Abstract
The activity of lanthionine-containing peptide antibiotics (lantibiotics) is based on different killing mechanisms which may be combined in one molecule. The prototype lantibiotic nisin inhibits peptidoglycan synthesis and forms pores through specific interaction with the cell wall precursor lipid II. Gallidermin and epidermin possess the same putative lipid II binding motif as nisin; however, both peptides are considerably shorter (22 amino acids, compared to 34 in nisin). We demonstrate that in model membranes, lipid II-mediated pore formation by gallidermin depends on membrane thickness. With intact cells, pore formation was less pronounced than for nisin and occurred only in some strains. In Lactococcus lactis subsp. cremoris HP, gallidermin was not able to release K+, and a mutant peptide, [A12L]gallidermin, in which the ability to form pores was disrupted, was as potent as wild-type gallidermin, indicating that pore formation does not contribute to killing. In contrast, nisin rapidly formed pores in the L. lactis strain; however, it was approximately 10-fold less effective in killing. The superior activity of gallidermin in a cell wall biosynthesis assay may help to explain this high potency. Generally, it appears that the multiple activities of lantibiotics combine differently for individual target strains.
Epidermin (1) and gallidermin (27) are lantibiotics produced by Staphylococcus epidermidis Tü 3298 and Staphylococcus gallinarum Tü 3928, respectively. Their antimicrobial activity spectra include a variety of pathogenic gram-positive bacteria, such as staphylococci and streptococci. Both peptides are active against Propionibacterium acnes (27), and preliminary clinical tests have demonstrated their potential for treatment of acne. Gallidermin and epidermin are structural analogues which differ at position 6 in the N-terminal part of the molecule (Leu in gallidermin and Ile in epidermin). As a common feature of lantibiotics, both contain dehydroalanine and dehydrobutyrine as well as lanthionine and methyllanthionine rings. Details on the chemistry and biosynthesis pathways of lantibiotics can be found in recent reviews (16, 21, 44).
Based on structural differences, two types of lantibiotics have been distinguished (26). Typical type A lantibiotics, e.g., epidermin, subtilin, and nisin, are screw-shaped, elongated, flexible, and amphipathic peptides with pore-forming activities (29, 42), whereas type B lantibiotics, e.g., mersacidin, are small and compact peptides which target specific components of the bacterial membrane (13).
The pore formation process of type A lantibiotics has been studied in detail using various physiological and artificial membrane systems (2, 17, 18, 35). Driessen et al. (18) suggested a “wedge model” for the nisin pore formation, in which the peptides induce local perturbation of the bilayer structure in a surface-bound configuration. This model was substantiated by further studies (8, 9); however, in model membrane systems, micromolar peptide concentrations are usually required to provoke perturbation effects. In contrast, many gram-positive bacteria have MICs in the nanomolar concentration range. An explanation for this discrepancy was provided when lipid II, the bactoprenol-bound cell wall precursor, was identified as a specific docking molecule which enabled pore formation in model membranes at nanomolar concentrations of nisin (14).
Subsequent studies of this unique phenomenon revealed that binding to lipid II promotes two bactericidal activities, pore formation and inhibition of peptidoglycan biosynthesis (11). Further structure-function studies identified the N-terminal double-ring system of nisin as the binding site for lipid II; an intact flexible hinge region and the C-terminal segment were found to be essential for pore formation (11, 52). These results were recently corroborated when the solution structure of the lipid II-nisin complex was solved (24). The N-terminal double-ring system of nisin was found to form a binding cage for the pyrophosphate linkage group of lipid II; obviously, undecaprenylpyrophosphate, when released after transglycosylation, can also be bound (6). Thus, nisin has the ability to interfere with the cell wall biosynthesis cycle simultaneously at various sites. Binding of lipid II leads to formation of a pore which is more stable and has a larger diameter than pores formed in the absence of lipid II (53). In addition, lipid II-independent mechanisms of action have been described, such as the induction of autolysis of staphylococci (4). These multiple activities are considered to result in the high potency of nisin towards some gram-positive strains.
Comparatively little is known about the mode of action of epidermin and gallidermin. Early studies indicated that they may impair membrane functions (2, 43) and that lipid II may play an important role in this process (14). The latter can be reasonably explained in light of the structural work mentioned above (6, 24), since the N-terminal double-ring system is almost completely conserved in both nisin and epidermin (Fig. 1). However, beyond the N-terminal double-ring system there is hardly any structural similarity between those two peptides. Ring C is completely missing in epidermin, and its flexible hinge region is longer. The C-terminal tail, which is essential for nisin pore formation (49), is absent from epidermin. Rather, its C terminus is compact due to a double-ring system involving an aminovinyl cysteine residue resulting from oxidative decarboxylation of the terminal ring (31). The solution structure identifies epidermin as an amphiphilic screw-shaped molecule with an overall length of 30 Å (19), which is considerably shorter than nisin (50 Å) (20). Such differences between nisin and epidermin provided an excellent basis for extending our knowledge of the molecular activities of lantibiotics, particularly since hybrid peptides with such drastic structural differences as exist between nisin and epidermin may be difficult to obtain through directed mutagenesis. It was of particular interest to study whether the same complexity of antimicrobial activity found for nisin would hold true for considerably smaller molecules such as epidermin and gallidermin.
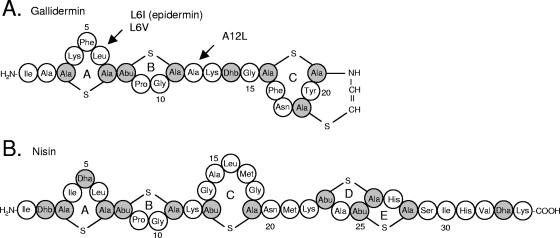
FIG. 1.
Primary structures of gallidermin, gallidermin mutant peptides, and epidermin (A) and the related lantibiotic nisin (B). Modifications of gallidermin mutants used in this study are indicated by arrows. Epidermin differs from gallidermin at position 6 (Ile). Dha, dehydroalanine; Dhb, dehydrobutyrine; Ala-S-Ala, lanthionine; Abu-S-Ala, β-methyllanthionine.
The results reported here demonstrate that the overall length of gallidermin is crucial for pore formation, which occurs only with certain strains. For nisin, which was able to form pores in the three indicator strains tested in this study, it was previously shown that antibiotic activity strongly increases when cell wall biosynthesis inhibition is combined with pore formation (52). Interestingly, in spite of pore formation not taking place with, e.g., Lactococcus lactis, epidermin was found to be more potent than nisin against this strain. Clearly, although there has been considerable progress in unraveling the complexity of lantibiotic activities and attributing specific mechanisms to defined segments of the molecules, more has to be learned on how these various activities combine to produce a given MIC of a particular test organism.
MATERIALS AND METHODS
Chemicals and materials.
All chemicals were of analytical grade or better. The phospholipids 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dipalmitoleoyl-sn-glycero-3-phosphocholine (DPoPC), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), and 1,2-dilauroyl-sn-glycero-3-phosphocholine (DLPC) were purchased from Avanti Polar Lipids, Inc. and stored at −20°C in chloroform. Cholesterol and carboxyfluorescein (CF) were purchased from Sigma (Steinheim, Germany). The protein concentration of membrane preparations was determined using the bicinchonic acid protein assay reagent (Pierce Chemical Corp., Bonn, Germany) with bovine serum albumin as a standard.
