Abstract
Multimodular penicillin-binding proteins (PBPs) are essential enzymes responsible for bacterial cell wall peptidoglycan (PG) assembly. Their glycosyltransferase activity catalyzes glycan chain elongation from lipid II substrate (undecaprenyl-pyrophosphoryl-N-acetylglucosamine-N-acetylmuramic acid-pentapeptide), and their transpeptidase activity catalyzes cross-linking between peptides carried by two adjacent glycan chains. Listeria monocytogenes is a food-borne pathogen which exerts its virulence through secreted and cell wall PG-associated virulence factors. This bacterium has five PBPs, including two bifunctional glycosyltransferase/transpeptidase class A PBPs, namely, PBP1 and PBP4. We have expressed and purified the latter and have shown that it binds penicillin and catalyzes in vitro glycan chain polymerization with an efficiency of 1,400 M−1 s−1 from Escherichia coli lipid II substrate. PBP4 also catalyzes the aminolysis (d-Ala as acceptor) and hydrolysis of the thiolester donor substrate benzoyl-Gly-thioglycolate, indicating that PBP4 possesses both transpeptidase and carboxypeptidase activities. Disruption of the gene lmo2229 encoding PBP4 in L. monocytogenes EGD did not have any significant effect on growth rate, peptidoglycan composition, cell morphology, or sensitivity to β-lactam antibiotics but did increase the resistance of the mutant to moenomycin.
Listeria monocytogenes is a food-borne pathogen whose ingestion causes listeriosis in patients with weakened immune systems (6, 9). It is sensitive to penicillins, but it has relatively high natural resistance to cephalosporins and fosfomycin (16). Multiresistant L. monocytogenes strains have been isolated from human patients, presumably resulting from the acquisition of plasmids and transposons carrying antibiotic resistance genes from Enterococcus and Streptococcus (6, 27, 31). Therefore, under selection pressure it may not be very long before this pathogen develops resistance to other antibiotics, including penicillins (6).
Peptidoglycan (PG) is the major constituent of the bacterial cell envelope (32). Its strength enables bacteria to withstand the high osmotic pressure within the cells. PG assembly is carried out by multimodular penicillin-binding proteins (PBPs) (11) that belong to either class A or class B, depending on the structure and the catalytic activity of their N-terminal module (11). The C-terminal penicillin-binding module of both classes has transpeptidase (TP) activity catalyzing peptide cross-linking between two adjacent glycan chains (11, 32). In class A PBPs, the N-terminal module is responsible for the glycosyltransferase (GT) activity leading to glycan chain elongation (30), whereas in class B PBPs the N-terminal domain is involved in interactions with other proteins during cell morphogenesis (21).
L. monocytogenes has five PBPs (34), including two of class A (PBP1 and PBP4), two of class B (PBP2 and PBP3), and one that is monofunctional (PBP5) (19). PBP3 was proposed to be the lethal target for β-lactams in L. monocytogenes (34), whereas increased expression of PBP4 together with a histidine kinase was associated with resistance to nisin (12, 13), an antibacterial peptide which exerts its action by forming pores in the cytoplasmic membrane through interaction with the PG precursor lipid II (4). Although hundreds of proteins with GT-TP architecture have been identified, very few of them have been isolated and only a few of their properties have been characterized so far (3, 8, 28, 30). Escherichia coli PBP1b is the only class A PBP for which both GT and TP activities have been clearly demonstrated in vitro with lipid II, the undecaprenyl-pyrophosphoryl-N-acetylglucosamine-N-acetylmuramic acid-pentapeptide, as a substrate (23, 30). E. coli PBP1b has the highest GT activity reported for any GT enzyme, with efficiencies of 39,000 M−1 s−1 (30) and 250,000 M−1 s−1 (28) in the presence of Mg2+ or Ca2+, respectively. The transpeptidase activity of PBP1b was demonstrated with lipid II substrate after its polymerization into peptidoglycan followed by high-performance liquid chromatography (HPLC) analysis of lysozyme hydrolysis products (30). This method requires large amounts of lipid II substrate, which is the limiting factor in the study of the GT enzymes. Using a simpler transpeptidase assay based on thiolesters as donor substrates and d-Ala as the acceptor (1), PBP1b catalyzes the transfer of the acyl moiety of these substrates on the amino group of the acceptor nucleophile (30).
In this work, we present the enzymatic properties of PBP4 from the pathogenic bacterium L. monocytogenes. The GT and TP activities were demonstrated in vitro using E. coli lipid II and the thiolester substrate analogue benzoyl-Gly-thiolactate in the presence of d-Ala, respectively. An L. monocytogenes EGD lmo2229 mutant was constructed by allelic replacement with an erythromycin resistance determinant, and the effect of the mutation on bacterial growth, sensitivity to antibiotics, PG composition, and cell morphology was examined.
MATERIALS AND METHODS
Substrates and antibiotics.
[14C]benzylpenicillin (54 nCi nmol−1) was from Amersham International (Buckinghamshire, United Kingdom). Fluoresceyl ampicillin was prepared as previously described (20). Unlabeled β-lactam antibiotics were from Sigma. Benzoyl-Gly-thiolactate (Bz-Gly-Thl), benzoyl-Gly-thiolglycolate (Bz-Gly-Thg), benzoyl-d-Ala-thiolglycolate (Bz-d-Ala-Thg), and Ac2-l-Lys-d-Ala-d-Ala, were described previously (1). The meso-[14C]diaminopimelic acid (A2pm)-labeled C55 lipid II intermediate [N-acetyl glucosaminyl-N-acetylmuramoyl (l-Ala-γ-d-Glu-(l)-meso-A2pm-(l)-d-Ala-d-Ala)-pyrophosphate-undecaprenol] was prepared essentially as described previously (33). Moenomycin was a gift from Aventis (Romainville, France). Vancomycin and nisin were purchased from Sigma-Aldrich.
