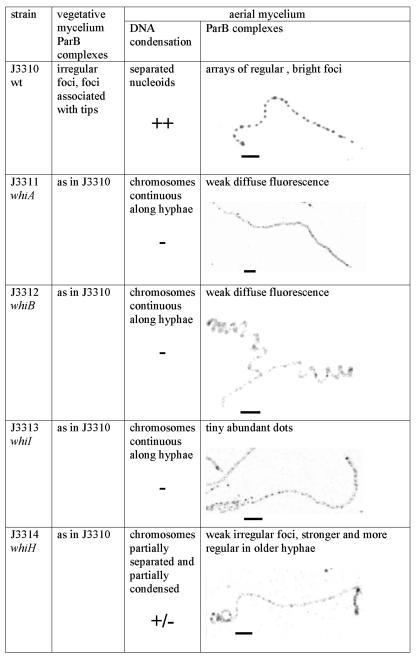
Since the formation of ParB complexes in aerial hyphae coincides with sporulation-associated DNA condensation and septation, we investigated the assembly of such complexes in nonsporulating mutants. An egfp-apra cassette was introduced downstream of parB in four early whi mutant strains, to give J3311 (whiA disruption), J3312 (whiB disruption), J3313 (whiI disruption), and J3314 (whiH disruption). Comparing ParB-EGFP fluorescence in these strains to that in wild-type strain J3310, we found wild-type complexes in the vegetative mycelium of the mutants but not in the aerial mycelium (Fig. 2). Fluorescence was very weak and diffuse in aerial hyphae of J3311 (whiA disruption) and J3312 (whiB disruption). The aerial hyphae of J3313 (ΔwhiI) displayed tiny, abundant fluorescent dots, but they were not regularly spaced and their signal intensity was about half of the intensity of the J3310 foci. Similarly, some irregularly spaced and rather weak foci (about three times weaker than the J3310 signals) could be distinguished in aerial mycelium of J3314 (whiH disruption), and in older cultures (more than 48 h) some hyphae could be found with brighter and more regular foci.
FIG. 2.
Comparison of DNA condensation and assembly of ParB complexes in the wild type (wt) and in whi mutants. Images show examples of EGFP fluorescence in aerial hyphae. Scale bars, 5 μm.
Efficient activation of the developmentally specific parABp2 promoter is dependent on whi gene products.
Clearly the efficient formation of sporulation-associated ParB-EGFP foci depended on all four whi genes. Two explanations might account for this: the whi genes might be necessary either for adequate levels of ParB or for the correct assembly of the complexes. We therefore investigated the activity of the parAB promoters in whiA, whiB, whiI, and whiH deletion mutants by using S1 nuclease protection analysis of RNA samples isolated at different time points from growing and differentiating surface cultures (Fig. 3). In all of these experiments, control S1 digestions were carried out with a probe for hrdB, whose expression is approximately constitutive (7) and which is often used as a combined semiquantitative standard and control of RNA quality.
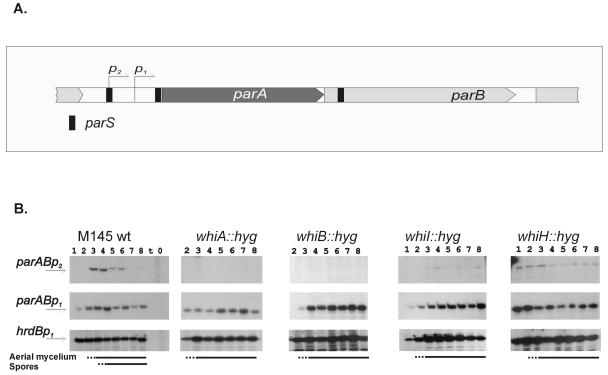
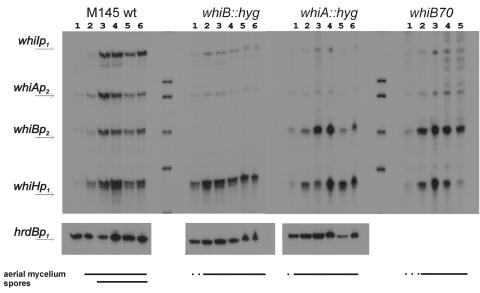
FIG. 3.