Bacterial strains and culture conditions.
Lantibiotic-producing strains Staphylococcus epidermidis Tü 3298, Staphylococcus gallinarum Tü 3928, Staphylococcus epidermidis EMS6 pCU gdmA L6V, and Staphylococcus epidermidis EMS6 pCU gdmA A12L were cultivated in tryptic soy broth (Merck, Darmstadt, Germany) at 37°C with aeration. The mutant strains (38) were kindly provided by F. Götz, Tübingen. Micrococcus flavus DSM 1790 and Staphylococcus simulans 22 were grown in tryptic soy broth at 30°C and 37°C, respectively, with aeration, and Lactococcus lactis subsp. cremoris HP was grown in M17 broth plus 0.5% glucose (Oxoid, Basingstoke, England) at 30°C without aeration.
Preparative purification of lantibiotics.
Producer strains were grown for 24 h. Cells were harvested by centrifugation (10,000 × g, 10 min) and the peptides were extracted from the culture supernatant as described previously (15). Chloroform was added to the supernatant fluid (0.1:1, vol/vol), stirred vigorously for 1 h at 4°C, and centrifuged (10,000 × g, 10 min) for phase separation. The precipitate formed at the interface between the chloroform and culture supernatant fluid was lyophilized. The crude extract was resuspended in 30% acetonitrile-0.1% trifluoroacetic acid (TFA) and applied to a preparative high-performance liquid chromatography column (Nucleosil 100-C18; 10 μm, 225 by 20 mm [inner diameter]; Schambeck SFD, Bad Honnef, Germany). The column was equilibrated with buffer A (H2O, 0.1% [vol/vol] TFA), and peptides were eluted using a linear gradient of 20 to 60% buffer B (acetonitrile, 0.1% [vol/vol] TFA) at a flow rate of 12 ml/min. For further purification, a semipreparative (Nucleosil 100-5C18; 250 by 8.6 mm [inner diameter]) and/or analytical (Nucleosil 100-3C18; 250 by 4.6 mm [inner diameter]) column was used. Electrospray mass spectrometry was used to confirm the correct mass and the purity of the peptides. Stock solutions were prepared in 0.05% acetic acid and stored at −20°C.
In vitro lipid II synthesis and purification.
Synthesis and purification of the lipid-bound cell wall precursor were performed as described previously (45). Briefly, lipid II was synthesized in vitro using membrane preparations of Micrococcus flavus DSM 1790. Membranes were isolated from lysozyme-treated cells by centrifugation (40,000 × g), washed twice in 50 mM Tris-HCl, 10 mM MgCl2, pH 7.5, and stored under liquid nitrogen until use. The analytical assay was performed in a total volume of 150 μl containing 400 to 800 μg of membrane protein, 10 nmol undecaprenylphosphate (C55-P), 100 nmol UDP-N-acetylmuramyl pentapeptide (UDP-MurNAc-PP), and 100 nmol UDP-N-acetylglucosamine (UDP-GlcNAc) in 60 mM Tris-HCl, 5 mM MgCl2, pH 8, and 0.5%(wt/vol) Triton X-100. UDP-MurNAc-PP was purified as described previously (28). For purification of milligram quantities of lipid II, the analytical procedure was scaled up by a factor of 250. Reaction mixtures were incubated for 1 h at 30°C, and lipids were extracted with the same volume of n-butanol-6 M pyridine-acetate (2:1, vol/vol), pH 4.2. Purification of lipid II was performed on a DEAE-cellulose column (0.9 by 25 cm, DEAE SS type; Serva, Heidelberg, Germany) and eluted with a linear gradient of chloroform-methanol-water (2:3:1, vol/vol/vol) to chloroform-methanol-300 mM ammonium bicarbonate (2:3:1, vol/vol/vol). Lipid II-containing fractions were identified by thin-layer chromatography (TLC) (60F254 silica plates; Merck) using chloroform-methanol-water-ammonia (88:48:10:1) as the solvent (40). Spots were visualized by phosphomolybdic acid staining. The concentration of purified lipids was determined as inorganic phosphate after treatment with perchloric acid (41).
Inhibition of in vitro lipid II synthesis.
Inhibition of in vitro lipid II formation was analyzed using the analytical lipid II synthesis assay as described above, with the addition of radioactively labeled [14C]UDP-GlcNAc (9.21 GBq/mmol; Amersham Pharmacia Biotech., Braunschweig, Germany). Gallidermin, epidermin, and the mutant peptides [L6V]gallidermin and [A12L]gallidermin were added to the reaction mixture in molar ratios as indicated (0.5:1, 1:1, and 1.5:1, referring to the total amount of 10 nmol C55-P). After incubation for the indicated time periods at 30°C (1 min to 1 h), lipids were extracted from the reaction mixture and separated by TLC (see above). Radioactively labeled spots were visualized with iodine vapor, excised, and quantified by beta-scintillation counting (1900 CA Tri-Crab scintillation counter; Packard, Zurich, Switzerland).
Inhibition of the FemX reaction.
The assays for synthesis of lipid II-Gly1 (45) were performed in a total volume of 100 μl containing 5 nmol lipid II, 10 μg His-tagged glycyl-tRNA-synthetase, 25 μg tRNA, and 2.7 μg His-tagged FemX in 100 mM Tris-HCl, 20 mM MgCl2 (pH 7.5), 0.8% Triton X-100, 2 mM ATP, and 50 nmol [U-14C]glycine (3.7 GBq/mmol; Pharmacia Biotech.). For testing the impact of the lantibiotics in the FemX reaction, the substrate lipid II and the peptides (in a molar ratio of 1:2) were preincubated for 15 min before addition of the reaction mixture. After incubation for 60 min at 30°C, 50 μl from the reaction mixture was analyzed by TLC (60F254 silica plates; Merck) using solvent B (butanol, acetic acid, water, pyridine; 15:3:12:10, vol/vol/vol/vol). Radioactively labeled spots were analyzed as described above.
MIC determinations.
MIC determinations were carried out in microtiter plates. M. flavus DSM 1790 and S. simulans 22 were grown in half-concentrated Mueller-Hinton broth (Oxoid). Lactococcus lactis subsp. cremoris HP was grown in M17 broth plus 0.5% glucose (Oxoid). Serial twofold dilutions of the peptides were made in the growth medium of the respective indicator strain. Bacteria were added to give a final inoculum of 105 CFU/ml in a volume of 0.2 ml. Incubation conditions were 30°C for 24 h for M. flavus DSM 1790, 37°C for 16 h for S. simulans 22, and 30°C for 16 h for L. lactis subsp. cremoris HP. The MIC was read as the lowest peptide concentration causing inhibition of visible growth; results given are means of three independent determinations.
Preparation of unilamellar vesicles.
