Abstract
Ribosomal protein S12 contains a highly conserved aspartic acid residue that is posttranslationally β-methylthiolated. Using mass spectrometry, we have determined the modification states of several S12 mutants of Thermus thermophilus and conclude that β-methylthiolation is not a determinant of the streptomycin phenotype.
Streptomycin and streptomycin-resistant mutants have played a seminal role in the elucidation of the decoding process of protein synthesis (reviewed in reference 19). Genetic and biochemical analyses of ribosomes from streptomycin-resistant mutants implicated ribosomal protein S12 as the determinant of the various streptomycin phenotypes, including resistance, dependence, and pseudodependence (reviewed in references 11 and 17). Such mutations have been localized in the three-dimensional structure of the Thermus thermophilus 30S ribosomal subunit to reside within two highly conserved loops centered around residues P90 and K42 (Escherichia coli numbering used throughout) (Fig. 1) (7).
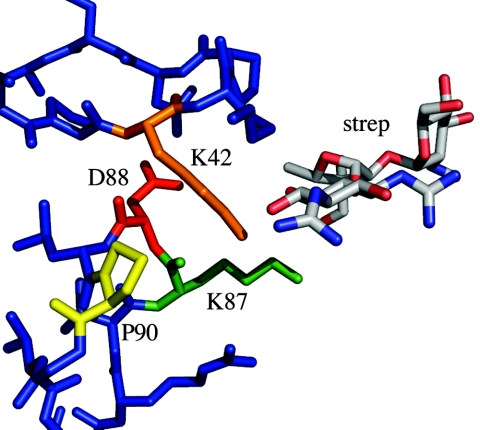
FIG. 1.
The region surrounding D88 in S12, as determined in the structure of the T. thermophilus 30S subunit complexed with antibiotics (7) (Protein Data Bank accession no. 1FJG). The conserved K42- and P90-containing loops of S12 are shown in blue. D88 is highlighted in red, P90 in yellow, K87 in green, and K42 in orange. The streptomycin (strep) binding site is proximal to these residues and is shown as tricolor sticks. This figure was illustrated with PyMol (9).
It has only recently been discovered by use of mass spectrometry that E. coli ribosomal protein S12 is posttranslationally modified via a β-methylthiolation at position D88 (Fig. 2A), near the streptomycin binding site and in the midst of residues altered in streptomycin-resistant mutants (14). This modification has also been found to occur in the phototrophic bacterium Rhodopseudomonas palustris (22), and we have identified it for the extremely thermophilic bacterium T. thermophilus (24). Posttranscriptional modifications of rRNA residues have been shown to affect resistance to various antibiotic classes (reviewed in reference 8). For example, ksgA mutants are resistant to kasugamycin due to the loss of N6-dimethylation of two conserved adenosines in 16S rRNA (13), while methylation of rRNA in the peptidyltransferase center (21) or in the decoding region (3) confers resistance to erythromycin and aminoglycosides, respectively (reviewed in references 20 and 26).
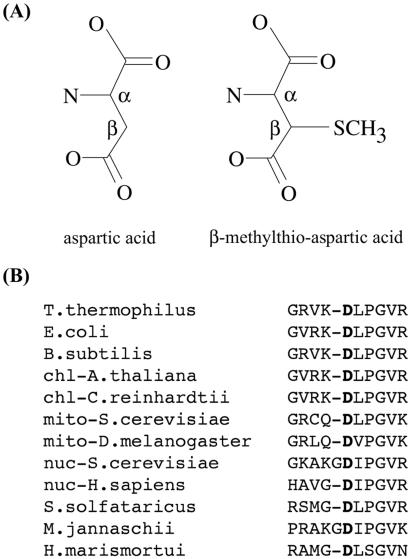
FIG. 2.