Transcriptional activity of the parAB promoter in whi mutants. (A) parAB promoter region. (B) S1 nuclease protection analysis of parAB transcripts in the wild-type strain and in whi gene disruption mutants. Total RNA was extracted from cultures growing on MS agar at the corresponding time points: 1, 18 h; 2, 24 h; 3, 36 h; 4, 48 h; 5, 60 h; 6, 72 h; 7, 84 h; 8, 96 h. A control reaction with yeast tRNA was included in the lane labeled “t.” hrdB is the control for a constitutively expressed gene. The lines at the bottom indicate time points at which aerial mycelium and spore chains were detected. Transcripts from parABp1 and parABp2 were detected with the same end-labeled probe and are therefore comparable.
The constitutive parABp1 promoter was not affected in the nonsporulating strains. However, parABp2 transcription, which in the wild-type M145 strain was strongly upregulated at the time of sporulation septation, was abolished in whiA and whiB deletion strains; in whiI and whiH mutants, its activity was detectable even at the earliest time point but stayed at a low and fairly unvarying level and, unlike the wild-type situation, was not switched off at later time points.
Thus, either the whi gene products themselves or a developmental signal(s) absent from the whi mutants is necessary for efficient activation of parABp2, and the consequent reduced level of ParB may be insufficient for the formation of the large number of complexes usually occurring during sporulation septation. Moreover, the eventual downregulation of parABp2 associated with spore maturation also appeared to be dependent on whiH and whiI.
Formation of regularly spaced ParB-EGFP complexes in aerial hyphae does not require sporulation septation.
Since all four whi mutants tested had severe defects in sporulation septation, the reduced parB expression and inefficient ParB-EGFP complex formation in their aerial hyphae might involve some kind of morphological coupling of parAB transcription to the initiation of sporulation septation. To investigate this further, we used a strain, J2418, that is deficient in sporulation-associated ftsZ expression. In S. coelicolor, ftsZp2 (one of three promoters) is upregulated before sporulation septation to provide enough protein for the efficient and synchronous formation of multiple Z rings (16). Strain J2418 contains an ftsZp2 promoter mutation that abolishes the effective sporulation-specific increase of ftsZ expression. The strain is therefore largely defective in sporulation septation but is expected to be unaffected in expression of whiA, whiB, whiH, and whiI.
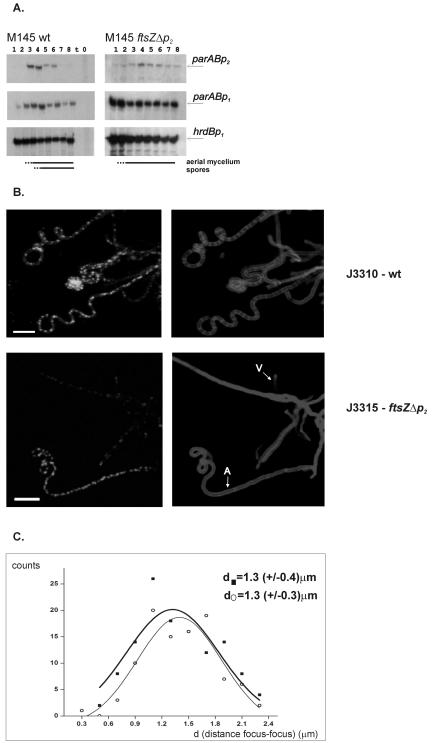
An S1 protection assay of parAB in J2418 showed that it was induced at the time corresponding to aerial hyphal maturation but that upregulation was less efficient than in the wild type (Fig. 4A). Moreover, a low-level signal was detected even at early time points, and there was not a complete shutdown in the later time points. We interpret the latter observation as indicating that sporulation septation itself participates in the signal cascade that results in the shutdown of parABp2. The early expression, which was also seen with whiI and whiH mutants, may be a consequence of inevitable differences in the inoculum consisting of aerial hyphal fragments from the inoculum of spores used for the wild type. Many previous analyses of expression of different genes in these mutants have shown similar early expression (e.g., in reference 1). We also used the ftsZp2 mutant to investigate whether sporulation septation played any role in the positioning of ParB foci. We constructed J3315, a J2418 (ftsZΔp2) derivative expressing the ParB-EGFP fusion protein, and compared ParB-EGFP fluorescence in J3315 and in the wild type, J3310. Bright ParB-EGFP foci were seen in some aerial hyphae of strain J3315 (Fig. 4B), with spacing (1.3 ± 0.4 μm) similar to that in the wild-type strain J3310 (1.3 ± 0.3 μm), although somewhat less regular (Fig. 4C). Notably, aerial hyphae containing ParB foci were much less frequently seen than in J3310. This may have been due either to their increased transience or to a more asynchronous appearance. Summarizing our microscopic and transcriptional analysis, ParB complex formation is not tightly dependent on septation. Circumstantial evidence indicates that septation is not tightly coupled to ParB complex formation either, since mutants disrupted in parB form abundant spore chains. However, the shutdown of parABp2 activity that usually accompanies spore maturation does appear to depend on a sporulation septation checkpoint.