Plasmid construction.
Escherichia coli XL1-Blue (Stratagene) was used for the construction of the plasmids. The lmo2229 gene encoding the penicillin-binding protein 4 (PBP4) (UniProt accession no. Q8Y547) was amplified by PCR from chromosomal DNA of L. monocytogenes EGD using the primers 5′-CGCCGCATGCTTATGGACAAATTCAAACAG-3′ and 5′-CGCGGATCCTTGATTACCTATCGAATC-3′ containing SphI and BamHI sites (underlined), respectively. The amplified SphI-lmo2229-BamHI fragment was then inserted into pQE-70 (QIAGEN) expression plasmid and sequenced. This construct resulted in the fusion of a C-terminal six-His tag to PBP4 (PBP4-H6), and the recombinant plasmid was named pQE-lmo2229. The lmo2229 gene was under the control of the T5 promoter/lac operator.
Construction of L. monocytogenes EGD/Δlmo2229 mutant.
A 690-bp DNA sequence of the gene lmo2229 corresponding to the polypeptide G356-M607 (TP4) in the TP domain of PBP4 was amplified by PCR using the primers BLJ (5′-GCGACAACCGGGCTCCAGCAGCTATTACTAAAGCC-3′) and BLO (5′-TGCAAATTGCGGAGTATAGCCAGCTAGCAGAACCA-3′) and the plasmid pQE-lmo2229 as a template. The resulting fragment was introduced into the plasmid pCR-Blunt (Invitrogen) and then excised using the EcoRI sites flanking the inserted fragment and subcloned into the plasmid pAUL-A (temperature-sensitive origin of replication) (5) to give rise to pAUL-TP4. The resulting pAUL-TP4 plasmid was introduced into L. monocytogenes EGD by electroporation, and transformants were selected for erythromycin resistance (1 μg ml−1) at 30°C. Integration of the plasmid pAUL-TP4 into the chromosome was realized by allelic replacement at the restrictive temperature (42°C) (5). The genotype of the L. monocytogenes EGD/Δlmo2229 mutant was confirmed by PCR and Southern blotting. PCR verification was performed using the primer BLJ at the 5′ end of the TP4 insert and the downstream antisense primer (within pAUL-A) 5′-AGCGGATAACAATTTCACACAGG-3′ or the upstream sense primer 5′-CCCAGTCACGACGTTGTAAAACG-3′ and the primer BLO at the 3′ end of the insert.
For Southern blot analysis, the 690-bp insert was labeled with digoxigenin, using the DIG-High Prime kit (Boehringer Mannheim), and used as probe for hybridization with the chromosomal DNA from the parental or the mutant strains previously digested with EcoRI.
Characterization of L. monocytogenes EGD/Δlmo2229 mutant.
The growth rate of L. monocytogenes EGD and the EGD/Δlmo2229 mutant was monitored for 6 h at 30, 37, or 42°C in tryptic soy broth (TSB) medium (Difco Laboratories) containing the appropriate antibiotic when required. Cell morphology analysis was performed on an exponentially growing culture by scanning electron microscopy with a LEO 1430VP instrument at a magnification of ×6,300.
The MICs were determined after 24 h and 48 h of incubation of L. monocytogenes EGD or the EGD/Δlmo2229 mutant at 37°C in a series of TSBs inoculated with 50-μl precultures (1 × 104 CFU/ml) and containing twofold dilutions of different β-lactam antibiotics (penicillin, imipenem, cephalothin, and cefotaxime), nisin, vancomycin, or moenomycin.
The crude cell wall from L. monocytogenes EGD or EGD/Δlmo2229 was isolated by boiling the cells in 4% sodium dodecyl sulfate (SDS) followed by washing with water and treatment with α-amylase and pronase (26). After a second step of 1% SDS extraction and washing with water, the wall was extracted with 5 to 10% trichloroacetic acid at 4°C for 48 h to remove teichoic acids (18). The purified PG was N acetylated with acetic anhydride in saturated solution of NaHCO3 for 24 h at 4°C as previously described (15). Finally, the sample was washed five times with water, digested with muramidase, and analyzed by HPLC as described elsewhere (26).
Membrane fractions from the two strains were prepared as described previously (24) and used for GT activity and penicillin binding assays (see below).
The susceptibility of the two strains to autolysis and the course of cell wall turnover were studied as previously described (25, 29).
Expression and purification of PBP4-H6.