(A) Structures of aspartic acid and the modified β-methylthio-aspartic acid. (B) Amino acid alignment of the P90 loop, showing the conservation of D88 (in boldface) throughout three domains of life. Bacteria are represented by T. thermophilus, E. coli, and B. subtilis; chloroplast (chl) S12 sequences are shown for Arabidopsis thaliana and Chlamydomonas reinhardtii; mitochondrial (mito) S12 sequences are shown for Saccharomyces cerevisiae and Drosophila melanogaster; and eukaryotic nuclear (nuc) S12 sequences are represented by S. cerevisiae and Homo sapiens. A protein-protein BLAST search with T. thermophilus S12 returned 300 nonredundant bacterial S12 proteins. Chloroplast, mitochondrial, and nuclear S12 sequences were retrieved from 345 eukaryotic matches. Thirty-four sequences were exhaustive of the archaeal S12 proteins retrieved, and here they are represented by Sulfolobus solfataricus, Methanococcus jannaschii, and Haloarcula marismortui.
Interestingly, loss of modification of a ribosomal protein has not yet been shown to affect antibiotic sensitivity. Nevertheless, the proximity of S12 residue D88 to residues altered in streptomycin-resistant mutants raises the possibility that such mutations might confer resistance indirectly by inhibiting β-methylthiolation of D88. To address this question, we performed matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS) to establish the modification status of ribosomal protein S12 in a series of streptomycin-resistant and streptomycin-dependent mutants of T. thermophilus.
Loss of β-methylthiolation in a subset of S12 mutants.
We examined the modification states of several S12 mutants of T. thermophilus IB-21 (ATCC 43815) (16) by MALDI-TOF MS as described previously (23). Wild-type S12 was determined to have a mass of 14,519 ± 6 Da (Table 1), consistent with our previous report (24), indicating loss of the initial methionine and the presence of the β-methylthiolation. Considering the proximity to D88, we sought to determine if the streptomycin-resistant mutants K87R and K87E (12) were modified at D88. MALDI-TOF MS analysis indicates that D88 is β-methylthiolated in both K87R and K87E mutants (Table 1).
TABLE 1.
Molecular masses of wild-type and mutant S12 proteinsa
| Wild type or S12 mutation(s) | Phenotype | Observed mass (Da) | Predicted change in mol wt due to:
|
Observed change in mass vs wild type | D88 β-methylthiolation | |
|---|---|---|---|---|---|---|
| Amino acid change only | Amino acid change and loss of β-methylthiolation | |||||
| Wild type | Strsb | 14,519 ± 6 | + | |||
| K87R | Strr | 14,545 ± 1 | +28 | −19 | +27 | + |
| K87E | Strr | 14,517 ± 4 | −1 | −48 | −2 | + |
| P90R | Strd | 14,533 ± 5 | +59 | +12 | +15 | − |
| P90W | Strd | 14,552 ± 2 | +88 | +41 | +33 | − |
| P90L | Strd | 14,531 ± 2 | +15 | −32 | +12 | + |
| P90E | Strd | 14,550 ± 4 | +30 | −17 | +30 | + |
| P90M | Strd | 14,554 ± 9 | +33 | −14 | +35 | + |
| P90A | Strr | 14,490 ± 6 | −27 | −74 | −28 | + |
| P90C | Strr | 14,521 ± 2 | +5 | −42 | +3 | + |
| P90G | Strr | 14,477 ± 6 | −41 | −88 | −41 | + |
| K42T P90R | Strr | 14,506 ± 9 | +31 | −16 | −13 | − |
| K42T | Strr | 14,490 ± 3 | −28 | −75 | −28 | + |
The theoretical (predicted) mass of wild-type S12 is 14,516 Da.
Strs, streptomycin sensitivity.
We have previously used site-directed mutagenesis and gene replacement to generate T. thermophilus ribosomal mutants harboring the S12 mutations P90R, P90L, P90E, P90M, and P90W; these bulky side chain substitutions at P90 confer a streptomycin dependence (Strd) phenotype (6). In the same study, we constructed P90A, P90G, and P90C mutants, with smaller side chain substitutions that confer streptomycin resistance (Strr). MALDI-TOF MS analysis of these P90 mutant ribosomes indicated that β-methylthiolation of D88 is not retained in all mutants. P90R and P90W ribosomes lack the modification, whereas β-methylthiolation is retained in the other P90 mutants (Table 1). It is interesting that the mutants which have lost the modification are a subset of the Strd strains and that the modification is not strictly correlated with a dependence phenotype. The lack of modification of D88 in the P90R and P90W mutants suggests a steric hindrance imposed by these very large residues on the ability of the enzyme to modify the aspartic acid side chain, whereas modification is still possible for the smaller side chain substitutions at P90.
