Abstract
Gentamicin accumulates in the lysosomes of kidney proximal tubular cells and causes apoptosis at clinically relevant doses. Gentamicin-induced apoptosis can be reproduced with cultured renal cells, but only at high extracellular concentrations (1 to 3 mM; 0.4 to 1.2 g/liter) because of its low level of uptake. We recently showed that gentamicin-induced apoptosis in LLC-PK1 cells involves a rapid (2-h) permeabilization of lysosomes and activation of the mitochondrial pathway of apoptosis (10 h). We now examine whether the delivery of gentamicin to the cytosol by electroporation would sensitize LLC-PK1 cells to apoptosis. Cells were subjected to eight pulses (1 ms) at 800 V/cm (square waves) in the presence of gentamicin (3 μM to 3 mM; 1.2 mg/liter to 1.2 g/liter); returned to gentamicin-free medium; and examined at 8 h for their Bax (a marker of mitochondrial pathway activation) contents by Western blotting and competitive reverse transcriptase PCR and at 24 h for apoptosis by 4′,6′-diamidino-2′-phenylindole staining (confirmed by electron microscopy) and for necrosis (by determination of lactate dehydrogenase release). Nonelectroporated cells were incubated with gentamicin for 8 and 24 h. Significant increases in Bax levels (8 h) and apoptosis (24 h) were detected with 0.03 mM (13.2 mg/liter) gentamicin in electroporated cells compared with those achieved with 2 mM (928 mg/liter) in incubated cells. The increase in the Bax level was not associated with an increase in the level of its mRNA but was associated with the accumulation of ubiquitinated forms (probably as a result of impairment of its degradation by the proteasome). Assay of cell-associated gentamicin showed a marked, immediate, but transient accumulation in electroporated cells, whereas a slow, steady uptake was detected in incubated cells. The data indicate that cytosolic gentamicin triggers apoptosis. Sequestration of gentamicin in lysosomes would, to some extent, protect against apoptosis.
Aminoglycoside antibiotics have long been known to cause acute renal failure in patients, in association with histological and functional signs of proximal tubule toxicity (1). Yet, the underlying molecular mechanisms still remain poorly defined (15). Aminoglycosides enter proximal tubular cells by pinocytosis from the luminal pole and accumulate in lysosomes (18, 27, 62), where they cause a conspicuous phospholipidosis (29, 32). Maneuvers that prevent gentamicin pinocytic uptake (59) and/or the development of lysosomal phospholipidosis (28) protect against nephrotoxicity. Together with the fact that the corresponding morphological and biochemical alterations develop early on in animals or humans treated with clinically relevant doses (10, 32), this was the main argument that linked aminoglycoside cell toxicity to its lysosomal storage and the ensuing organelle dysfunction (40). Gentamicin, indeed, is a highly polar compound and is therefore largely considered to be unable to pass across membranes and to act outside of lysosomes. Besides lysosomal changes, however, the proximal tubules of animals treated with gentamicin also show clear signs of apoptosis (11, 34). This effect of gentamicin, together with the lysosomal alterations described above, can be reproduced in vitro with both renal and nonrenal cells (2, 12). These, however, must be exposed to high concentrations of gentamicin because of poorly efficient drug uptake (12). Using LLC-PK1 cells, an accepted model of renal proximal tubular cells for the study of aminoglycoside toxicity (24, 64, 66, 67, 72), we observed that gentamicin causes an early permeabilization of lysosomes before activation of the mitochondrial pathway of apoptosis can be detected (61). In parallel, recent data indicate that a minor but probably significant proportion of the gentamicin accumulated by cells can reach the cytosol and the mitochondria by trafficking through the Golgi apparatus and the endoplasmic reticulum (56). We also know that apoptosis can be triggered in the kidney by cytosolic signals, such as an increase in the cytosolic Bax protein content, which activates the so-called mitochondrial pathway of apoptosis (54). This has raised the question whether it is not the cytosolic gentamicin that is released from or transported out of lysosomes that triggers apoptosis. This has now been tested directly by taking advantage of the technique of electroporation, which allows the direct delivery of membrane-impermeant solutes into cells (14). Our results show that LLC-PK1 cells undergo extensive apoptosis when they are subjected to electroporation in the presence of concentrations of gentamicin about 100-fold lower than what is necessary if cells are incubated with the drug. We also show that this process is associated with an increase in the cell content of the proapoptogenic protein Bax, which acts upstream of the mitochondria in the cascade that leads to apoptosis (71). The data therefore imply that the lysosomal sequestration of gentamicin could actually protect cells from its apoptogenic effect and thereby mitigate its toxicity.