FIG. 4.
Lack of dependence of ParB complex formation on sporulation septation. (A) S1 nuclease protection analysis of parAB transcripts in J2418. (B) Distribution of ParB-EGFP foci in aerial mycelium of the wild-type strain J3310 and an ftsZp2 deletion mutant (J3315) deficient in septum formation. Images show ParB-EGFP fluorescence (left) and cell walls stained with WGA conjugate (right). V, example of vegetative hyphae; A, example of aerial hyphae. Scale bar, 5 μm. (C) Gaussian distribution of distances measured between ParB-EGFP complexes in arrays of ParB-EGFP foci in the aerial hyphae of strains J3310 (wild type) (○) and J3315 (▪).
Regulatory preamble to the activation of parABp2.
To investigate further the nature of the strong dependence of parABp2 on the whiA and whiB genes, we carried out surveys of the transcriptional interdependence of these two genes on each other and of their interplay with the whiH and whiI genes (areas that have not been investigated in previous studies of whi gene expression). The cultures studied were M145 and its whiA and whiB disruption mutants (15), together with a nonisogenic whiB point mutant (whiB70) (8). The latter strain was used in order to extend the results with the whiB::hyg mutant, in which the absence of the entire whiB gene made it impossible to evaluate the activity of the developmentally regulated whiB promoter.
In a first experiment, RNA samples from the M145 control strain were hybridized to mixed probes for the developmentally regulated promoters of all four whi genes, in order to facilitate comparisons of their time courses of expression. In each case, there was a weak signal at the earliest time points, sharply increasing in strength at the time point at which spore formation became obvious (though a slight increase in all four, and particularly whiH, was also detected in the preceding sample, corresponding to the first emergence of aerial mycelium) (Fig. 5).
FIG. 5.
Transcriptional analysis of whi genes in whiA and whiB mutants. Shown are S1 nuclease protection analyses of whi transcripts in the wild-type strain and in whi gene disruption mutants. Total RNA was extracted from cultures growing on MS agar at the corresponding time points: 1, 24 h; 2, 36 h; 3, 48 h; 4, 72 h; 5, 96 h; 6, 120 h. The lines at the bottom indicate time points at which aerial mycelium and spore chains were detected.
In the whiA mutant, expression of the sporulation-associated whiAp2 promoter was reduced to a constitutive low level, as if the WhiA protein were needed in a positive feedback circuit for the upshift associated with sporulation. On the other hand, sporulation-associated whiBp2 transcription was somewhat increased, although still developmentally regulated, suggesting that WhiA is involved in negative regulation of whiB. In both whiB mutants, whiAp2 expression was low and constitutive, indicating that WhiB contributes in some way to whiA autoinduction. Judging by the use of whiB70 RNA, whiB seems to be slightly overexpressed in a whiB mutant, as if WhiB contributed to its own repression (note that overexpression of whiB in the whiB70 mutant and in a whiA point mutant was also reported in an early study, but only one time point was examined in that work [46]). Interestingly, both whiB and whiA were needed for the sporulation-associated upshift in whiI expression but not for that of whiH.
DISCUSSION
Previously it was shown that parB is required for proper chromosome segregation during sporulation, a finding reinforced by strong induction of the parABp2 promoter at the time of sporulation (29). The use of a ParB-EGFP fusion revealed that ParB forms nucleoprotein complexes over the oriC region of the chromosome that behave differently in S. coelicolor vegetative and aerial hyphae (24). In aerial hyphae, the complexes assist DNA partitioning at the time of sporulation. Here, we have related formation of the ParB complexes to transcriptional activity of the two parAB promoters in the wild type and in different developmental mutants.