These results indicate that loss of β-methylthiolation of D88 is not a prerequisite for streptomycin dependence but do not exclude the possibility that loss of modification is sufficient on its own to confer a dependence phenotype. This possibility is excluded by our analysis of the modification state of the S12 double mutant K42T P90R. This mutant was derived from the P90R mutant in selections for streptomycin independence. The K42T second-site mutation suppresses the streptomycin dependence phenotype, resulting in streptomycin resistance (6) (Fig. 1). We found that the double mutant is not β-methylthiolated at D88 (Table 1), indicating that K42T does not suppress dependence by restoration of the D88 modification. Additionally, this emphasizes that loss of β-methylthiolation does not inevitably result in a streptomycin dependence phenotype. As anticipated, K42T alone was found to be modified at D88 (Table 1), consistent with the observation that an E. coli K42R Strr mutant is not otherwise altered (27).
It may seem surprising that the modification state of the K87 mutants (especially the large R substitution) is unaffected, considering that position 87 is closer in primary sequence to D88 than position 90; however, the side chain of K87 points away from D88 (Fig. 1). It is possible that the enzyme recognition may not involve position 87 but, rather, more-C-terminal residues, including P90. A more detailed understanding awaits identification of the gene(s) encoding the modifying enzyme(s), followed by biochemical analysis of the modification reaction.
Mutagenesis of D88 is not tolerated.
A definitive role for the D88 modification would best be determined using null alleles of the gene or genes involved in the modification. This would allow the generation of modification-deficient ribosomes which are otherwise unaltered. However, these genes have not been identified for any organism, making D88 mutagenesis a reasonable approach. We have the ability to generate S12 point mutants, which would enable us to infer limited substrate specificity. By the same manner in which we are successfully able to generate many mutants in this region of S12, we attempted to generate D88 mutants by gene replacement using several selection schemes. In brief, the chromosomal rpsL (S12) gene can be replaced with a mutant allele by using a suitable positive selection, for example, streptomycin resistance (6). We attempted to transform wild-type T. thermophilus (15) with a nonreplicating pUC18 plasmid bearing various rpsL D88 mutations by selecting for resistance to streptomycin (50 and 100 μg/ml) or paromomycin (20 μg/ml). Using the same plasmids in a separate selection, we employed an Strd strain (rpsL P90R) and selected for drug independence (6) in the event that D88 mutants would not exhibit a drug resistance phenotype.
We wished to change D88 in a manner that might provide insight into the substrate requirement of the enzyme and that might dissect the role of the aspartic acid residue from that of the modification. Specifically, we attempted to replace D88 with G (no side chain), A (a β carbon only), E (structurally similar to the negatively charged D), N (an available β-hydrocarbon and an uncharged amide group), and M (essentially, a γ-methylthiolation). However, multiple attempts failed to generate any D88 mutants. The ease with which we were able to generate many other mutants in the proximal region and throughout S12, coupled with our inability to mutagenize D88, suggests that alteration of this residue is lethal.
A strong argument for the importance of residue D88 is its high evolutionary conservation. By a BLAST search (www.ncbi.nlm.nih.gov/BLAST) (1), more than 675 S12 sequences were retrieved, encompassing bacterial, archaeal, organellar, and nuclear eukaryotic sources (Fig. 2B). Results of the search revealed the conservation of D88 in all S12 proteins except Bacillus subtilis 168, in which it was reported as an N. To address the likely possibility that this was an error in sequencing or annotation, we sequenced the S12 gene from four strains of B. subtilis, including strain 168 and three wild-type isolates, all obtained from the Bacillus Genetic Stock Center (BGSC). The strains included 168 (BGSC no. 1A1) (4), B. subtilis subsp. spizizenii NRRL B-23049 (BGSC no. 2A8) (18), N10 (BGSC no. 3A17) (10), and ATCC 21332 (BGSC no. 3A22) (2). Upon sequencing both strands of DNA, we found that all four strains have D at position 88. Incidentally, we detected another sequencing error and determined position 91 to be a conserved G, rather than the reported R, in the four strains (Fig. 2B). Without exception, 679 S12 sequences have a conserved D88.