Large unilamellar vesicles were prepared for carboxyfluorescein and potassium efflux experiments by the extrusion technique (23, 34). Large unilamellar vesicles were made of phosphatidylcholine with different carbon chain lengths (DOPC, DPoPC, DMPC, or DLPC). When indicated, vesicles were made with 50% cholesterol (molar ratio) with and without 0.1 mol% lipid II (referring to the total amount of phospholipids). To prepare liposomes, lipid solvents were removed under vacuum in an exsiccator. Multilamellar liposomes were made by the addition of CF or KCl solutions to the dry lipids, followed by vigorous stirring. Then, unilamellar vesicles were produced by repeated extrusion (Lipex Extruder; Northern Lipids, Canada) of the multilamellar vesicles through polycarbonate filters with a pore size of 400 nm (Isopore membrane filters; Millipore, Ireland). Following the extrusion, vesicles were passed through a Sephadex G-50 column to remove untrapped CF or KCl. The concentration of phospholipids in the final liposome suspension was determined according to the method described by Rouser et al. (41).
Carboxyfluorescein efflux experiments.
CF-loaded vesicles were prepared with 50 mM CF and then diluted in 1.5 ml K+ buffer (50 mM MES [morpholinoethanesulfonic acid]-KOH, pH 6.0, 100 mM K2SO4) in a final concentration of 25 μM phospholipid (or, when vesicles were prepared with 50% cholesterol, 12.5 mM phospholipid) on a phosphorous basis. After addition of the peptide, the increase of fluorescence intensity was measured at 520 nm (excitation at 492 nm) on an RF-5301 spectrophotometer (Shimadzu, Duisburg, Germany) at room temperature. Peptide-induced leakage was documented relative to the total amount of marker release after disintegration of the vesicles by addition of 10 μl of 20% Triton X-100.
Potassium efflux experiments.
Potassium-loaded vesicles were made of DOPC with and without 0.1% lipid II in KCl buffer (300 mM KCl, 30 mM MES, 20 mM Tris, pH 6.5) and diluted in 2 ml choline buffer (300 mM choline chloride, 30 mM MES, 20 mM Tris, pH 6.5) in a final concentration of 250 μM phospholipid on a phosphorous basis. Peptide-induced potassium efflux was monitored using a microprocessor pH meter (pH 213; Hanna Instruments, Kehl, Germany) with an MI-442 potassium electrode and MI-409F reference electrode. Peptide-induced leakage was expressed relative to the total amount of potassium recorded after addition of 46 μl 30% octylglycoside (final concentration of 0.7%). Before each experiment, the electrodes were calibrated with standard solutions containing 0.01, 0.1, or 1 mM KCl in buffer, and calculations of potassium efflux in percent were performed as described previously (37).
Potassium release from whole cells.
Cells were harvested at an optical density at 600 nm (OD600) of 1.0 to 1.5 (3,300 × g, 5°C, 3 min), washed with 50 ml cold choline buffer, and resuspended in the same buffer to an OD600 of 30. The concentrated cell suspension was kept on ice and used within 30 min. For each measurement the cells were diluted in choline buffer (25°C) to an OD600 of about 3. Peptide-induced leakage was expressed relative to the total amount of potassium release induced by addition of 1 μM nisin (data not shown).
RESULTS
Studies on unilamellar vesicles.
Previous experiments had shown that, at nanomolar concentrations, nisin and epidermin were able to induce marker release from lipid II-containing multilamellar liposomes made of egg yolk phosphatidylcholine and 50% cholesterol (14). To gain more insight into structural and functional aspects of the lipid II-mediated pore formation process, it was necessary to use a better-defined membrane system. Thus, we prepared large unilamellar vesicles (consisting of only one bilayer of phospholipids and possessing a homogeneous size of 400 nm) made of phospholipids with uniform acyl chain length.
Such liposomes, when made of pure DOPC (acyl chains of C18:1), were not impaired by nisin or by epidermin in concentrations up to 1 μM. When 0.1 mol% lipid II was added to DOPC liposomes, unexpectedly, gallidermin was not able to release carboxyfluorescein even at a concentration of 1 μM (data not shown). In control experiments conducted with nisin, rapid marker release was detected, as reported before (52). We confirmed that gallidermin was able to bind to lipid II by sequential addition of nisin after gallidermin; in this case, nisin was not able to induce any detectable marker leakage, most likely because lipid II binding sites were already occupied by gallidermin.
We also tested for K+ leakage to exclude the possibility that gallidermin-induced pores could be smaller than nisin-induced pores and too narrow to promote CF leakage (CF has a molecular radius of approximately 1 nm). However, significant K+ release could not be detected (data not shown). These data suggested fundamental differences between nisin and gallidermin in their ability to form pores. Since the most obvious difference on the structural level is the overall length (30 Å for epidermin and 50 Å for nisin), we prepared membranes from phosphatidylcholine with shorter acyl chains, i.e., DPoPC (C16:1), DMPC (C14:0), and DLPC (C12:0). Such liposome membranes may spontaneously leak CF over a broad temperature range (7) and need to be stabilized by addition of 50% cholesterol (3).
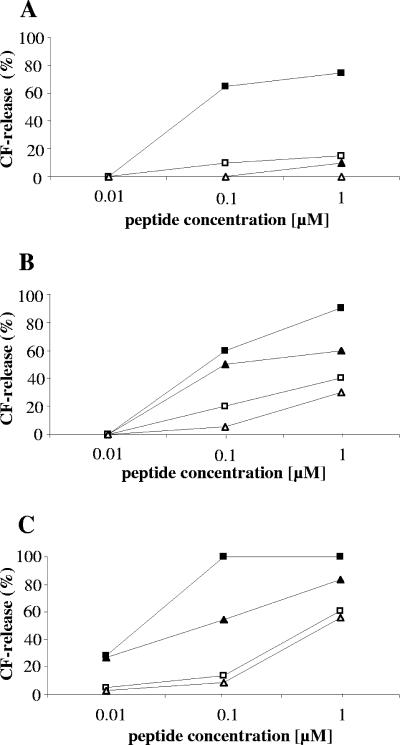
When such liposomes were made from DPoPC and supplemented with 0.1 mol% lipid II, gallidermin was still not able to release CF (Fig. 2A). In contrast, its ability to induce pores became detectable with liposomes made of DMPC and DLPC (Fig. 2B and C), although the rate of marker release was significantly lower than that for nisin. However, these thin membranes became increasingly unstable as demonstrated with the undoped control membranes, which became more susceptible towards the lantibiotics (Fig. 2). Notwithstanding this, our results demonstrate that membrane thickness is crucial for gallidermin pore formation and raised the question of whether pore formation would be an integral part of the antibiotic activity of gallidermin in vivo.
FIG. 2.
Activities of gallidermin (triangles) and nisin (squares) on unilamellar liposomes made of phospholipids with decreasing chain length. Filled symbols, liposomes with 0.1 mol% lipid II; open symbols, liposomes without lipid II. Liposomes were stabilized with 50% cholesterol and used at a final concentration of 12.5 μM phospholipid on a phosphorous basis. Carboxyfluorescein release from (A) DPoPC (C16:1), (B) DMPC (C14:0), and (C) DLPC (C12:0) was determined 2.5 min after addition of peptide. The 100% leakage level was determined by addition of Triton X-100.
Pore formation by gallidermin in intact cells.