MATERIALS AND METHODS
Cells.
All experiments were performed with LLC-PK1 (Lilly Laboratories Culture-pig kidney) cells, clone ATCC CL-101, that originated from and that displayed the attributes of proximal tubular cells (60). They were cultivated in Dubelcco's modified Eagle's medium supplemented with 10% fetal calf serum in an atmosphere of 95% air-5% CO2; subcultured twice a week; and used at about 80% confluence to avoid cell detachment, which spontaneously takes place after the cells reach confluence. Cells grown under these conditions are not polarized and, in contrast to LLC-PK1 cells grown on inserts to trigger their polarization (47), do not express megalin (17), a protein that causes receptor-mediated endocytosis of gentamicin in renal tubular cells in vivo (42). Previous studies showed that gentamicin is taken up by fluid endocytosis in LLC-PK1 cells (i.e., without evidence of membrane binding) under our conditions of culture (12).
Electroporation.
We follow the general technique developed for delivery of membrane-impermeant drugs in mammalian cells (14, 26, 53). In brief, subconfluent cells were detached by trypsinization (5 g/liter trypsin, 2 g/liter sodium EDTA, 8. g/liter NaCl), centrifuged at 1,000 rpm (5810 R centrifuge equipped with an A-4-62 rotor; Eppendorf AG, Hamburg, Germany), and resuspended in electroporation buffer (10 mM Na2HPO4/KH2PO4, pH 7.2, 250 mM sucrose, 1 mM MgCl2) in the presence of a suitable concentration of gentamicin (0 to 3 mM). One hundred microliters of cell suspension (roughly corresponding to 50 μg of cell protein) was placed in electroporation cuvettes (model 620; BTX-Genetronics Inc., San Diego, CA) made of two embedded aluminum electrodes 2 mm apart. The cells were exposed to 8 square-wave pulses (PA-4000 device; Cyto Pulse Sciences, Inc., Columbia, MD) with an electric field of 800 V/cm for 1 ms (with a 1-Hz repetition frequency) and were thereafter left for 15 min at room temperature in the same medium. They were then dispersed in 5 ml of gentamicin-free, complete culture medium and transferred into cell culture dishes for incubation at 37°C for up to 24 h. These conditions were selected based on preliminary pilot studies that assessed the efficiency of the cell penetration of two nonpermeant tracers (trypan blue and lucifer yellow) compared with the release of the cytosolic enzyme lactate dehydrogenase (as a marker of cell membrane leakage). For the sake of conciseness, cells treated according to this protocol are consistently referred to as “electroporated.”
Incubation.
The cells were continuously exposed to gentamicin at 37°C in complete cell culture medium at the indicated concentrations and for the indicated times. Cells treated according to this protocol are consistently referred to as “incubated.”
Detection of apoptosis and necrosis.
Apoptotic cells were enumerated after they were stained with 4′,6′-diamidine-2′-phenylindole (DAPI) to reveal the characteristic nuclear changes of apoptosis (condensation and fragmentation of the nuclear material), as described earlier (61). Necrosis was assessed by measuring the amount of the lactate dehydrogenase activity released in the medium (43).
Measurement of gentamicin cell content.
Cells were collected by scrapping them into ice-cold phosphate-buffered saline (PBS) after three successive washes with the same buffer and were subjected to brief sonication. Gentamicin was then assayed by a disk-plate microbiological technique using Bacillus subtilis (ATCC 6633) as the test organism, as described previously (12, 68). Our routine assay had a limit of detection of 0.1 mg/liter, with a range of linearity up to 64 mg/liter (R2 = 0.994 ± 0.005 [n = 6]). The protein content of the samples was measured by the Folin-Ciocalteu method (38). All gentamicin cell contents were expressed as μg/mg cell protein. The apparent cellular gentamicin concentration was then calculated based on a cell volume of 5 μl per mg protein (68). In previous studies, we showed that samples from control cells spiked with amounts of gentamicin similar to those detected here in electroporated or incubated cells gave readings within 90% of the expected values.