PBP4-H6 was expressed in the host strain Escherichia coli M15 harboring plasmid pREP4, which carries the lacI repressor, providing tight regulation of the expression (QIAGEN). E. coli M15/pREP4 was transformed with the expression plasmid pQE-lmo2229, and the transformants were grown at 37°C in Luria-Bertani (LB) medium containing 100 μg ampicillin ml−1 and 25 μg kanamycin ml−1. When the absorbance reached a value of 0.6 to 0.7 at 600 nm, the cultures were supplemented with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) and maintained for an additional 3 h. The cells were suspended in 10 mM Tris-malate buffer (pH 6.8) containing 10 mM MgCl2 and protease inhibitor (2 mM phenylmethylsulfonyl fluoride or Pefabloc) (GP buffer) and disrupted by sonication. The particulate fraction was washed with GP buffer containing 1 M NaCl and incubated overnight in GP buffer containing 150 mM NaCl and 2% Triton X-100 at 4°C. After centrifugation at 40,000 × g at 4°C, the soluble fraction was loaded onto a 5-ml Ni2+ nitrilotriacetic acid-agarose column (QIAGEN), which was preequilibrated with 50 mM sodium phosphate buffer (pH 8.0) containing 300 mM NaCl, 1% Triton X-100, and 10 mM imidazole (buffer C). After washing the column with 20 mM imidazole in buffer C (10 volumes), PBP4-H6 was eluted with 250 mM imidazole in the same buffer. The protein was dialyzed against 50 mM sodium phosphate buffer (pH 8.0) containing 300 mM NaCl and stored at −20°C. The protein was found to undergo proteolysis during the purification (see below).
N-terminal sequencing and immunoblotting.
PBP4-H6 samples were submitted to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to Immobilon-P polyvinylidene difluoride membrane (Millipore) for N-terminal protein sequencing, using a 477 pulsed liquid sequencer (Perkin-Elmer, Applied Biosystems Division, Foster City, Calif.), or for immunodetection with anti-His tag antibodies and alkaline phosphatase-conjugated secondary antibody according to the Bio-Rad protocol.
Glycosyl transfer reaction.
Typical GT assays were performed under the following conditions. meso-[14C]A2pm lipid II (1 to 5 μM; 0.126 μCi nmol−1) and PBP4-H6 (0.17 μM) or membranes (100 μg total proteins) were incubated in a mixture of 50 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 0.5% decyl polyethylene glycol, 12% 1-octanol, 15% dimethyl sulfoxide, and 10−4 M penicillin (buffer D) for 30 min at 30°C. The reaction products were separated by an overnight chromatography on Whatman no. 1 filter paper in isobutyric acid-1 M ammonia (5:3) (29). Under these conditions, the polymerized non-cross-linked peptidoglycan remained immobile on the chromatograms while the lipid II substrate migrated with the solvent. The radioactive compounds were detected with PhosphorImager scanner and analyzed with Quantity One software (Bio-Rad).
Interaction of PBP4 with β-lactams and thiolester substrates.
The interaction between PBP4 (E) and β-lactams (I) was analyzed on the basis of the three-step reaction (10):
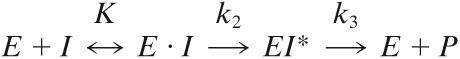 |
where K is the dissociation constant of the Henri-Michaelis complex E · I, k2/K (M−1 s−1) is the second order rate constant of acyl enzyme EI* formation, k2 is the first rate constant of enzyme acylation, k3 is the first rate constant of the acyl enzyme breakdown, and P is the reaction product.
Determination of k2/K.
Purified PBP4-H6 (2 μM final concentration) and fluoresceyl ampicillin (5 × 10−5 to 8 × 10−5 M) were incubated for various times at 37°C. The reaction was stopped by the addition of SDS-PAGE loading buffer and submitted to electrophoresis. The fluorescent complexes were detected with the PhosphorImager scanner and analyzed with Quantity One software (Bio-Rad).
Effect of thiolesters on fluoresceyl ampicillin binding.
PBP4 (2 μM final concentration) was incubated with different concentrations (0.1 to 20 mM) of the donor substrates (Bz-Gly-thiolactate, Bz-Gly-thioglycolate, Bz-d-Ala-thioglycolate, or A2-l-Lys-d-Ala-d-Ala) in 50 mM sodium phosphate buffer, pH 7.0, 150 mM NaCl, and 1% Triton X-100 (buffer B) in the presence or absence of 50 mM d-Ala as acceptors for 12 min at 37°C. The reaction mixes were then treated with 10−4 M fluoresceyl ampicillin for 1 min (∼50% of PBP4 was acylated under these conditions) and then analyzed as described above.
Acyltransferase activity of PBP4 with thiolester substrate.
PBP4-H6 samples (5 μM) and Bz-Gly-thiolactate (5 mM) were incubated in the presence or absence of 50 mM d-Ala for 2 h at 37°C in a total volume of 100 μl of buffer B. Fifty microliters of a fivefold dilution of the reaction mixture was analyzed by HPLC using an ET250/8/4 Nucleosil-5 C18 column (Macherey Nagel). The elution was realized with a 0 to 30% gradient of solvent B at a flow rate of 1 ml min−1; solvent A was 0.1% trifluoroacetic acid, and solvent B was 0.1% trifluoroacetic acid in acetonitrile (17).
Heterologue complementation assay.
In order to test whether L. monocytogenes PBP4-H6 was functional in E. coli, E. coli strain EJ801, which is defective in PBP1b and possesses a thermosensitive (42°C) PBP1a (14), was used for the heterologue complementation assay. This strain grows as rod-shaped cells at 30°C, but it fails to grow at 42°C unless an active class A PBP is present. The plasmid pQE-lmo2229, encoding PBP4-H6, was introduced in EJ801, and growth was monitored in the presence or absence of 0.1 or 1 mM IPTG for 4 h at 42°C.
RESULTS
Expression and purification of PBP4-H6.