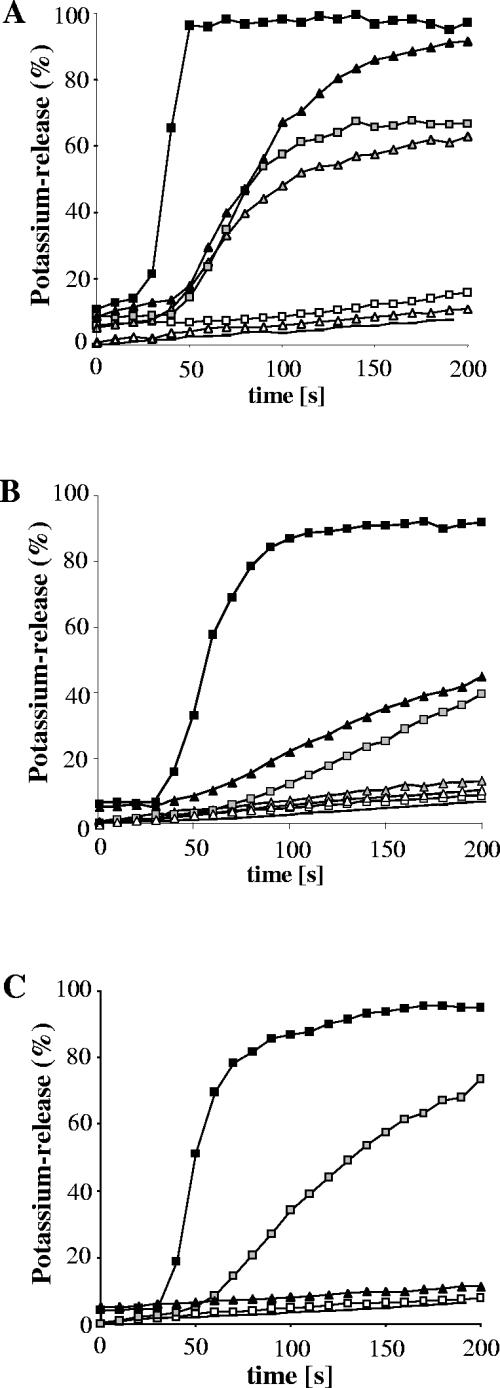
The lipid composition of bacterial membranes differs from species to species and varies considerably with growth conditions, in particular temperature. We used the potassium electrode system to monitor gallidermin-induced efflux from three different indicator strains, i.e., Staphylococcus simulans 22, with intermediate gallidermin susceptibility, and Micrococcus flavus DSM 1790 and Lactococcus lactis subsp. cremoris HP, with high susceptibility (Table 1). Pore formation was most pronounced in M. flavus. Both gallidermin and nisin induced complete and rapid leakage of potassium at a concentration of 500 nM, although with nisin controls, efflux was clearly faster (Fig. 3A). At a concentration of 50 nM (which corresponds to 25 times the MIC for nisin and 8 times the MIC for gallidermin), both peptides were equally effective, whereas no leakage was observed at 5 nM.
TABLE 1.
Antimicrobial activities of gallidermin, epidermin, and gallidermin mutant peptides
| Peptide |
M. flavus DSM 1790
|
S. simulans 22
|
L. lactis subsp. cremoris HP
|
|||
|---|---|---|---|---|---|---|
| MIC (μM) | Potassium releasea | MIC (μM) | Potassium release | MIC (μM) | Potassium release | |
| Epidermin | 0.002 | Yes | 0.15 | —b | 0.002 | — |
| Gallidermin | 0.002 | Yes | 0.15 | Yes | 0.005 | No |
| [L6V]gallidermin | 0.002 | Yes | 0.15 | — | 0.009 | — |
| [A12L]gallidermin | 0.037 | No | 2.3 | — | 0.009 | — |
| Nisin | 0.006 | Yes | 0.19 | Yes | 0.048 | Yes |
FIG. 3.
Potassium release from Micrococcus flavus DSM 1790 (A), Staphylococcus simulans 22 (B), and Lactococcus lactis HP (C) induced by gallidermin (triangles) and nisin (squares). Peptides were added at 30 seconds, and the potassium release was monitored with a potassium electrode. Potassium leakage is expressed relative to the total amount of potassium released after addition of 1 μM nisin (100% value). Peptides were applied at 500 nM (black symbols), 50 nM (gray symbols), or 5 nM (white symbols). Lines without symbols are baselines.
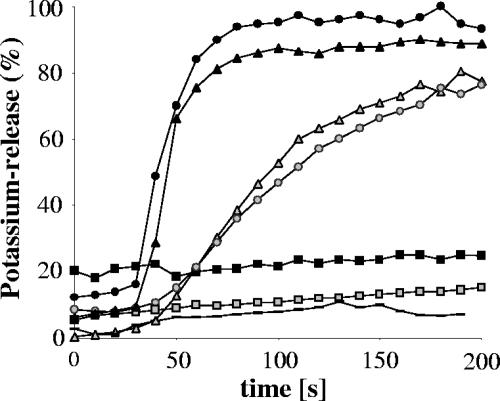
Compared to wild-type activity, gallidermin variant peptides, i.e., epidermin (Fig. 4) and [L6V]gallidermin (data not shown), with an amino acid exchange at position 6 to Ile or Val, exhibited an unaltered capability to form pores. In contrast, mutation in the flexible hinge region of the peptide, i.e., replacement of Ala by Leu at position 12 ([A12L]gallidermin), led to a total loss of the pore-forming activity (Fig. 4). Interestingly, the antimicrobial activity of the non-pore-forming mutant against M. flavus and S. simulans 22 was clearly reduced (Table 1), underlining the important role of the central flexible region for the mechanism of pore formation.
FIG. 4.
Potassium release from Micrococcus flavus cells induced by gallidermin (triangles) epidermin (circles), and [A12L]gallidermin (squares). Peptides were applied at 500 nM (black symbols) or 50 nM (gray symbols); the line without symbols is the baseline.
In the staphylococcal and lactococcal strains, pore formation appeared to contribute less to killing than in the case of M. flavus. In staphylococcal cells, despite MICs in the same range, gallidermin had to be 10 times more concentrated (500 nM) than nisin (50 nM) to induce comparable K+ release levels (Fig. 3B). An even more striking result was obtained with L. lactis. Although this strain is highly susceptible against gallidermin in vivo, we observed no potassium leakage even at the highest concentration (500 nM). Nisin pore formation in this strain was unaltered. However, the pore-forming activity of gallidermin against the three indicator strains tested does not correlate with the antimicrobial activity in vivo (Table 1), indicating that further mechanisms may be involved in its antibiotic activity.
Inhibition of in vitro lipid II biosynthesis.