Formation of ParB complexes during vegetative growth and sporulation depends on the differential activities of two parAB promoters.
The provision of ParB at levels appropriate for the assembly of the complexes observed in different hyphal types is associated temporally with the differentially expressed activities of two promoters for the parAB operon. The natural constitutive level of parABp1 transcription is required and sufficient for normal complex formation in vegetative mycelium, while the induction of parABp2 transcription is necessary and sufficient both for presporulation complex formation in aerial hyphae and for proper partitioning of chromosomes into prespore compartments. Although parABp1 is not needed for complex formation during sporulation and therefore is dispensable for DNA segregation into spores, we cannot exclude that it may be active in aerial hyphae. Several other Streptomyces promoters have been shown to be subject to temporal and spatial regulation during colony development. For example, sigF (43) and sigHp2 (27) activities are restricted to sporulating aerial hyphae, and redD is active only in substrate hyphae (50). Circumstantial evidence points to more such cases, but the case of the promoter region of ftsZ is particularly similar to that of parAB (16). In in vitro transcription experiments with RNA polymerase purified from S. coelicolor, the transcript from parABp2 was absent while the transcript from parABp1 was present (L. Servin-Gonzalez and D. Jakimowicz, unpublished data), suggesting the requirement for an activator to transcribe the parABp2 promoter. However, it is still possible that signals necessary for parABp2 induction in aerial hyphae of sporulating strains may operate by relieving repression rather than by direct transcriptional activation.
parABp2 transcription is controlled by the developmental regulatory network that coordinates sporulation.
parABp1 is constitutively expressed, and its activity was not changed in four nonsporulating mutants (whiA, whiB, whiH, and whiI). Consistent with this, the formation of ParB-EGFP foci in vegetative hyphae of all these mutants was no different from that of the wild-type strain. In contrast, parABp2 showed clear evidence of developmental control, which correlated well with the degree of impairment in the formation of sporulation-associated ParB-EGFP foci in the whi mutants and with the sporulation-associated chromosome partitioning defect of a parABp2 mutant.
The complete absence of p2 expression and the presumably resultant absence of presporulation ParB foci in the whiA and whiB mutants is particularly striking and strongly suggests that WhiA and/or WhiB may be directly implicated in controlling parABp2 transcription. Low levels of WhiA and WhiB are probably present in nondifferentiating mycelium, in view of the activity of additional, apparently constitutive promoters for the corresponding genes (2, 49); however, either because WhiA and WhiB are present at insufficient levels or because additional regulatory factors are involved, parABp2 is not activated.
The ability of WhiA and WhiB to bring about the shutdown of aerial growth before sporulation septation (15) may be mediated partly via an effect of ParB complexes on preventing the initiation of further rounds of replication, but this cannot be the whole story, since sporulation was not significantly impaired in a parB mutant (apart from the reduced regularity of DNA partitioning).
As the aerial mycelium matures, there are much higher levels of expression of whiA and whiB, while other possible accessory developmental regulators of parABp2, such as WhiH and WhiI, are also more abundant, permitting strong parABp2 expression. We found that mutations in whiH or whiI diminish parABp2 activity, keeping it at a fairly unvarying low level, possibly reflecting some kind of modulating role for WhiH and WhiI. This could involve a direct effect of WhiI and WhiH on parABp2 promoter activity or be more indirect, perhaps involving morphological or physiological checkpoints. Such checkpoints would have to precede chromosome condensation, since ParB foci form before DNA condensation takes place (24).
parABp2 transcription and ParB complex formation are not tightly coupled with sporulation septation.