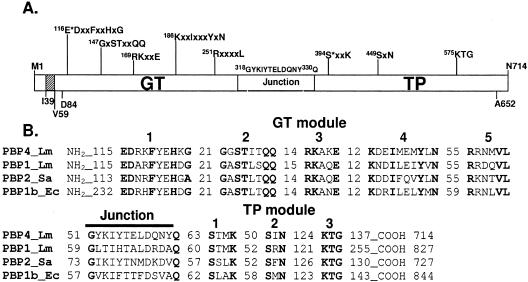
The multimodular class A PBP4 (714 amino acids) (Fig. 1) has a short cytosolic tail (Met1 to His38) followed by a predicted transmembrane anchor segment (Ileu39 to Val59). The N-terminal GT module (Asp84 to Gly318) contains five conserved motifs that characterize the GT51 family, which includes the GT module of class A PBPs and the monofunctional GTs (11; P. M. Coutinho and B. Henrissat [1999], Carbohydrate-Active Enzymes server at http://afmb.cnrs-mrs.fr/CAZY/GT_51.html). The Glu116 of motif 1 is the proposed catalytic residue of the GT domain by similarity to E. coli PBP1b Glu233 (30). The C-terminal penicillin-binding/TP module (Gln330 to Ala652) contains the three conserved motifs of the penicilloyl serine transferase superfamily (11); the active-site serine is Ser394.
FIG. 1.
Modular organization of PBP4 and comparison of its amino acid sequence with those of other class A PBPs. (A) The catalytic GT and TP modules are separated by the linker motif (G318 to Q330). The conserved motifs of each module are shown. E116 is the proposed catalytic residue of the GT domain, and S394 is the active serine of the TP domain. The protein contains a short cytosolic tail (M1 to 38) followed by one predicted membrane anchor segment (I39 to V59). (B) Amino acid sequence alignment of L. monocytogenes PBP4 (Q8Y547) with L. monocytogenes (Lm) PBP1 (Q8Y610), S. aureus (Sa) PBP2 (Q53724), and E. coli (Ec) PBP1b (P02919). The conserved motifs and the number of amino acids between them or at the protein end are shown.
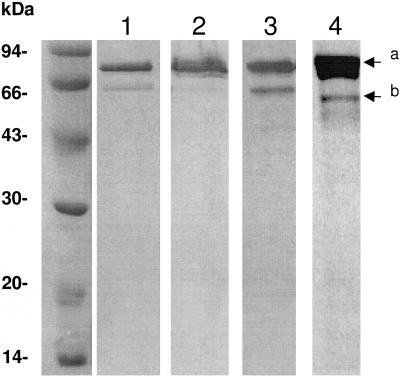
In order to characterize the GT and TP activities of PBP4 in vitro, the full-length protein, fused to a C-terminal His tag, was expressed in E. coli and purified in the presence of Triton X-100 on an Ni2+-affinity column (see Materials and Methods). The yield was about 3 mg per liter of culture, and the protein was 90% pure. Analysis of the purified protein by SDS-PAGE shows a major band with an expected molecular mass of ∼78 kDa and a minor band of ∼62 kDa (Fig. 2). The two bands bound penicillin and were recognized by anti-His tag antibodies (Fig. 2), suggesting that the lower band resulted from the degradation of the full-length protein at the N-terminal end.
FIG. 2.
SDS-PAGE analysis of PBP4-H6. Lane 1, Coomassie blue staining of purified PBP4-H6 (4 μg); lanes 2 and 3, Western blot analysis using anti-His tag antibodies of cell extract from 100 μl of culture at an A600 of 1 (lane 2) or purified protein (4 μg) (lane 3); lane 4, fluorography of purified PBP4-H6 (5 μg) labeled with 100 μM [14C]benzylpenicillin. a, full-length PBP4-H6; b, degradation product which has lost 169 residues from its N terminus.
N-terminal sequencing of the ∼62-kDa degradation product revealed that it started with the sequence K170AKEIFMARE, which is within the third conserved motif (underlined) of the GT module R169K170AKE. The cleavage occurs during purification as immunoblotting analysis of total cell extract before purification shows only one band recognized by anti-His tag antibodies (Fig. 2). The use of protease inhibitors during purification could not prevent proteolysis. Mutations could not be envisaged to disrupt this site because alteration of the third conserved motif may change the properties of the protein.
The glycosyltransferase activity of PBP4-H6.
GT activity was monitored with lipid II substrate isolated from E. coli, the pentapeptide of which is similar to that found in L. monocytogenes. Purified PBP4-H6 (0.17 μM), the transpeptidase activity of which was inactivated with 10−4 M penicillin, was incubated with increasing concentrations of meso-[14C]A2pm lipid II (1 to 5 μM) in buffer D as described in Materials and Methods. The penicillin had no effect on the GT activity of the enzyme. The initial rates of radioactive peptidoglycan synthesis from three experiments were determined. The enzyme efficiency kcat/Km value was ∼1,400 M−1 s−1. The GT activity was completely inhibited by 0.4 μM moenomycin or 20 μM vancomycin (molar ratio to lipid II of 10:1) used at antibiotic/enzyme molar ratios of 2:1 and 100:1, respectively.
The acyltransferase activity of PBP4-H6. (i) Penicillin binding.
The affinity of the PBP4-H6 for fluoresceyl ampicillin was determined by incubating the protein with different concentrations of the antibiotic for different times, and the acylation rate k2/K value of 124.2 ± 20 M−1 s−1 was determined (see Materials and Methods). The k2/K value of E. coli PBP1b for fluoresceyl ampicillin was 20 M−1 s−1, showing that PBP4 is sixfold more sensitive to fluoresceyl ampicillin than PBP1b.
(ii) Transpeptidation.