Detection of Bax and ubiquitinated Bax.
The cells were washed three times with ice-cold PBS and resuspended in lysis buffer (10 mM N-2-hydroxyethyl piperazine-N′-2-ethane sulfonic acid [HEPES], 2 mM EDTA, 0.1% 3-[(3-cholamidopropyl) dimethyl-ammonio]-1-propane sulfonate [CHAPS], 5 mM 1,4-dithiothreitol [DTT]) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/ml pepstatin A, 10 μg/ml aprotinin, 20 μg/ml leupeptin). The samples were then subjected to five successive cycles of freezing-thawing (dry ice, acetone, 37°C water bath) and centrifuged at 12,000 rpm for 20 min (Eppendorf 5415C centrifuge). The supernatants were collected, and 10 μg of protein (as measured by a modified Bradford method [51]) was mixed with Laemmli buffer for detection of Bax by Western blot analysis, as described previously (61), and by use of a rabbit anti-Bax antibody raised against a synthetic peptide corresponding to amino acids 44 to 62 of human Bax (1:500; Calbiochem Immunochemicals catalog no. 196821; Merck KGaA, Darmstadt, Germany) and peroxidase-labeled anti-rabbit immunoglobulin G (IgG). Bands were revealed by using the SuperSignal West Pico chemiluminescence substrate (Pierce, Rockford, IL). The films were scanned with a commercial flatbed digitizer operated at a resolution of 600 dpi, and the images were subjected to densitometric analysis for the bands corresponding to the monomer of Bax (21 kDa [48]) by using Image J software (version 1.3.1; available from the Research Service Branch of the National Institute of Mental Health [http://rsb.info.nih.gov/ij]). For detection of ubiquitinated Bax, the lysis buffer was supplemented with 1 mg/liter ubiquitin aldehyde and 10 μM Z-Leu-Leu-Leu-aldehyde (MG132; an inhibitor of the proteasome) and protease inhibitors (pepstatin, aprotinin, leupeptin). Fifty micrograms of protein was then immunoprecipitated by addition of 3 μl of undiluted anti-Bax antibody, followed by an overnight incubation at 4°C in a total volume of 1 ml. The mixture was then mixed with 30 μl protein A-agarose beads for 2 h at 4°C and centrifuged at 12,500 rpm (Eppendorf 5415C centrifuge) for 5 min at 4°C. The pellets were washed twice with ice-cold lysis buffer, once in 0.5 M NaCl, and once with PBS and were finally resuspended in Laemmli buffer for Western blot analysis. Ubiquitinated proteins were identified by incubating the membranes for 24 h at 4°C with a mouse monoclonal antibody (FK2; 1:1,000) directed against ubiquitinated proteins (but not free ubiquitin) and obtained from Biomol International LP, Plymouth, PA. Visualization was obtained with peroxidase-labeled anti-mouse IgG, and the bands were revealed and quantified as described above by using the 47- to 55-kDa region corresponding to the ubiquitinated forms of Bax (35, 50).
Quantitative measurement of Bax mRNA.