Sporulation septation is probably not the key route through which parABp2 is activated, since promoter activity was only slightly impaired, if at all, in a mutant largely lacking the sporulation septa because of a mutation in the sporulation-specific ftsZp2 promoter. parABp2 activity in the ftsZp2-deficient mutant was high enough to provide sufficient protein for the formation of ParB complexes in aerial hyphae. This contrasts with a report that in B. subtilis multinucleate filamentous cells depleted of FtsZ, Spo0J complexes were scattered (35). Thus, S. coelicolor is exceptional not only in its ability to survive deletion of ftsZ (36) but also in its loose coupling of DNA segregation to sporulation septation. A dependence of positions of ParB foci on the positioning of FtsZ rings was not entirely ruled out, but the spacing of foci was only marginally less regular in the sporulation septation-deficient ftsZp2 promoter mutant. It remains to be examined whether there is any dependence of the positioning of FtsZ rings on the ParB-oriC complex, but we note that the size of prespore compartments seemed to be more variable in parB mutants, either null (29) or defective in the putative DNA binding region of ParB (24). What is unambiguous is that during sporulation, neither ParB focus formation nor FtsZ ring formation per se depends on the other process.
Regulatory network controlling parABp2.
The finding that sporulation-specific parAB expression is completely dependent on whiA and whiB, with some degree of dependence on whiI, led us to analyze the effects of whiA and whiB mutations on sporulation regulatory genes. In the time courses examined, even though we evaluated several genes in a single reaction tube, we could not clearly distinguish any differences in the time of onset of transcription. However, the time intervals between samples were large (12 h) in relation to the overall time for aerial growth and sporulation to be accomplished (probably less than 20 h), so it remains possible that there are real differences in expression kinetics between the genes.
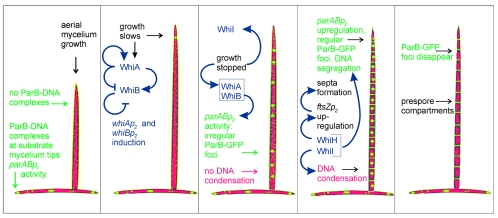
The developmentally associated increased expression of whiA, whiB, and whiI—and therefore, presumably, the increased abundance of WhiA, WhiB, and WhiI—appears to result from the regulatory interactions of these proteins and the corresponding genes. We propose the following working model, which extends earlier models (e.g., reference 11) (Fig. 6). WhiB, now shown to be a redox-sensitive protein containing a 4Fe-4S cluster (25), is an autorepressor in its reduced state and an activator of whiA, while WhiA is an autoactivator and a repressor of whiB (conceivably, WhiA and WhiB may actually interact at both promoters). Thus, whiA and whiB mutants overtranscribe whiBp2 and undertranscribe whiAp2. When an aerial hypha stops extending, a transient redox shock associated with the sudden change in physiology oxidizes WhiB, eliminating its autorepressing activity and increasing WhiB levels significantly. The increased WhiB levels contribute to the activation of whiAp2 and thus initiate the accumulation of WhiA. The increasing amount of WhiA further stimulates whiAp2 expression, and WhiA builds up to levels high enough to influence the regulation of other genes, including parB (parABp2 promoter).
FIG. 6.
Model of parAB promoter expression during S. coelicolor development in relation to the regulatory network of whi genes, assembly of ParB complexes, DNA partitioning, and sporulation. Blue arrows indicate whi gene autoregulation and their regulation of sporulation processes. Color coding is as follows: red, DNA; green, ParB; yellow, cell wall.
It was also noticeable that whiI expression was reduced in whiA and whiB mutants. Since WhiI is a response regulator-like protein (albeit somewhat atypical) (1), it is likely to change its activity during development in response to a signal. We postulate that this signal may not be sufficiently strong in whiA and whiB mutants, so any effects of WhiI that depend on the signal may not be manifested. One of these effects, it appears, is relief from autorepression; thus, the low-level expression of whiI in whiA and whiB mutants indicated by our present data would be a prediction of this model. Low-level expression of whiI might itself be expected to have developmental consequences, including effects on sporulation-associated DNA condensation (1).
Conclusions.
In summary, we have shown that formation of two different types of ParB complexes in vegetative and aerial hyphae depends on the differential activity of two parAB promoters, one of which is dependent on several sporulation regulatory genes. Further studies are necessary to find the signals that regulate ParB complex formation. In addition to control at the level of gene expression, other factors/checkpoints may influence the assembly of the complex, perhaps associated with proper growth cessation. It is also plausible that interaction of ParB with some cellular component present only in aerial hyphae is a prerequisite for proper complex assembly and/or localization. Such a component(s) could itself depend on whi gene products.