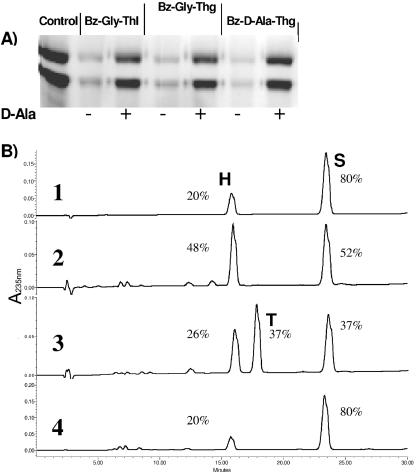
Preincubation of PBP4-H6 with thiolester (0.1 to 20 mM) donors for 12 min at 37°C inhibited the binding of fluoresceyl ampicillin to the enzyme upon subsequent incubation for 1 min with the β-lactam (see Materials and Methods). Nearly complete inhibition was seen with 5 mM Bz-Gly-thiolactate, Bz-Gly-thioglycolate, and Bz-d-Ala-thioglycolate (Fig. 3A). Penicillin binding occurred when preincubation of the thiolester with the PBP4-H6 was performed in the presence of 50 mM d-alanine as the acceptor, which accelerates the deacylation rate of the reaction. The results indicate a competition between the thiolester and fluoresceyl ampicillin acting as carbonyl donors and between water and the amino group of d-alanine acting as nucleophile acceptors of the transpeptidation reaction (Fig. 3A). The peptide Ac2-l-Lys-d-Ala-d-Ala (20 mM) did not have any effect on fluoresceyl ampicillin binding to PBP4-H6 (data not shown). In order to demonstrate the transpeptidation activity of PBP4-H6, the carbonyl donor substrate Bz-Gly-thiolactate (5 mM) and d-Ala acceptor (50 mM) were incubated with 5 μM PBP4-H6 for 2 h at 37°C. The products of the reaction were analyzed by HPLC using a C18 column (see Materials and Methods). Three peaks corresponding to the carbonyl donor substrate Bz-Gly-thiolactate (S), the hydrolyzed product Bz-Gly (H), and the aminolysed product Bz-Gly-d-Ala (T) were identified (Fig. 3B). Addition of benzylpenicillin (10−3 M) inhibited the transpeptidation reaction, and the transfer product (T) was not detected (Fig. 3B). Incubation of Bz-Gly-thiolactate substrate in the presence of d-Ala without the addition of the enzyme (in the same buffer conditions) resulted in 20% of spontaneous hydrolysis. When the enzyme was incubated with Bz-Gly-thiolactate in the absence of d-Ala, no transpeptidation product was detected and interestingly the hydrolysis product (H) increased from 20% to 48%. These results demonstrate that L. monocytogenes PBP4 catalyzes both transpeptidase and carboxypeptidase reactions.
FIG. 3.
Acyltransferase activity of PBP4-H6. (A) SDS-PAGE analysis. Shown is the effect of preincubation of the purified PBP4-H6 with thiolester donor substrates, in the presence (+) and in the absence (−) of d-Ala (acceptor), on subsequent binding of fluoresceyl ampicillin to the enzyme. In this experiment, more than 50% of the protein had lost the C-terminal 1-169 peptide. (B) HPLC analysis of the reaction products. Rows: 1, Bz-Gly-thiolactate substrate incubated for 2 h with d-Ala without added enzyme; 2, substrate incubated for 2 h with PBP4-H6 without d-Ala; 3, substrate and d-Ala incubated for 2 h in the presence of PBP4-H6; 4, the same condition as in row 3 with 10−3 M penicillin. S, Bz-Gly-thiolactate substrate; H, Bz-Gly hydrolysis product; T, Bz-Gly-d-Ala transfer product. The retention times for S, H, and T were 15.9, 17.6, and 23.4 min, respectively. Quantification (%) was performed by measuring the areas of the various peaks.
L. monocytogenes EGD/Δlmo2229 mutant.
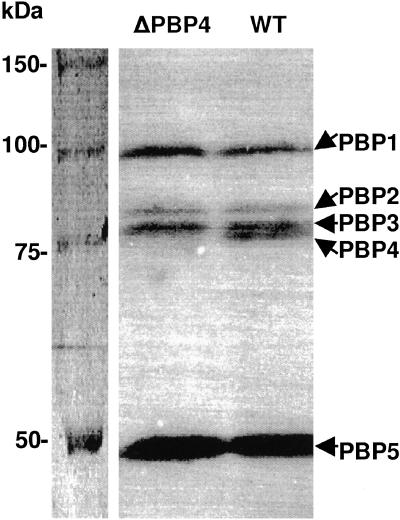
The chromosomal L. monocytogenes EGD gene lmo2229 was inactivated by plasmid integration, resulting in the interruption of the sequence encoding the TP module of PBP4 (see Materials and Methods). Furthermore, binding of the [14C]penicillin to membrane proteins of the mutant showed that PBP4 was not present (Fig. 4). The GT activity in L. monocytogenes EGD/Δlmo2229 membranes was decreased twofold compared to that in the wild-type membranes (0.016 nmol of disaccharide unit per mg of protein per min versus 0.029 nmol/mg/min).
FIG. 4.
Inactivation of PBP4 in L. monocytogenes EGD. Shown are the results of SDS-PAGE and fluorography analysis of membranes (100 μg total proteins) isolated from L. monocytogenes EGD/Δlmo2229 (ΔPBP4) and L. monocytogenes EGD (wild type [WT]) after labeling with 10−4 M [14C]benzylpenicillin.