At present, we are unable to distinguish between a requirement for aspartic acid or a requirement for β-methylthiolation at position 88. Our results indicate that loss of modification is tolerated in certain non-wild-type contexts, e.g., when P90 is mutated to R or W. These bulky residues may serve to suppress a local structural defect resulting from the absence of modification. The importance of both aspartic acid and β-methylthiolation is suggested by the remarkable conservation of D88, the presence of the modification in distantly related organisms, and the absence of mutations at D88, both in the literature and by our site-directed efforts. However, loss of modification of ribosomal protein L11 by the PrmA methyltransferase has no apparent functional defect, despite the phylogenetic conservation of this modification (5, 25). A clearer understanding of the role of β-methylthiolation of ribosomal protein S12 awaits identification of the modifying enzyme(s) and generation of a null mutant. Additionally, comprehensive mass spectrometric analyses of ribosomes from other organisms are warranted in order to establish the extent of the evolutionary conservation of the modification. Nonetheless, we show here that the β-methylthiolation of D88 in S12 is not a determinant of the streptomycin phenotype.
Nucleotide sequence accession numbers.
The B. subtilis S12 sequences were deposited in GenBank with the accession numbers DQ284750, DQ284751, DQ284752, and DQ284753.
Acknowledgments
We thank Daniel Zeigler at the Bacillus Genomic Stock Center for supplying the B. subtilis strains.
This work has been supported by grants from the National Institutes of Health to A.E.D. (GM19756) and to P.A.L. (GM58843).
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Arima, K., A. Kakinuma, and G. Tamura. 1968. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 31:488-494. [DOI] [PubMed] [Google Scholar]
- 3.Beauclerk, A. A., and E. Cundliffe. 1987. Sites of action of two ribosomal RNA methylases responsible for resistance to aminoglycosides. J. Mol. Biol. 193:661-671. [DOI] [PubMed] [Google Scholar]
- 4.Burkholder, P. R., and N. H. Giles. 1947. Induced biochemical mutants in Bacillus subtilis. Am. J. Bot. 34:345-348. [PubMed] [Google Scholar]
- 5.Cameron, D. M., S. T. Gregory, J. Thompson, M.-J. Suh, P. A. Limbach, and A. E. Dahlberg. 2004. Thermus thermophilus L11 methyltransferase, PrmA, is dispensable for growth and preferentially modifies free ribosomal protein L11 prior to ribosome assembly. J. Bacteriol. 186:5819-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr, J. F., S. T. Gregory, and A. E. Dahlberg. 2005. Severity of the streptomycin resistance and streptomycin dependence phenotypes of ribosomal protein S12 of Thermus thermophilus depends on the identity of highly conserved amino acid residues. J. Bacteriol. 187:3548-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter, A. P., W. M. Clemons, D. E. Brodersen, R. J. Morgan-Warren, B. T. Wimberly, and V. Ramakrishnan. 2000. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature 407:340-348. [DOI] [PubMed] [Google Scholar]
- 8.Cundliffe, E. 1990. Recognition sites for antibiotics within rRNA, p. 479-490. In W. E. Hill, A. E. Dahlberg, R. A. Garrett, P. B. Moore, D. Schlessinger, and J. R. Warner (ed.), The ribosome: structure, function, and evolution. American Society for Microbiology, Washington, D.C.
- 9.DeLano, W. L. 2002. The PyMol molecular graphics system. DeLano Scientific, San Carlos, Calif.