We used the competitive reverse transcriptase PCR (RT-PCR) technique using an internal standard (7). RNA was extracted by using the TRIzol reagent (Life Technologies, Gaithersburg, MD) and treated with 0.8 kU of DNase I (QIAGEN GmbH, Hilden, Germany) for 15 min at room temperature. The RNA was purified with the clean-up RNeasy Mini Kit (QIAGEN), the purity was assessed by spectrophotometry (ratio of absorbance at 260 nm/absorbance at 280 nm in water of at least 1.7 to 1.8), and the quantity of RNA was calculated by determination of its absorbance at 260 nm (assuming a specific absorption coefficient of 0.025 ml μg−1 cm−1). cDNA synthesis was carried out with the reverse transcription system of Promega (Promega Corp., Madison, WI) with 5 μg of RNA. The internal standard was prepared by RT-PCR and was designed to be smaller than the target amplicon in the competitive RT-PCR. The forward primer contained an internal sequence of bax preceded by a more upstream sequence in the gene (underlined), which corresponds to the full-length bax forward primer sequence (underlined) (5′-CAGCTCTGAGCAGATCATGAAGACAGGACAGTAACATGGAGCTGCA-3′), and the sequence shown below as the reverse primer. The PCR product was subjected to agarose gel electrophoresis, and the amplicon (sBax; which was used as the internal standard) was cut and extracted by using a QIAquick gel extraction kit (QIAGEN). The DNA was quantified by performing electrophoresis with the MassRuler DNA ladder (Fermentas International Inc., Burlington, Ontario, Canada). Low-range intensities were assessed by a Bio-Rad Gel Doc analyzer operated with Quantity One software. The estimation of the Bax mRNA concentration was performed by mixing the reverse transcription products of the samples with various amounts of internal standard (from 1 to 1,000 ag) and with the primers (full-length bax sequence) 5′-CAGCTCTGAGCAGATCATGAAGACA-3′ (forward primer) and 5′-GCCCATCTTCTTCCAGATGGTGAGC-3′ (reverse primer) (final primer concentrations, 0.2 μM) with 0.18 mM deoxynucleoside triphosphate mixture in PCR buffer (7.5 mM Tris-HCl [pH 9]), 0.2 mM MgCl2, 5 mM KCl, 2 mM (NH4)2SO4, and 2.5 U of polymerase (BioTools B & M Labs, S.A. Madrid, Spain). The PCR profile was 95°C for 45 s, 60°C for 45 s, and 72°C for 2 min for 30 cycles, followed by termination incubation at 72°C for 7 min. After PCR, aliquots of the reaction products were analyzed by electrophoresis in a 1.8% agarose gel. The gel was then stained in electrophoresis buffer containing 0.5 μg/ml ethidium bromide and washed in 1 mM MgSO4. The amount of mRNA was estimated by comparing the relative intensities of the amplified internal standard and of the cDNA of interest (Bax). The relative amounts of DNA corresponding to sBax (internal standard) and Bax were measured by using the Bio-Rad Gel Doc analyzer.
Statistical analyses.
The significance of the differences between paired values was tested by Student's t test (Excel 2000; Microsoft, Inc., Bellevue, WA). Correlations were calculated by using GraphPad Prism version 4.02 for Windows (GraphPad Software, San Diego, CA).
Materials.
Dulbecco's modification of Eagle's minimum essential medium and trypsin-EDTA were purchased from Life Technologies, Paisley, United Kingdom. Gentamicin sulfate was provided as Geomycine (the branded product registered for clinical use in Belgium) by Glaxo-SmithKline Belgium (on behalf of Schering-Plough Corp., Kenilworth, NJ); conversion of grams to moles was done by using a mean molecular weight of 464.4 (sulfate salt form), based on the average contribution of the main subcomponents (C-1, 30%; C-1a, 20%; C-2, 50%) required by the European Pharmacopoeia (65). Aprotinin, HEPES, CHAPS, PMSF, DTT, ubiquitin-aldehyde, and Z-Leu-Leu-Leu-aldehyde (MG132) were purchased from Sigma-Aldrich (St. Louis, MO). DAPI and protein A-agarose beads came from Roche Diagnostics (F. Hoffmann-La Roche Ltd., Basel, Switzerland).
RESULTS
Validation of electroporation conditions.
The efficacy of electroporation for the delivery of nonpermeant solutes was assessed by examining the penetration of trypan blue and lucifer yellow added to the electroporation buffer. Fifteen minutes after electroporation, 64.3% ± 6.5% (trypan blue) and 63.4% ± 6.0% (lucifer yellow) of the cells were homogeneously stained. In parallel, the effective resealing of the pericellular membrane was assessed through the measurement of the release of the cytosolic enzyme lactate dehydrogenase into the culture medium. A typical value of release, recorded 15 min after electroporation, was 11.3% ± 2.1% of the total cell content, whereas the value was 9.1% ± 2.3% for nonelectroporated cells. Electroporated cells were also examined under an electron microscope to verify the absence of alterations of the main subcellular organelles and the intactness of the pericellular membrane. No significant difference from the control (nonelectroporated) cells was noticed.
Morphological demonstration of apoptosis.