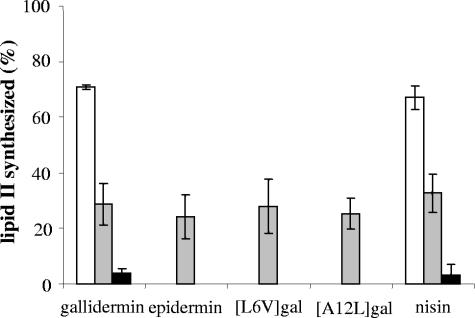
The ability of gallidermin and gallidermin variants to bind to lipid I and subsequently to inhibit the formation of lipid II was analyzed using an in vitro lipid II synthesis assay with radiolabeled UDP-GlcNAc. The conversion of the substrate (C55-P) to lipid II was clearly inhibited by addition of wild-type gallidermin in a concentration-dependent manner (Fig. 5). Almost the same levels of inhibition were obtained after addition of nisin as a control, of epidermin, or of the mutant peptides [L6V]gallidermin and [A12L]gallidermin (substrate/peptide ratio of 1:1) (Fig. 5), demonstrating that neither the described changes in the first ring nor the reduced flexibility in the hinge region affects the binding of gallidermin to lipid I.
FIG. 5.
Inhibition of in vitro lipid II synthesis by gallidermin, epidermin, gallidermin mutant peptides, and nisin. Gallidermin and nisin were tested at molar ratios of 0.5:1 (white bars), 1:1 (gray bars), and 1.5:1 (black bars) with regard to the primary substrate C55-P (10 nmol). Epidermin and gallidermin variants were tested only at a 1:1 (lantibiotic/substrate) molar ratio. The amount of lipid II synthesized by M. flavus membranes in an assay without addition of lantibiotics was taken as 100%. Inhibition of lipid II formation results from complexation of the peptides with the intermediate substrate lipid I. Mean values and standard deviations from three independent experiments are shown.
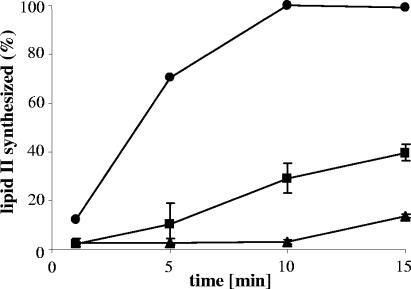
We also tested the inhibition of in vitro lipid II biosynthesis at a fixed peptide/C55-P ratio (1:1) over time. In such an assay, gallidermin was superior to nisin (Fig. 6) in that it completely blocked synthesis of lipid II within the first 10 minutes of incubation. These results suggest that gallidermin may have a higher binding affinity to lipid I than nisin, which might help to explain the excellent in vivo activity of gallidermin against several strains in spite of its inability to form pores.
FIG. 6.
Impact of gallidermin (triangles) and nisin (squares) on the kinetics of in vitro lipid II synthesis. Peptides were added at 10 nmol to the synthesis assay in 150 μl containing 10 nmol C55-P. Lipid II synthesized after 15 min of incubation in the absence of the peptides (circles) was taken as 100%. Mean values and standard deviations from three independent experiments are shown.
Inhibition of lipid II-Gly1 biosynthesis.
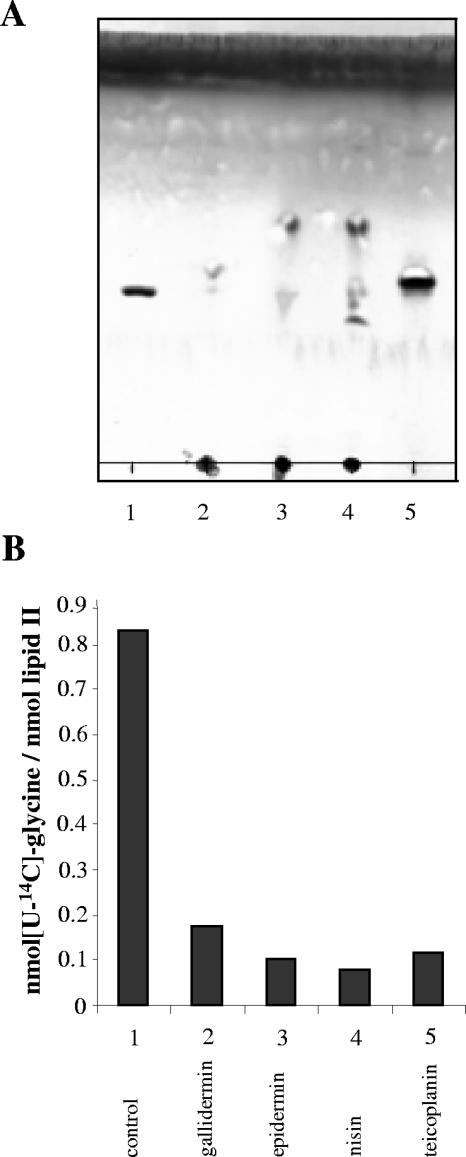
In a search for further inhibitory activities, we also tested whether gallidermin may interfere with subsequent steps of cell wall precursor biosynthesis, such as the formation of the interpeptide bridge. The initial step of pentapeptide bridge formation in staphylococci is the addition of a glycine residue to the stem peptide of lipid II catalyzed by the peptidyl-transferase FemX. The activity of FemX can be analyzed in vitro, using the purified enzyme as well as lipid II and the charged Gly-tRNA as substrates (45). In this assay approximately 0.8 mol glycine was introduced per mol of lipid II in the absence of inhibitors, which is close to the theoretical value of 1. In contrast, when a glycopeptide antibiotic such as teicoplanin was added, no radiolabeled glycine was detected in lipid II (Fig. 7A and B, lane and bar 5). Glycopeptides are known to bind to lipid II at the d-Ala-d-Ala terminus of the pentapeptide chain (36), which obviously not only blocks the transglycosylation reaction but also interferes with the activity of FemX. When lantibiotics were added to the reaction mix at a molar ratio of 1:2 (lipid II:peptide), lipid II was not found to migrate in the TLC system used. Rather, lipid II was found together with the lantibiotics at the application spot. Apparently, and in contrast to teicoplanin, all three lantibiotics equally form a stable complex with lipid II which does not dissociate in the TLC solvent system. Moreover, lipid II isolated from the application spot did not contain any radiolabeled glycine, demonstrating the strong inhibition of the FemX reaction by nisin, gallidermin, and epidermin.
FIG. 7.
Inhibition of in vitro lipid II-Gly1 synthesis by gallidermin, epidermin, nisin, and teicoplanin (lanes and bars 2 to 5, respectively). (A) TLC of reaction mixtures of purified lipid II incubated with [U-14C]glycine in the presence of recombinant tRNA synthetase and purified tRNA with FemX in the absence (lane-1) or in the presence (lanes 2 to 5) of the peptides. (B) Ratio of [U-14C]glycine incorporated per lipid II as determined from the TLC presented in panel A. The results of one representative experiment are shown.
DISCUSSION
In this study we have demonstrated that the type A lantibiotics gallidermin and epidermin are able to kill bacteria at nanomolar concentrations through complex mechanisms of action. We found that the antibiotic activity of gallidermin against two of the indicator strains used in this study, i.e., M. flavus and S. simulans, can be well explained by a dual mode of action, which involves pore formation and cell wall inhibition by specific interaction with cell wall precursor lipid II according to the model described for nisin (52). Using the potassium release assay, we observed that gallidermin forms pores in the membrane of both strains and that the rate of pore formation (Fig. 3) is reflected in the MIC values (Table 1), i.e., higher release rates were obtained for the more susceptible strain, M. flavus. With a mutant peptide in which a bulky amino acid side chain was introduced into the flexible hinge region ([A12L]gallidermin) (38), we observed both loss of the pore-forming activity (Fig. 4) and significantly reduced in vivo activity (Table 1), demonstrating that pore formation is an important factor contributing to the overall killing mechanism for this strain. The remaining antimicrobial activity of the hinge region mutant peptide can be well explained by the unaltered ability to bind to lipid I and lipid II and thus to inhibit cell wall biosynthesis.