The growth rates of L. monocytogenes EGD and the EGD/Δlmo2229 mutant were compared at 30, 37, or 42°C in TSB medium over a period of 6 h. The generation times of the mutant compared to the wild type were 110 versus 97, 101 versus 72, and 140 versus 102 at 30°C, 37°C, and 42°C, respectively; in all cases, the growth of the mutant was slower than that of the parental strain. Analysis of cell morphology using scanning electron microscopy showed that there were no significant differences in cell shape or size, nor was there any tendency to form filaments. These results suggest that PBP1 and PBP4 have redundant functions, as do functionally equivalent PBPs in other bacteria.
The MICs of penicillin, imipenem, cephalothin, cefotaxime, vancomycin, and nisin for L. monocytogenes EGD/Δlmo2229 mutant and the wild type were similar. In contrast, the moenomycin MIC for the mutant was fourfold higher than that for the wild type (2.5 μg ml−1 compared to 0.6 μg ml−1 for the wild type), suggesting that mutation of the lmo2229 gene affected the GT domain.
The PG from the L. monocytogenes EGD/Δlmo2229 mutant and the wild-type strain were prepared, digested with muramidase, and analyzed by HPLC (see Materials and Methods). The results (not shown) indicate that the muropeptide profiles from the two strains were undistinguishable.
Comparisons of the susceptibility of the L. monocytogenes EGD/Δlmo2229 mutant and the parent strain to autolysis (induced by Triton X-100 or lysozyme) and measurement of the turnover of prelabeled cell wall with [3H]GlcNAc were carried out. The susceptibilities of the two strains to autolysis were comparable (data not shown), suggesting that the structure of the peptidoglycan did not change significantly. Furthermore, the rate of cell wall turnover was slightly slower in the mutant than in the wild type (1.4-fold). The results indicate that the synthesis and content of peptidoglycan in the mutant were lower than those in the parent strain (in agreement with the in vitro experiment); this decrease did not seem to affect the properties of the mutant cell wall significantly.
Heterologous complementation assay.
E. coli strain EJ801, which is defective in PBP1b and has a thermosensitive PBP1a (14), was used for the heterologous complementation assay with PBP4. This strain grows as rod-shaped cells at 30°C, but it fails to grow at 42°C unless an active class A PBP is present (E. coli PBP1b). When plasmid pQE-lmo2229 encoding PBP4-H6 was introduced into E. coli EJ801, the cells were able to grow at 30°C but stopped growing at 42°C and lysed, indicating that L. monocytogenes EGD PBP4 was not functional in E. coli.
DISCUSSION
The class A PBP4 from L. monocytogenes was expressed in E. coli in fusion with a C-terminal His tag. The purified protein catalyzes peptidoglycan polymerization in vitro using E. coli lipid II as substrate. The enzyme catalytic efficiency was 28-fold lower than that of E. coli PBP1b (1,400 M−1 s−1 versus 39,000 M−1 s−1), measured under the same conditions (30), and was within twofold that of S. aureus PBP2 (3,400 M−1 s−1) (3). The GT activity of PBP4 remains linear up to 5 μM substrate, suggesting that the enzyme has a high Km value (>5 μM) responsible for its lower efficiency compared to that of E. coli PBP1b (Km = 1.8 ± 0.8 μM). S. pneumoniae PBP2a has high Km value of 40.6 μM. Its catalytic efficiency is 6 orders of magnitude lower than that of L. monocytogenes PBP4 (kcat/Km of 1 × 10−3 M−1 s−1) (8).
PBP4-H6 also catalyzes the aminolysis (d-Ala as acceptor) and hydrolysis of thiolester donor substrates; these two reactions are specifically inhibited by penicillin, showing that PBP4 has both TP and carboxypeptidase activities. The peptide Ac2-l-Lys-d-Ala-d-Ala has no effect on penicillin binding to PBP4. The same result was obtained with E. coli PBP1b, suggesting that the GT and TP activities are coupled and/or the peptide should be attached to nascent peptidoglycan.
The protein undergoes proteolysis during purification within the third conserved motif of the GT domain, between residues Arg169 and Lys170. Unless the two fragments stay together after proteolysis, the polypeptide Lys170-Asn714 product should not have GT activity as a consequence of the loss of motifs 1 and 2 and the first residue of motif 3. The first motif contains the putative catalytic Glu116, equivalent to the catalytic Glu233 of E. coli PBP1b, the mutation of which resulted in an inactive protein. Thus, the observed activity was due to the full-length protein. As Streptococcus pneumoniae PBP1b depleted of the first two motifs binds weakly to lipid II substrate (7), the degradation product of PBP4 should not bind or only weakly bind to the substrate. The degradation product Lys170-Asn714 binds penicillin indistinguishably from the full-length protein and most likely retains transpeptidase activity as well, because thiolester compounds inhibit penicillin binding to both species and an activity was regained in the presence of d-Ala used as acceptor in the TP reaction. These latter results indicate that deacylation is rate limiting in the absence of d-Ala.