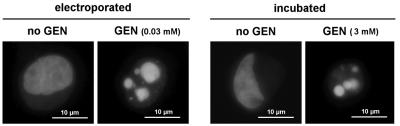
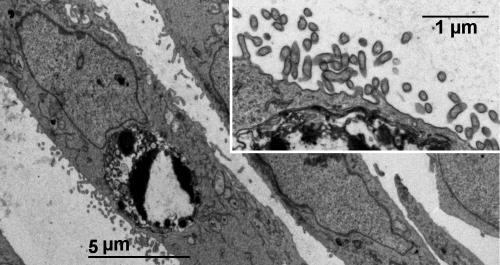
Cells subjected to electroporation in the presence of increasing concentrations of gentamicin were examined 24 h after electroporation for morphological evidence of apoptosis (DAPI staining). As shown in Fig. 1, cells electroporated in the presence of low concentrations of gentamicin (0.03 mM; 13.9 mg/liter) already showed typical images of apoptosis, consisting of the fragmentation and condensation of the nuclear material. These images were similar to those seen for cells incubated with gentamicin for 24 h but at considerably higher concentrations (3 mM; 1.39 g/liter), as described previously (61). The occurrence of apoptosis in cells electroporated in the presence of low concentrations of gentamicin was confirmed by electron microscopy, which revealed typical images, such as the condensation of chromatin material abutting the nuclear membrane (Fig. 2). These cells did not show a visible alteration of their pericellular membrane (inset of Fig. 2). Similar images were seen in the present investigation for cells incubated with gentamicin at high concentrations (1 to 3 mM; data not shown) and in our previous study (12).
FIG.1.
Staining of nuclei of LLC-PK1 cells by DAPI. Electroporated (left panel), cells were electroporated in the absence of gentamicin (no GEN) or in the presence of gentamicin (GEN) at the concentration shown (0.03 mM; 13.9 mg/liter) and were examined 24 h later; incubated (right panel), cells were maintained for 24 h in the absence of gentamicin (no GEN) or in the presence of gentamicin (GEN) at the concentration shown (3 mM; 1.39 g/liter). In the absence of gentamicin, both electroporated and incubated cells show diffuse finely reticulated staining characteristic of the euchromatin of diploid interphase animal cells. In contrast, cells electroporated or incubated in the presence of gentamicin show typical changes associated with apoptosis, consisting of the condensation and fragmentation of the nuclear material. A color version of this figure is available for download at http://www.facm.ucl.ac.be/public/AAC-0897-05-Figure-1-color.jpg.
FIG. 2.
Electron microscopy of LLC-PK1 cells electroporated in the presence of 0.1 mM (46.4 mg/liter) gentamicin and examined 24 h later. The main image shows a typical nucleus demonstrating apoptotic features consisting of condensed and fragmented chromatin clumps abutting the perinuclear membrane. The inset shows that the pericellular membrane of that cell is intact (with many, normal-looking microvilli).
Quantitative analysis of apoptosis.
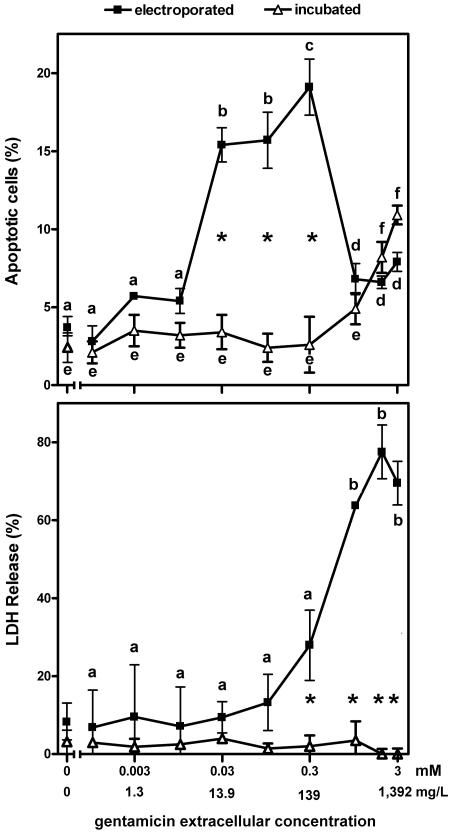
Figure 3 shows the percentage of apoptotic cells (as assessed by DAPI staining) among cells electroporated in the presence of increasing concentrations of gentamicin and examined after 24 h of culture in drug-free medium in comparison with that among cells incubated for 24 h with increasing concentrations of gentamicin. It appears that the electroporated cells were about 100-fold more sensitive to gentamicin-induced apoptosis than the incubated cells. The frequency of apoptotic cells among the electroporated cells, however, reached a maximum at 0.3 mM (139 mg/liter) and sharply declined thereafter. With high gentamicin concentrations, indeed, most samples showed a large proportion of cells undergoing pycnotic nuclear degeneration (characterized by highly condensed but not fragmented nuclear material), suggesting the occurrence of massive necrosis. This was confirmed by measurement of the release of lactate dehydrogenase, as shown in the lower panel of Fig. 3. Cells incubated with gentamicin did not show necrosis within the range of concentrations investigated here (up to 3 mM [1.39 g/liter]). Yet, incubation of the cells with higher concentrations (from 4 mM [1.85 g/liter]) caused detectable necrosis (data not shown), as reported previously (61).