Acknowledgments
We thank Klas Flardh for providing strain J2418, Luis Servin-Gonzales for help with the in vitro transcription assay, and Bertolt Gust for helpful advice.
D.J. was supported by a Marie Curie Fellowship of the European Community program Human Potential, under contract HPMF-CT-2002-01676, and by Marie Curie Reintegration Grant MERG-6-CT-2005-014851, and S.M. was supported by grant 208/P10321 from the Biotechnological and Biological Research Council.
REFERENCES
- 1.Ainsa, J. A., H. D. Parry, and K. F. Chater. 1999. A response regulator-like protein that functions at an intermediate stage of sporulation in Streptomyces coelicolor A3(2). Mol. Microbiol. 34:607-619. [DOI] [PubMed] [Google Scholar]
- 2.Ainsa, J. A., N. J. Ryding, N. Hartley, K. C. Findlay, C. J. Bruton, and K. F. Chater. 2000. WhiA, a protein of unknown function conserved among gram-positive bacteria, is essential for sporulation in Streptomyces coelicolor A3(2). J. Bacteriol. 182:5470-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartosik, A. A., and G. Jagura-Burdzy. 2005. Bacterial chromosome segregation. Acta Biochim. Pol. 52:1-34. [PubMed] [Google Scholar]
- 4.Bartosik, A. A., K. Lasocki, J. Mierzejewska, C. M. Thomas, and G. Jagura-Burdzy. 2004. ParB of Pseudomonas aeruginosa: interactions with its partner ParA and its target parS and specific effects on bacterial growth. J. Bacteriol. 186:6983-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 6.Bignell, C., and C. M. Thomas. 2001. The bacterial ParA-ParB partitioning proteins. J. Biotechnol. 91:1-34. [DOI] [PubMed] [Google Scholar]
- 7.Buttner, M. J., K. F. Chater, and M. J. Bibb. 1990. Cloning, disruption, and transcriptional analysis of three RNA polymerase sigma factor genes of Streptomyces coelicolor A3(2). J. Bacteriol. 172:3367-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chater, K. F. 1972. A morphological and genetic mapping study of white colony mutants of Streptomyces coelicolor. J. Gen. Microbiol. 72:9-28. [DOI] [PubMed] [Google Scholar]
- 9.Chater, K. F. 1993. Genetics of differentiation in Streptomyces. Annu. Rev. Microbiol. 47:685-713. [DOI] [PubMed] [Google Scholar]
- 10.Chater, K. F. 1998. Taking a genetic scalpel to the Streptomyces colony. Microbiology 144:1465-1478. [DOI] [PubMed] [Google Scholar]
- 11.Chater, K. F. 2001. Regulation of sporulation in Streptomyces coelicolor A3(2): a checkpoint multiplex? Curr. Opin. Microbiol. 4:667-673. [DOI] [PubMed] [Google Scholar]
- 12.Chater, K. F., C. J. Bruton, K. A. Plaskitt, M. J. Buttner, C. Mendez, and J. D. Helmann. 1989. The developmental fate of S. coelicolor hyphae depends upon a gene product homologous with the motility sigma factor of B. subtilis. Cell 59:133-143. [DOI] [PubMed] [Google Scholar]
- 13.Davis, N. K., and K. F. Chater. 1992. The Streptomyces coelicolor whiB gene encodes a small transcription factor-like protein dispensable for growth but essential for sporulation. Mol. Gen. Genet. 232:351-358. [DOI] [PubMed] [Google Scholar]
- 14.Flardh, K. 2003. Growth polarity and cell division in Streptomyces. Curr. Opin. Microbiol. 6:564-571. [DOI] [PubMed] [Google Scholar]
- 15.Flardh, K., K. C. Findlay, and K. F. Chater. 1999. Association of early sporulation genes with suggested developmental decision points in Streptomyces coelicolor A3(2). Microbiology 145:2229-2243. [DOI] [PubMed] [Google Scholar]
- 16.Flardh, K., E. Leibovitz, M. J. Buttner, and K. F. Chater. 2000. Generation of a non-sporulating strain of Streptomyces coelicolor A3(2) by the manipulation of a developmentally controlled ftsZ promoter. Mol. Microbiol. 38:737-749. [DOI] [PubMed] [Google Scholar]
- 17.Gerdes, K., J. Moller-Jensen, and R. Bugge Jensen. 2000. Plasmid and chromosome partitioning: surprises from phylogeny. Mol. Microbiol. 37:455-466. [DOI] [PubMed] [Google Scholar]