The disruption of the chromosomal lmo2229 gene leads to the absence of detectable PBP4 in the mutated strain. While the mutant has a slightly reduced growth rate, no significant differences in terms of morphology or peptidoglycan composition were found between the parental strain and the mutant. The sensitivity of L. monocytogenes EGD/Δlmo2229 to β-lactams and vancomycin was similar to that of the wild type. The results show that PBP4 does not contribute to the natural resistance of L. monocytogenes to cephalosporins and monobactams, which is due to the low affinity of PBP3 and PBP5 for those antibiotics (24, 34). Similarly, inactivation of lmo2229 had no effect on the sensitivity of the L. monocytogenes EGD/Δlmo2229 mutant to nisin compared to the wild type; a slight increase in nisin sensitivity was previously reported in L. monocytogenes strain 412 upon lmo2229 disruption (12). Increased resistance to nisin in strain L. monocytogenes 412N was caused by enhanced expression of PBP4 turned on by the histidine kinase HPK1021, whose level was also higher than that of the wild-type strain. On the other hand, the L. monocytogenes EGD/Δlmo2229 mutant was fourfold more resistant to moenomycin than the parental strain, confirming the sensitivity of the protein to this inhibitor observed in vitro. This result, together with lower GT activity observed in the mutant membranes, suggests that PBP4 is more sensitive to this inhibitor than PBP1 and significantly contributes to peptidoglycan synthesis in L. monocytogenes.
Taken together, the results obtained with the mutant suggest that PBP4 is dispensable and that, as in other bacteria, its absence can be compensated for by the second class A PBP, PBP1, although the latter seems to be more resistant to moenomycin than PBP4. In E. coli, at least one class A PBP, PBP1a or PBP1b, is required, whereas the viability of Bacillus subtilis and Enterococcus faecalis was not impaired in the absence of all class A PBPs (2, 22). In order to check the importance of the class A PBPs in L. monocytogenes, mutants lacking PBP1 and both PBP1 and PBP4 need to be constructed.
L. monocytogenes PBP4 has both GT and TP activities in vitro, and because of the similarities between the peptidoglycan structures of listeriae and E. coli, one could expect PBP4 to be functional in E. coli. This possibility was tested by heterologous complementation in E. coli strain EJ801 at 42°C. Interestingly, PBP4 was unable to complement for the loss of PBP1a and PBP1b of E. coli (21% identity between PBP1b and PBP4), suggesting that the listerial protein may have a localization defect and/or fail to engage in specific interactions with other proteins in E. coli which are necessary for its function in vivo. Although the class A PBPs most likely have similar catalytic centers, the protein surfaces involved in protein-protein interactions may be different and species specific.
Acknowledgments
This work was supported in part by the Belgian State, Prime Minister's Office, Science Policy programming (IAP no. P5/33), the Action de Recherche Concertées (grant 03/08-297), and European Commission grant LSHM-CT-2004-512138. J.Z. was supported by a travel grant from CEMERA, Warsaw University, and M.T. was supported by a return grant from the Belgian Science Policy.
REFERENCES
- 1.Adam, M., C. Damblon, M. Jamin, W. Zorzi, V. Dusart, M. Galleni, A. el Kharroubi, G. Piras, B. G. Spratt, W. Keck et al. 1991. Acyltransferase activities of the high-molecular-mass essential penicillin-binding proteins. Biochem. J. 279:601-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbeloa, A., H. Segal, J.-E. Hugonnet, N. Josseaume, L. Dubost, J.-P. Brouard, L. Gutmann, D. Mengin-Lecreulx, and M. Arthur. 2004. Role of class A penicillin-binding proteins in PBP5-mediated β-lactam resistance in Enterococcus faecalis. J. Bacteriol. 186:1221-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, D., C. Leimkuhler, L. Chen, D. Walker, D. Kahne, and S. Walker. 2005. Kinetic characterization of the glycosyltransferase module of Staphylococcus aureus PBP2. J. Bacteriol. 187:2215-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brotz, H., M. Josten, I. Wiedemann, U. Schneider, F. Gotz, G. Bierbaum, and H. G. Sahl. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30:317-327. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty, T., M. Leimeister-Wächter, E. Domann, M. Hartl, W. Goebel, T. Nichterlein, and S. Notermans. 1992. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 174:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charpentier, E., and P. Courvalin. 1999. Antibiotic resistance in Listeria spp. Antimicrob. Agents Chemother. 43:2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Guilmi, A. M., A. Dessen, O. Dideberg, and T. Vernet. 2003. Functional characterization of penicillin-binding protein 1b from Streptococcus pneumoniae. J. Bacteriol. 185:1650-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Guilmi, A. M., A. Dessen, O. Dideberg, and T. Vernet. 2003. The glycosyltransferase domain of penicillin-binding protein 2a from Streptococcus pneumoniae catalyzes the polymerization of murein glycan chains. J. Bacteriol. 185:4418-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farber, J. M., and P. I. Peterkin. 1991. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 55:476-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frère, J. M., M. Nguyen-Distèche, J. Coyette, and B. Joris. 1992. Mode of action: interaction with the penicillin-binding proteins, p. 148-197. In M. I. Page (ed.), The chemistry of β-lactams. Blackie Academic and Professional, London, United Kingdom.