FIG. 3.
Influence of gentamicin concentration on apoptosis (upper panel) and necrosis (lower panel) in LLC-PK1 cells. Apoptosis was assessed by enumeration of typical apoptotic nuclei (DAPI staining; see Fig. 1). Necrosis was assessed by measuring the release of the cytosolic enzyme lactate dehydrogenase (LDH) in the culture medium (shown as the percentage of the total amount in cells plus medium). Electroporated, cells were subjected to electroporation in the presence of gentamicin at the concentrations showed in the abcissa, returned to gentamicin-free medium, and examined 24 h later; incubated, cells were cultivated for 24 h in the presence of gentamicin at the concentrations shown on the abscissa. The 0 value on the abscissa corresponds to cells electroporated (closed symbols) or incubated (open symbols) in the absence of gentamicin. Values are means ± standard deviations (n = 3). Statistical analysis was performed by the use of analysis of variance, and the values of the datum points with different letters in the electroporated cell or the incubated cell panels are significantly different from those of all other points in the same group (P < 0.05; for lactate dehydrogenase release [lower panel], there is no significant difference among any of the datum points for incubated cells); the asterisks show the significant differences (P < 0.05) between groups (electroporated versus incubated cells).
Penetration and retention of gentamicin in cells.
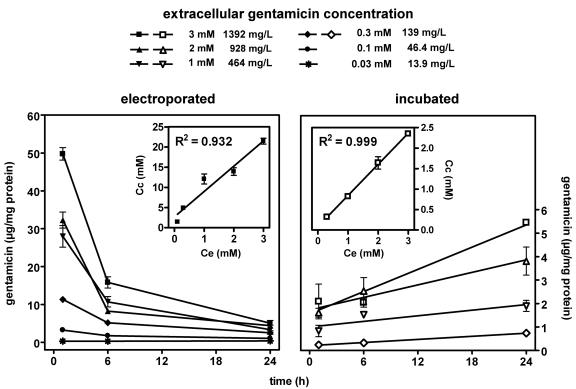
Figure 4 shows the variation of the apparent cellular gentamicin content in cells electroporated in the presence of the drug (at increasing concentrations) and monitored for the next 24 h compared with that in cells incubated with increasing concentrations of the drug for up to 24 h (while the two processes are very different, we present the data together since comparison of the data is instructive). With electroporated cells (left panel of Fig. 4), there was a marked and immediate accumulation of gentamicin that was proportional to its extracellular concentration, with an apparent cellular concentration to extracellular concentration ratio of approximately 6 (see the slope of the correlation shown in the inset of the left panel of Fig. 4). The amount of gentamicin found to be associated with electroporated cells exposed to high extracellular concentrations of gentamicin (0.3 mM [139 mg/liter] or higher) quickly decreased over the next 6 h and decreased more slowly thereafter to reach values about 8- to 10-fold lower than the original ones after 24 h. In contrast, the amount of gentamicin found in cells electroporated in the presence of 0.1 mM (46.4 mg/liter) gentamicin decreased to only about 30% of the original amount within 24 h. No decrease was seen from cells incubated with only 0.03 mM gentamicin (13.9 mg/liter). This behavior was in sharp contrast to what was observed with cells incubated with gentamicin (right panel of Fig. 4). In accordance with our previous results (12), the uptake of gentamicin proceeded almost linearly with time and was proportional to the extracellular concentration (resulting in a mean, stable clearance of 4.44 μl per mg of cell protein and per day). The apparent cellular concentration to extracellular concentration ratio of gentamicin in incubated cells was about 0.8 after 24 h (see the slope of the correlation shown in the inset of the right panel of Fig. 4), in accordance with the findings of our previous studies (12).