- 18.Gerdes, K., J. Moller-Jensen, G. Ebersbach, T. Kruse, and K. Nordstrom. 2004. Bacterial mitotic machineries. Cell 116:359-366. [DOI] [PubMed] [Google Scholar]
- 19.Glaser, P., M. E. Sharpe, B. Raether, M. Perego, K. Ohlsen, and J. Errington. 1997. Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 11:1160-1168. [DOI] [PubMed] [Google Scholar]
- 20.Gust, B., G. L. Challis, K. Fowler, T. Kieser, and K. F. Chater. 2003. PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. USA 100:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopwood, D. A., K. F. Chater, and M. J. Bibb. 1995. Genetics of antibiotic production in Streptomyces coelicolor A3(2), a model streptomycete. Biotechnology 28:65-102. [DOI] [PubMed] [Google Scholar]
- 22.Ireton, K., N. W. Gunther, and A. D. Grossman. 1994. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 176:5320-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakimowicz, D., K. Chater, and J. Zakrzewska-Czerwinska. 2002. The ParB protein of Streptomyces coelicolor A3(2) recognizes a cluster of parS sequences within the origin-proximal region of the linear chromosome. Mol. Microbiol. 45:1365-1377. [DOI] [PubMed] [Google Scholar]
- 24.Jakimowicz, D., B. Gust, J. Zakrzewska-Czerwinska, and K. F. Chater. 2005. Developmental-stage-specific assembly of ParB complexes in Streptomyces coelicolor hyphae. J. Bacteriol. 187:3572-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakimowicz, P., M. R. Cheesman, W. R. Bishai, K. F. Chater, A. J. Thomson, and M. J. Buttner. 2005. Evidence that the Streptomyces developmental protein WhiD, a member of the WhiB family, binds a [4Fe-4S] cluster. J. Biol. Chem. 280:8309-8315. [DOI] [PubMed] [Google Scholar]
- 26.Kelemen, G. H., G. L. Brown, J. Kormanec, L. Potuckova, K. F. Chater, and M. J. Buttner. 1996. The positions of the sigma-factor genes, whiG and sigF, in the hierarchy controlling the development of spore chains in the aerial hyphae of Streptomyces coelicolor A3(2). Mol. Microbiol. 21:593-603. [DOI] [PubMed] [Google Scholar]
- 27.Kelemen, G. H., P. H. Viollier, J. Tenor, L. Marri, M. J. Buttner, and C. J. Thompson. 2001. A connection between stress and development in the multicellular prokaryote Streptomyces coelicolor A3(2). Mol. Microbiol. 40:804-814. [DOI] [PubMed] [Google Scholar]
- 28.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, Conn.
- 29.Kim, H. J., M. J. Calcutt, F. J. Schmidt, and K. F. Chater. 2000. Partitioning of the linear chromosome during sporulation of Streptomyces coelicolor A3(2) involves an oriC-linked parAB locus. J. Bacteriol. 182:1313-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonard, T. A., J. Moller-Jensen, and J. Lowe. 2005. Towards understanding the molecular basis of bacterial DNA segregation. Philos. Trans. R. Soc. Lond. B 360:523-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis, P. J., and J. Errington. 1997. Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localization with the SpoOJ partitioning protein. Mol. Microbiol. 25:945-954. [DOI] [PubMed] [Google Scholar]
- 32.Lewis, R. A., C. R. Bignell, W. Zeng, A. C. Jones, and C. M. Thomas. 2002. Chromosome loss from par mutants of Pseudomonas putida depends on growth medium and phase of growth. Microbiology 148:537-548. [DOI] [PubMed] [Google Scholar]
- 33.Lin, D. C., and A. D. Grossman. 1998. Identification and characterization of a bacterial chromosome partitioning site. Cell 92:675-685. [DOI] [PubMed] [Google Scholar]
- 34.Lin, D. C., P. A. Levin, and A. D. Grossman. 1997. Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 94:4721-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marston, A. L., and J. Errington. 1999. Dynamic movement of the ParA-like Soj protein of B. subtilis and its dual role in nucleoid organization and developmental regulation. Mol. Cell 4:673-682. [DOI] [PubMed] [Google Scholar]