Generally, the pore formation capacity of gallidermin was reduced compared to that of nisin and clearly depended on membrane thickness. Only when liposome membranes were made of phospholipids with C14 or shorter acyl chains was pore formation observed, which was facilitated when lipid II was incorporated into the bilayer. The thickness of the lipid bilayer in vesicles made of monounsaturated phosphorylcholines has been shown to depend on the number of acyl chain carbons and can be calculated according to dL ≅ (15.9 ± 1.4) + n(1.5 ± 0.2) Å (30), so that, e.g., the thickness of a membrane made of PC14:1 is approximately 37 Å. Since incorporation of cholesterol is known to increase the thickness of membranes prepared from short-chain fatty acid phospholipids (47), the C14 liposomes used in our study can be estimated to be close to 40 Å. For the DOPC membranes, which were prepared without cholesterol, a thickness of 43 Å can be assumed. Based on these figures, it can be expected that nisin, with an overall length of 50 Å, is able to form pores, while epidermin and gallidermin, with an overall length of 30 Å, in theory may be considered to be too short to span a membrane. Currently, there is not enough information on the architecture of lipid II-mediated pores to conclusively discuss the molecular basis for the (albeit clearly reduced) ability of gallidermin and epidermin to form pores. For nisin, it has been suggested that lipid II is an integral part of the pore (10). If this holds true for gallidermin, the composite pore aggregate may compensate for the theoretical size deficiency of this lantibiotic. Also, the Shai-Matsusaki-Huang model, which describes the non-target-mediated membrane poration by amphiphilic peptides, proposes a membrane-thinning effect before the actual poration process takes place (54). In addition, the changes induced by cholesterol and possibly yet-undefined effects of lipid II on the properties of model membranes may facilitate pore formation by gallidermin.
With intact cells, pore formation was only observed in the staphylococcal and micrococcal strains, whereas in the Lactococcus lactis strain no gallidermin-induced potassium leakage occurred. In addition, [A12L]gallidermin, which lost its pore-forming activity in Micrococcus flavus (Fig. 4), was as active as wild-type gallidermin against this strain (Table 1), indicating that indeed in lactococci pore formation does not contribute to killing. Interestingly, L. lactis membranes have been reported to contain considerable proportions of phospholipids with 16, 18, and 19 C atoms; the average acyl chain length had been calculated to be 17.1 (25). In contrast, for several staphylococcal species (32), for S. aureus (48, 50), and for Micrococcus luteus (51), a high proportion of C15 and very little C17 or longer acyl chains have been reported. Whether these figures are sufficient to explain the observed differences in pore formation capacity of gallidermin remains to be studied. Very little is known about the inhomogeneous distribution of membrane lipids or the occurrence of lipid raft-like structures in bacteria, which could lead to localized areas of reduced membrane thickness. Also, the distribution of lipid II in membranes is unknown, and it is conceivable that it concentrates at sites of de novo cell wall synthesis. In such spots, specific local environments that might be favorable for pore formation by a specific lantibiotic in a given species could occur.
Remarkably, epidermin and gallidermin were 10 to 20 times more potent against L. lactis than nisin in spite of the missing pore formation capacity. In the in vitro lipid II biosynthesis assay, gallidermin was more efficient in blocking the addition of N-acetylglucosamine to lipid I. It should be noted that this reaction takes place at the inner leaflet of the cytoplasmic membrane so that epidermin and nisin would have to cross the membrane in order to inhibit this reaction. Experimental proof for translocation of lantibiotics across the cytoplasmic membrane is lacking so far. However, it has been demonstrated that cationic amphiphilic peptides do cross membranes and act on cytoplasmic targets (for a review, see reference 12), and it is conceivable that this also happens with lantibiotics; interactions with bactoprenol might even be helpful for translocation. In such a context, a small molecule such as gallidermin/epidermin might translocate more easily than nisin and thus might more strongly inhibit cytoplasmic cell wall biosynthesis reactions such as lipid II synthesis and the subsequent formation of the pentaglycine interpeptide bridge. On the other hand, the higher affinity for lipid I could also be reflected in a higher affinity of gallidermin for lipid II, and this may enable stronger inhibition of transglycosylation which occurs on the outside, where lipid II is readily accessible.
Structural information on the interaction of lantibiotics with the cell wall precursor so far is restricted to lipid II. Hsu et al. (24) described the pyrophosphate moiety of lipid II as the primary binding site for nisin. They identified six hydrogen bonds between backbone amides of rings A and B of nisin and the pyrophosphate moiety. Bonev et al. (6) demonstrated that nisin can also bind bactoprenol pyrophosphate; however, the affinity was considerably lower than that for the complete lipid II molecule. This demonstrates that for high-affinity binding of nisin additional interactions must take place, presumably between the N-acetylmuramyl moieties, whereas the pentapeptide side chain and the isoprenoid moiety may not be involved. Evidence for the interaction of the lantibiotics with lipid I stems mainly from the observation that lipid II biosynthesis is strongly blocked; structural analysis of a lantibiotic-lipid I complex has not been reported. The A- and B-ring system of nisin, which has been shown to be responsible for binding lipid II, in particular the pyrophosphate moiety, is conserved in nisin, subtilin, epidermin/gallidermin, and a number of structural variants, such as mutacin 1140, mutacin I, ericin A, and streptin. A structural element in gallidermin/epidermin that could be responsible for higher affinity, compared to nisin, is Lys in position 4 (instead of Ile in nisin), which provides an additional positive charge that may enhance binding to the pyrophosphate moiety.
The antimicrobial activity of gallidermin, as reflected in the MIC of a given strain, may also be influenced by lipid II-independent factors, e.g., the lantibiotics nisin and Pep5 have been shown to induce autolysis of susceptible staphylococcal cells, resulting in massive cell wall degradation. The cationic peptides activate the autolysins by displacing them from their binding sites in the peptidoglycan (4, 5). Also, binding and nontargeted pore formation by nisin in the absence of lipid II were shown to depend on the overall negative surface charge of the cytoplasmic membrane (8). This phenomenon was also described for antimicrobial defense peptides (for a review, see reference 33), and Staphylococcus aureus achieves resistance to such peptides by modifying anionic phosphatidylglycerol in the cytoplasmic membrane with positively charged l-lysine (46). In addition, the charge of the cell wall influenced sensitivity towards cationic antimicrobial peptides, since staphylococcal mutants with increased sensitivity were shown to have an altered teichoic acid structure. The mutant teichoic acids lacked d-alanine, as a result of which the cells carried an increased negative surface charge (39). Thus, the net charge of the bacterial envelope, which is modulated during the cell cycle and under varying physiological conditions, is an important contributor to the susceptibility of bacteria towards cationic peptides and needs to be considered when modes of action and MICs are correlated.