FIG. 4.
Cell contents in gentamicin as a function of its extracellular concentration and time. Electroporated (left panel), cells were subjected to electroporation in the presence of gentamicin at the concentrations indicated by the closed symbols at the top of the graphs and returned to drug-free medium for incubation at 37°C for the times indicated on the abscissa; incubated (right panel), cells were incubated at 37°C in the presence of gentamicin at the concentrations indicated by the open symbols at the top of the graphs for the times indicated on the abscissa. Values are means ± standard deviations (n = 3). (Insets) Correlation between the apparent cellular concentration (Cc) and the extracellular concentration (Ce) measured 1 h after electroporation for electroporated cells (left; slope, 6.3 ± 0.5) and after 24 h for incubated cells (right; slope, 0.76 ± 0.02).
It is interesting that cells electroporated in the presence of 0.03 mM (13.9 mg/liter) gentamicin showed an apparent drug concentration (measured after 1 h) of about 0.3 μg/mg protein and that these cells developed extensive apoptosis (16%) within the next 24 h. By comparison, in this time frame, cells incubated for 24 h with 3 mM gentamicin developed about 11% apoptosis, for an apparent cell gentamicin content of about 5.4 μg/mg when it was measured at 24 h.
Increase in the cell content of the proapoptotic Bax protein.
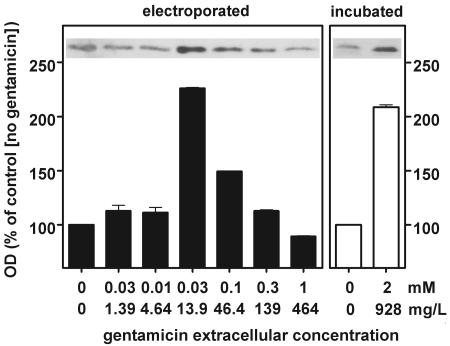
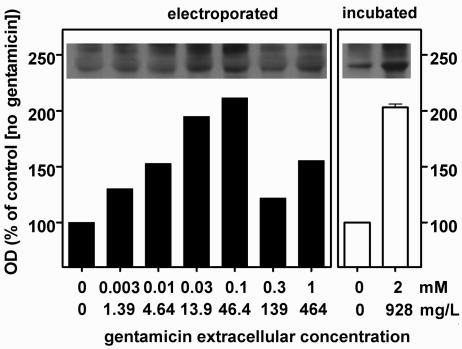
Apoptosis induced by cytotoxic agents is increasingly reported to be associated with a larger cell content of the proapoptotic protein Bax (4, 52, 57). In pilot studies with cells incubated with gentamicin (2 mM), we observed a significant increase in the level of Bax from 8 h of incubation, and this progressed over time up to 32 h. We therefore looked for a similar increase in cells electroporated in the presence of gentamicin. Figure 5 shows that there was a marked increase in the cell content of Bax 8 h after electroporation at extracellular gentamicin concentrations of 0.03 and 0.1 mM. At 0.03 mM, this increase (about twofold) was similar to that seen with cells incubated for 8 h with 2 mM gentamicin. The origin of this increase was then examined in terms of Bax overexpression and/or decreased degradation. Competitive RT-PCR failed to reveal significant changes in the cell content of Bax mRNA (data not shown). In contrast, Fig. 6 shows that the ubiquitinated forms of Bax were significantly increased in cells electroporated in the presence of gentamicin as well as in cells incubated with gentamicin, largely in parallel with the increases in Bax itself.
FIG. 5.