- 36.McCormick, J. R., E. P. Su, A. Driks, and R. Losick. 1994. Growth and viability of Streptomyces coelicolor mutant for the cell division gene ftsZ. Mol. Microbiol. 14:243-254. [DOI] [PubMed] [Google Scholar]
- 37.Mohl, D. A., J. Easter, Jr., and J. W. Gober. 2001. The chromosome partitioning protein, ParB, is required for cytokinesis in Caulobacter crescentus. Mol. Microbiol. 42:741-755. [DOI] [PubMed] [Google Scholar]
- 38.Mohl, D. A., and J. W. Gober. 1997. Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell 88:675-684. [DOI] [PubMed] [Google Scholar]
- 39.Ogasawara, N., and H. Yoshikawa. 1992. Genes and their organization in the replication origin region of the bacterial chromosome. Mol. Microbiol. 6:629-634. [DOI] [PubMed] [Google Scholar]
- 40.Omura, S., H. Ikeda, J. Ishikawa, A. Hanamoto, C. Takahashi, M. Shinose, Y. Takahashi, H. Horikawa, H. Nakazawa, T. Osonoe, H. Kikuchi, T. Shiba, Y. Sakaki, and M. Hattori. 2001. Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc. Natl. Acad. Sci. USA 98:12215-12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paget, M. S., L. Chamberlin, A. Atrih, S. J. Foster, and M. J. Buttner. 1999. Evidence that the extracytoplasmic function sigma factor σE is required for normal cell wall structure in Streptomyces coelicolor A3(2). J. Bacteriol. 181:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pogliano, K., J. Pogliano, and E. Becker. 2003. Chromosome segregation in Eubacteria. Curr. Opin. Microbiol. 6:586-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potuckova, L., G. H. Kelemen, K. C. Findlay, M. A. Lonetto, M. J. Buttner, and J. Kormanec. 1995. A new RNA polymerase sigma factor, sigma F, is required for the late stages of morphological differentiation in Streptomyces spp. Mol. Microbiol. 17:37-48. [DOI] [PubMed] [Google Scholar]
- 44.Ryding, N. J., G. H. Kelemen, C. A. Whatling, K. Flardh, M. J. Buttner, and K. F. Chater. 1998. A developmentally regulated gene encoding a repressor-like protein is essential for sporulation in Streptomyces coelicolor A3(2). Mol. Microbiol. 29:343-357. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 46.Schwedock, J., J. R. McCormick, E. R. Angert, J. R. Nodwell, and R. Losick. 1997. Assembly of the cell division protein FtsZ into ladder-like structures in the aerial hyphae of Streptomyces coelicolor. Mol. Microbiol. 25:847-858. [DOI] [PubMed] [Google Scholar]
- 47.Sharpe, M. E., and J. Errington. 1996. The Bacillus subtilis soj-spo0J locus is required for a centromere-like function involved in prespore chromosome partitioning. Mol. Microbiol. 21:501-509. [DOI] [PubMed] [Google Scholar]
- 48.Sherratt, D. J. 2003. Bacterial chromosome dynamics. Science 301:780-785. [DOI] [PubMed] [Google Scholar]
- 49.Soliveri, J., K. L. Brown, M. J. Buttner, and K. F. Chater. 1992. Two promoters for the whiB sporulation gene of Streptomyces coelicolor A3(2) and their activities in relation to development. J. Bacteriol. 174:6215-6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun, J., G. H. Kelemen, J. M. Fernandez-Abalos, and M. J. Bibb. 1999. Green fluorescent protein as a reporter for spatial and temporal gene expression in Streptomyces coelicolor A3(2). Microbiology 145:2221-2227. [DOI] [PubMed] [Google Scholar]
- 51.Webb, C. D., A. Teleman, S. Gordon, A. Straight, A. Belmont, D. C. Lin, A. D. Grossman, A. Wright, and R. Losick. 1997. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell 88:667-674. [DOI] [PubMed] [Google Scholar]
- 52.Wu, L. J. 2004. Structure and segregation of the bacterial nucleoid. Curr. Opin. Genet. Dev. 14:126-132. [DOI] [PubMed] [Google Scholar]