Taken together, the overall antimicrobial activity of gallidermin is a complex process that involves specific interactions with the cell wall precursor lipid II and possibly lipid I, leading to cell wall inhibition and, in some cases, to pore formation; lipid II-independent nontargeted interactions with the bacterial cell and possibly additional effects which remain to be identified may further contribute to killing. The multiple activities may combine differently for individual target bacteria and explain the range of sensitivities of various bacterial species (22). The combination of different modes of action can provide a new concept for the design of future multifunctional antibiotics to combat primarily multiresistant pathogens.
Acknowledgments
We are grateful to F. Götz for gallidermin and gallidermin mutant peptide producer strains and to Alessandro Tossi for mass determination of the peptides.
R. R. Bonelli received a fellowship from the Brazilian government agency CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior). Financial support was also obtained from the German Research Foundation (grant Sa 292/9-2, 9-4 to H. G. Sahl).
Footnotes
This paper is dedicated to Professor Hans-Georg Trüper on the occasion of his 70th birthday.
REFERENCES
- 1.Allgaier, H., G. Jung, R. G. Werner, U. Schneider, and H. Zahner. 1986. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur. J. Biochem. 160:9-22. [DOI] [PubMed] [Google Scholar]
- 2.Benz, R., G. Jung, and H. G. Sahl. 1991. Mechanism of channel-formation by lantibiotics in black lipid membranes, p. 359-372. In G. Jung and H. G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM Science Publishers, Leiden, The Netherlands.
- 3.Bhattacharya, S., and S. Haldar. 2000. Interactions between cholesterol and lipids in bilayer membranes. Role of lipid headgroup and hydrocarbon chain-backbone linkage. Biochim. Biophys. Acta 1467:39-53. [DOI] [PubMed] [Google Scholar]
- 4.Bierbaum, G., and H. G. Sahl. 1987. Autolytic system of Staphylococcus simulans 22: influence of cationic peptides on activity of N-acetylmuramoyl-l-alanine amidase. J. Bacteriol. 169:5452-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierbaum, G., and H. G. Sahl. 1985. Induction of autolysis of staphylococci by the basic peptide antibiotic Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch. Microbiol. 141:249-254. [DOI] [PubMed] [Google Scholar]
- 6.Bonev, B. B., E. Breukink, E. Swiezewska, B. De Kruijff, and A. Watts. 2004. Targeting extracellular pyrophosphates underpins the high selectivity of nisin. FASEB J. 18:1862-1869. [DOI] [PubMed] [Google Scholar]
- 7.Bramhall, J., J. Hofmann, R. DeGuzman, S. Montestruque, and R. Schell. 1987. Temperature dependence of membrane ion conductance analyzed by using the amphiphilic anion 5/6-carboxyfluorescein. Biochemistry 26:6330-6340. [DOI] [PubMed] [Google Scholar]
- 8.Breukink, E., C. van Kraaij, A. van Dalen, R. A. Demel, R. J. Siezen, B. de Kruijff, and O. P. Kuipers. 1998. The orientation of nisin in membranes. Biochemistry 37:8153-8162. [DOI] [PubMed] [Google Scholar]
- 9.Breukink, E., C. van Kraaij, R. A. Demel, R. J. Siezen, O. P. Kuipers, and B. de Kruijff. 1997. The C-terminal region of nisin is responsible for the initial interaction of nisin with the target membrane. Biochemistry 36:6968-6976. [DOI] [PubMed] [Google Scholar]
- 10.Breukink, E., H. E. van Heusden, P. J. Vollmerhaus, E. Swiezewska, L. Brunner, S. Walker, A. J. R. Heck, and B. de Kruijff. 2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 278:19898-19903. [DOI] [PubMed] [Google Scholar]
- 11.Breukink, E., I. Wiedemann, C. van Kraaij, O. P. Kuipers, H. Sahl, and B. de Kruijff. 1999. Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361-2364. [DOI] [PubMed] [Google Scholar]
- 12.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitor in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 13.Brötz, H., G. Bierbaum, K. Leopold, P. E. Reynolds, and H. G. Sahl. 1998. The lantibiotic mersacidin inhibits peptidoglycan synthesis by tageting lipid II. Antimicrob. Agents Chemother. 42:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brötz, H., M. Josten, I. Wiedemann, U. Schneider, F. Götz, G. Bierbaum, and H. G. Sahl. 1998. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics. Mol. Microbiol. 30:317-327. [DOI] [PubMed] [Google Scholar]
- 15.Burianek, L. L., and A. E. Yousef. 2000. Solvent extraction of bacteriocins from liquid cultures. Lett. Appl. Microbiol. 31:193-197. [DOI] [PubMed] [Google Scholar]
- 16.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacterial lantibiotics: strategies to improve therapeutic potential. Curr. Protein Pept. Sci. 6:61-75. [DOI] [PubMed] [Google Scholar]
- 17.Demel, R. A., T. Peelen, R. J. Siezen, B. de Kruijff, and O. P. Kuipers. 1996. Nisin Z, mutant nisin Z and lacticin 481 interactions with anionic lipids correlate with antimicrobial activity. A monolayer study. Eur. J. Biochem. 235:267-274. [DOI] [PubMed] [Google Scholar]
- 18.Driessen, A. J., H. W. van den Hooven, W. Kuiper, M. van de Kamp, H. G. Sahl, R. N. Konings, and W. N. Konings. 1995. Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry 34:1606-1614. [DOI] [PubMed] [Google Scholar]
- 19.Freund, S., G. Jung, O. Gutbrod, G. Folkers, W. A. Gibbons, H. Allgaier, and R. Werner. 1991. The solution structure of the lantibiotic gallidermin. Biopolymers 31:803-811. [DOI] [PubMed] [Google Scholar]
- 20.Goodman, M., D. E. Palmer, D. Mierke, S. Ro, K. Nunami, T. Wakamiya, K. Fukase, S. Horimoto, M. Kitazawa, H. Fujita, A. Kubo, and T. Shiba. 1991. Conformations of nisin and its fragments using synthesis, NMR and computer simulations, p. 59-75. In G. Jung and H. G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM Science Publishers, Leiden, The Netherlands.
- 21.Guder, A., I. Wiedemann, and H. G. Sahl. 2000. Posttranslationally modified bacteriocins—the lantibiotics. Biopolymers 55:62-73. [DOI] [PubMed] [Google Scholar]
- 22.Héchard, Y., and H. G. Sahl. 2002. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 84:545-557. [DOI] [PubMed] [Google Scholar]
- 23.Hope, M. J., M. B. Bally, G. Webb, and P. R. Cullis. 1985. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta 812:55-65. [DOI] [PubMed] [Google Scholar]
- 24.Hsu, S. T., E. Breukink, E. Tischenko, M. A. Lutters, B. De Kruijff, R. Kaptein, A. M. Bonvin, and N. A. van Nuland. 2004. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 11:963-967. [DOI] [PubMed] [Google Scholar]
- 25.In ’t Veld, G., A. J. M. Driessen, J. A. F. Op den Kamp, and W. N. Konings. 1991. Hydrophobic membrane thickness and lipid-protein interactions of the leucine transport system of Lactococcus lactis. Biochim. Biophys. Acta 1065:203-212. [DOI] [PubMed] [Google Scholar]
- 26.Jung, G. 1991. Lantibiotics: a survey, p. 1-34. In G. Jung and H. G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM Science Publishers, Leiden, The Netherlands.