- 11.Goffin, C., and J.-M. Ghuysen. 1998. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 62:1079-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravesen, A., B. Kallipolitis, K. Holmstrøm, P. E. Høiby, M. Ramnath, and S. Knøchel. 2004. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl. Environ. Microbiol. 70:1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravesen, A., K. Sorensen, F. M. Aarestrup, and S. Knochel. 2001. Spontaneous nisin-resistant Listeria monocytogenes mutants with increased expression of a putative penicillin-binding protein and their sensitivity to various antibiotics. Microb. Drug Resist. 7:127-135. [DOI] [PubMed] [Google Scholar]
- 14.Hara, H., and H. Suzuki. 1984. A novel glycan polymerase that synthesizes uncross-linked peptidoglycan in Escherichia coli. FEBS Lett. 168:155-160. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi, H., Y. Araki, and E. Ito. 1973. Occurrence of glucosamine residues with free amino groups in cell wall peptidoglycan from bacilli as a factor responsible for resistance to lysozyme. J. Bacteriol. 113:592-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hof, H. 2004. An update on the medical management of listeriosis. Expert Opin. Pharmacother. 5:1727-1735. [DOI] [PubMed] [Google Scholar]
- 17.Jamin, M., M. Adam, C. Damblon, L. Christiaens, and J. M. Frere. 1991. Accumulation of acyl-enzyme in DD-peptidase-catalysed reactions with analogues of peptide substrates. Biochem. J. 280:499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamisango, K., I. Saiki, Y. Tanio, H. Okumura, Y. Araki, I. Sekikawa, I. Azuma, and Y. Yamamura. 1982. Structures and biological activities of peptidoglycans of Listeria monocytogenes and Propionibacterium acnes. J. Biochem. 92:23-33. [DOI] [PubMed] [Google Scholar]
- 19.Korsak, D., W. Vollmer, and Z. Markiewicz. 2005. Listeria monocytogenes EGD lacking penicillin-binding protein 5 (PBP5) produces a thicker cell wall. FEMS Microbiol. Lett. 251:281-288. [DOI] [PubMed] [Google Scholar]
- 20.Lakaye, B., C. Damblon, M. Jamin, M. Galleni, S. Lepage, B. Joris, J. Marchand-Brynaert, C. Frydrych, and J. M. Frere. 1994. Synthesis, purification and kinetic properties of fluorescein-labelled penicillins. Biochem. J. 300:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrec-Fairley, M., A. Piette, X. Gallet, R. Brasseur, H. Hara, C. Fraipont, J. M. Ghuysen, and M. Nguyen-Disteche. 2000. Differential functionalities of amphiphilic peptide segments of the cell-septation penicillin-binding protein 3 of Escherichia coli. Mol. Microbiol. 37:1019-1031. [DOI] [PubMed] [Google Scholar]
- 22.McPherson, D. C., and D. L. Popham. 2003. Peptidoglycan synthesis in the absence of class A penicillin-binding proteins in Bacillus subtilis. J. Bacteriol. 185:1423-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa, J., S. Tamaki, S. Tomioka, and M. Matsuhashi. 1984. Functional biosynthesis of cell wall peptidoglycan by polymorphic bifunctional polypeptides. Penicillin-binding protein 1Bs of Escherichia coli with activities of transglycosylase and transpeptidase. J. Biol. Chem. 259:13937-13946. [PubMed] [Google Scholar]
- 24.Pierre, J., A. Boisivon, and L. Gutmann. 1990. Alteration of PBP 3 entails resistance to imipenem in Listeria monocytogenes. Antimicrob. Agents Chemother. 34:1695-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popowska, M., M. Kloszewska, S. Gorecka, and Z. Markiewicz. 1999. Autolysis of Listeria monocytogenes. Acta Microbiol. Pol. 48:141-152. [PubMed] [Google Scholar]
- 26.Poros-Gluchowska, J., M. Kloszewska, and Z. Markiewicz. 2003. Ampicillin resistance in Listeria monocytogenes acquired as a result of transposon mutagenesis. Acta Microbiol. Pol. 52:131-142. [PubMed] [Google Scholar]
- 27.Poyart-Salmeron, C., C. Carlier, P. Trieu-Cuot, A. L. Courtieu, and P. Courvalin. 1990. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 335:1422-1426. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz, B., J. A. Markwalder, S. P. Seitz, Y. Wang, and R. L. Stein. 2002. A kinetic characterization of the glycosyltransferase activity of Escherichia coli PBP1b and development of a continuous fluorescence assay. Biochemistry 41:12552-12561. [DOI] [PubMed] [Google Scholar]
- 29.Siwinska, M., M. Kloszewska, A. Loniewska, and Z. Markiewicz. 1999. Effect of benzylpenicillin on murein biosynthesis, turnover and structure in Listeria monocytogenes cells. Acta Microbiol. Pol. 48:331-340. [PubMed] [Google Scholar]
- 30.Terrak, M., T. K. Ghosh, J. van Heijenoort, J. Van Beeumen, M. Lampilas, J. Aszodi, J. A. Ayala, J. M. Ghuysen, and M. Nguyen-Disteche. 1999. The catalytic, glycosyl transferase and acyl transferase modules of the cell wall peptidoglycan-polymerizing penicillin-binding protein 1b of Escherichia coli. Mol. Microbiol. 34:350-364. [DOI] [PubMed] [Google Scholar]
- 31.Tsakris, A., A. Papa, J. Douboyas, and A. Antoniadis. 1997. Neonatal meningitis due to multi-resistant Listeria monocytogenes. J. Antimicrob. Chemother. 39:553-554. [DOI] [PubMed] [Google Scholar]
- 32.van Heijenoort, J. 2001. Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology 11:25R-36R. [DOI] [PubMed] [Google Scholar]
- 33.van Heijenoort, Y., M. Gómez, M. Derrien, J. Ayala, and J. van Heijenoort. 1992. Membrane intermediates in the peptidoglycan metabolism of Escherichia coli: possible roles of PBP 1b and PBP 3. J. Bacteriol. 174:3549-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vicente, M. F., J. C. Pérez-Daz, F. Baquero, M. Angel de Pedro, and J. Berenguer. 1990. Penicillin-binding protein 3 of Listeria monocytogenes as the primary lethal target for β-lactams. Antimicrob. Agents Chemother. 34:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]