- 10.El-Helow, E. R. 2001. Identification and molecular characterization of a novel Bacillus strain capable of degrading Tween-80. FEMS Microbiol. Lett. 196:119-122. [DOI] [PubMed] [Google Scholar]
- 11.Gorini, L. 1974. Streptomycin and misreading of the genetic code, p. 791-803. In M. Nomura, A. Tissières, and P. Lengyel (ed.), Ribosomes. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 12.Gregory, S. T., J. H. D. Cate, and A. E. Dahlberg. 2001. Streptomycin-resistant and streptomycin-dependent mutants of the extreme thermophile Thermus thermophilus. J. Mol. Biol. 309:333-338. [DOI] [PubMed] [Google Scholar]
- 13.Helser, T. L., J. E. Davies, and J. E. Dahlberg. 1971. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat. New Biol. 233:12-14. [DOI] [PubMed] [Google Scholar]
- 14.Kowalak, J. A., and K. A. Walsh. 1996. β-Methylthio-aspartic acid: identification of a novel posttranslational modification in ribosomal protein S12 from Escherichia coli. Protein Sci. 5:1625-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koyama, Y., T. Hoshino, N. Tomizuka, and K. Furukawa. 1986. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J. Bacteriol. 166:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristjansson, J. K., G. O. Hreggvidsson, and G. A. Alfredsson. 1986. Isolation of halotolerant Thermus spp. from submarine hot springs in Iceland. Appl. Environ. Microbiol. 52:1313-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurland, C. G., D. Hughes, and M. Ehrenberg. 1996. Limitations of translational accuracy, p. 979-1004. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 18.Nakamura, L. K., M. S. Roberts, and F. M. Cohan. 1999. Relationship of Bacillus subtilis clades associated with strains 168 and W23: a proposal for Bacillus subtilis subsp. subtilis subsp. nov. and Bacillus subtilis subsp. spizizenii subsp. nov. Int. J. Syst. Bacteriol. 49:1211-1215. [DOI] [PubMed] [Google Scholar]
- 19.Ogle, J. M., and V. Ramakrishnan. 2005. Structural insights into translational fidelity. Annu. Rev. Biochem. 74:129-177. [DOI] [PubMed] [Google Scholar]
- 20.Poehlsgaard, J., and S. Douthwaite. 2005. The bacterial ribosome as a target for antibiotics. Nat. Rev. Microbiol. 3:870-881. [DOI] [PubMed] [Google Scholar]
- 21.Skinner, R., E. Cundliffe, and F. J. Schmidt. 1983. Site of action of a ribosomal RNA methylase responsible for resistance to erythromycin and other antibiotics. J. Biol. Chem. 258:12702-12706. [PubMed] [Google Scholar]
- 22.Strader, M. B., N. C. VerBerkmoes, D. L. Tabb, H. M. Connelly, J. W. Barton, B. D. Bruce, D. A. Pelletier, B. H. Davidson, R. L. Hettich, F. W. Larimer, and G. B. Hurst. 2004. Characterization of the 70S ribosome from Rhodopseudomonas palustris using an integrated “top-down” and “bottom-up” mass spectrometric approach. J. Proteome Res. 3:965-978. [DOI] [PubMed] [Google Scholar]
- 23.Suh, M.-J., and P. A. Limbach. 2004. Investigation of methods suitable for the matrix-assisted laser desorption/ionization mass spectrometric analysis of proteins from ribonucleoprotein complexes. Eur. J. Mass Spectrom. 10:89-99. [DOI] [PubMed] [Google Scholar]
- 24.Suh, M.-J., D.-M. Hamburg, S. T. Gregory, A. E. Dahlberg, and P. A. Limbach. 2005. Extending ribosomal protein identifications to unsequenced bacterial stains using matrix-assisted laser desorption/ionization mass spectrometry. Proteomics 5:4818-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanet, A., J. A. Plumbridge, M. F. Guerin, and J. H. Alix. 1994. Ribosomal protein methylation in Escherichia coli: the gene prmA, encoding the ribosomal protein L11 methyltransferase, is dispensable. Mol. Microbiol. 14:947-958. [DOI] [PubMed] [Google Scholar]
- 26.Vester, B., and S. Douthwaite. 2001. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob. Agents Chemother. 45:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcox, S. K., G. S. Cavey, and J. D. Pearson. 2001. Single ribosomal protein mutations in antibiotic-resistant bacteria analyzed by mass spectrometry. Antimicrob. Agents Chemother. 45:3046-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]