- 27.Kellner, R., G. Jung, T. Horner, H. Zahner, N. Schnell, K. D. Entian, and F. Götz. 1988. Gallidermin: a new lanthionine-containing polypeptide antibiotic. Eur. J. Biochem. 177:53-59. [DOI] [PubMed] [Google Scholar]
- 28.Kohlrausch, U., and J. V. Höltje. 1991. One-step purification procedure for UDP-N-acetylmuramyl-peptide murein precursors from Bacillus cereus. FEMS Microbiol. Lett. 62:253-257. [DOI] [PubMed] [Google Scholar]
- 29.Kordel, M., F. Schüller, and H. G. Sahl. 1989. Interaction of the pore forming-peptide antibiotics Pep 5, nisin and subtilin with non-energized liposomes. FEBS Lett. 244:99-102. [DOI] [PubMed] [Google Scholar]
- 30.Kučerka, N., D. Uhriková, J. Teixeira, and P. Balgavý. 2004. Bilayer thickness in unilamellar phosphatidylcholine vesicles: small-angle neutron scattering using contrast variation. Physica B 350:e639-e642. [Google Scholar]
- 31.Kupke, T., C. Kempter, G. Jung, and F. Götz. 1995. Oxidative decarboxylation of peptides catalyzed by flavoprotein EpiD. Determination of substrate specificity using peptide libraries and neutral loss mass spectrometry. J. Biol. Chem. 270:11282-11289. [DOI] [PubMed] [Google Scholar]
- 32.Lambert, L. H., T. Cox, K. Mitchell, R. A. Rosselló-Mora, C. del Cueto, D. E. Dodge, P. Orkand, and R. J. Cano. 1998. Staphylococcus succinus sp. nov., isolated from Dominican amber. Int. J. Syst. Bacteriol. 48:511-518. [DOI] [PubMed] [Google Scholar]
- 33.Lohner, K., and S. E. Blondelle. 2005. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 8:241-256. [DOI] [PubMed] [Google Scholar]
- 34.Mayer, L. D., M. J. Hope, and P. R. Cullis. 1986. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim. Biophys. Acta 858:161-168. [DOI] [PubMed] [Google Scholar]
- 35.Moll, G. N., G. C. Roberts, W. N. Konings, and A. J. Driessen. 1996. Mechanism of lantibiotic-induced pore-formation. Antonie Leeuwenhoek 69:185-191. [DOI] [PubMed] [Google Scholar]
- 36.Nieto, M., and H. R. Perkins. 1971. Modifications of the acyl-d-alanyl-d-alanine terminus affecting complex-formation with vancomycin. Biochem. J. 123:789-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orlov, D. S., T. Nguyen, and R. I. Lehrer. 2002. Potassium release, a useful tool for studying antimicrobial peptides. J. Microbiol. Methods 49:325-328. [DOI] [PubMed] [Google Scholar]
- 38.Ottenwälder, B., T. Kupke, S. Brecht, V. Gnau, J. Metzger, G. Jung, and F. Götz. 1995. Isolation and characterization of genetically engineered gallidermin and epidermin analogs. Appl. Environ. Microbiol. 61:3894-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peschel, A., M. Otto, R. J. Jack, H. Kalbacher, G. Jung, and F. Götz. 1999. Inactivation of the dtl operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 274:8405-8410. [DOI] [PubMed] [Google Scholar]
- 40.Rick, P. D., G. L. Hubbard, M. Kitaoka, H. Nagaki, T. Kinoshita, S. Dowd, V. Simplaceanu, and C. Ho. 1998. Characterization of the lipid-carrier involved in the synthesis of enterobacterial common antigen (ECA) and identification of a novel phosphoglyceride in a mutant of Salmonella typhimurium defective in ECA synthesis. Glycobiology 8:557-567. [DOI] [PubMed] [Google Scholar]
- 41.Rouser, G., S. Fleischer, and A. Yamamoto. 1970. 2-Dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494-496. [DOI] [PubMed] [Google Scholar]
- 42.Ruhr, E., and H. G. Sahl. 1985. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob. Agents Chemother. 27:841-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahl, H. G. 1991. Pore formation in bacterial membranes by cationic lantibiotics, p. 347-358. In G. Jung and H. G. Sahl (ed.), Nisin and novel lantibiotics. ESCOM Science Publishers, Leiden, The Netherlands.
- 44.Sahl, H. G., and G. Bierbaum. 1998. Lantibiotics: biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 52:41-79. [DOI] [PubMed] [Google Scholar]
- 45.Schneider, T., M. M. Senn, B. Berger-Bächi, A. Tossi, H. G. Sahl, and I. Wiedemann. 2004. In vitro assembly of a complete, pentaglycine interpeptide bridge containing cell wall precursor (lipid II-Gly(5)) of Staphylococcus aureus. Mol. Microbiol. 53:675-685. [DOI] [PubMed] [Google Scholar]
- 46.Staubitz, P., H. Neumann, T. Schneider, I. Wiedemann, and A. Peschel. 2004. MprF-mediated biosynthesis of lysylphosphatidylglycerol, an important determinant in staphylococcal defensin resistance. FEMS Microbiol. Lett. 231:67-71. [DOI] [PubMed] [Google Scholar]
- 47.Tahara, Y., and Y. Fujiyoshi. 1994. A new method to measure bilayer thickness: cryo-electron microscopy of frozen hydrated liposomes and image simulation. Micron 25:141-149. [DOI] [PubMed] [Google Scholar]
- 48.Theodore, T. S., and C. Panos. Protein and fatty acid composition of mesosomal vesicles and plasma membranes of Staphlococcus aureus. J. Bacteriol. 116:571-576. [DOI] [PMC free article] [PubMed]
- 49.van Heusden, H. E., B. De Kruijff, and E. Breukink. 2002. Lipid II induces a transmembrane orientation of the pore-forming peptide lantibiotic nisin. Biochemistry 41:12171-12178. [DOI] [PubMed] [Google Scholar]
- 50.Ward, J. B., and H. R. Perkins. 1968. The chemical composition of the membrane protoplasts and L-forms of Staphylococcus aureus. Biochem. J. 106:391-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welby, M., and J. F. Tocanne. Evidence for the incorporation of a fluorescent anthracene fatty acid into the membrane lipids of Micrococcus luteus. Biochim. Biophys. Acta 689:173-176. [DOI] [PubMed]
- 52.Wiedemann, I., E. Breukink, C. van Kraaij, O. P. Kuipers, G. Bierbaum, B. De Kruijff, and H. G. Sahl. 2001. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. J. Biol. Chem. 276:1772-1779. [DOI] [PubMed] [Google Scholar]
- 53.Wiedemann, I., R. Benz, and H. G. Sahl. 2004. Lipid II-mediated pore formation by the peptide antibiotic nisin: a black lipid membrane study. J. Bacteriol. 186:3259-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]