Detection of the proapoptotic protein Bax by Western blot analysis of lysates of LLC-PK1 cells. The films were analyzed by densitometry (with measurements done in triplicate; the values shown are means ± standard deviations; when not visible, the standard deviation bars are smaller than the minimal resolution of the graph). Electroporated (left panel), cells were subjected to electroporation in the presence of gentamicin at the concentrations shown on the abscissa and returned to drug-free medium for 8 h at 37°C before they were collected and processed; incubated (right panel), cells were maintained at 37°C for 8 h in the presence of gentamicin at the concentrations indicated on the abscissa. The 0 value on the abscissa corresponds to cells electroporated (closed bars) or incubated (open bars) in the absence of gentamicin. OD, optical density.
FIG. 6.
Detection of the ubiquitinated forms of the proapoptotic protein Bax by Western blot analysis of lysates of LLC-PK1 cells. Bax was immunoprecipitated with an anti-Bax antibody, and the uniquitinated proteins were revealed on the membrane with an antibody directed against ubiquitinated proteins but not free ubiquitin. The films were analyzed by densitometry measurements made in triplicate; the values shown are means ± standard deviations; when not visible, the standard deviation bars are smaller than the minimal resolution of the graph). Electroporated (left panel), cells were subjected to electroporation in the presence of gentamicin at the concentrations shown on the abscissa and then returned to drug-free medium for 8 h at 37°C before they were collected and processed; incubated (right panel), cells were maintained at 37°C for 8 h in the presence of gentamicin at the concentrations indicated on the abscissa. The 0 value on the abscissa corresponds to cells electroporated (closed bars) or incubated (open bars) in the absence of gentamicin. OD, optical density.
DISCUSSION
The main finding of the present study is that gentamicin causes apoptosis in LLC-PK1 cells when they are subjected to electroporation with extracellular drug concentrations considerably lower than those needed to cause apoptosis in cells incubated with gentamicin. These concentrations (10 to 15 mg/liter) are both microbiologically and pharmacologically meaningful (12, 61). This takes place without evidence of gross cell damage (beyond what could be associated with apoptosis), based on morphological data and a lack of marked lactate dehydrogenase release. The absence of visible alterations of intracellular organelles upon electroporation is consistent with the fact that the effect of electric pulses on membrane permeability is critically dependent on the size of the corresponding structures. Lysosomes and mitochondria, for instance, typically require much more severe conditions than whole cells, namely, a 300-fold higher potential and much shorter pulse durations, to be significantly affected by electroporation (3).
Although considered a valid in vitro model for the study of aminoglycoside-induced toxicity, LLC-PK1 cells accumulate gentamicin much more slowly and less effectively than proximal tubular cells. As observed here and as discussed earlier (12), the rate of gentamicin uptake in LLC-PK1 cells cultivated as monolayers is indeed similar to that of validated tracers of fluid-phase pinocytosis. The situation is different in proximal tubular cells, where uptake proceeds through adsorptive pinocytosis because of the presence of acidic phospholipids (58) and of a specific binding site for polybasic compounds (megalin [45]) at the level of the brush border of proximal tubular cells. LLC-PK1 cells, however, seem to express megalin only when they are grown on inserts, i.e., under conditions that allow their polarization (47). Yet, apoptosis can be unambiguously demonstrated in LLC-PK1 cells grown as monolayers when the cellular concentration of gentamicin is made similar to that observed in the proximal tubules of animals treated with low doses of gentamicin (11), i.e., in a 2- to 20-μg/mg protein range. In the first analysis, it would seem to be reasonable to assume that electroporation simply allows the initial cell concentration of gentamicin to immediately reach the critical cell concentrations needed to trigger apoptosis.
There is, however, a major qualitative difference between gentamicin delivery to cells by pinocytosis (fluid or adsorptive) and delivery by electroporation. Pinocytosis (in cultured cells in vitro or in proximal tubular cells in vivo) should, indeed, direct the drug primarily to lysosomes, as observed for impermeant solutes (5) and for gentamicin in particular (18, 68). In contrast, the data obtained with trypan blue and lucifer yellow indicate that electroporation could allow gentamicin to directly gain access to the cytosol. Yet, we showed that incubation of LLC-PK1 cells with gentamicin causes a destabilization of lysosomes before the activation of the so-called apoptosis mitochondrial pathway becomes evident (61). This led us to conclude that it is the gentamicin leaking from lysosomes that eventually causes apoptosis in cells incubated with this drug (61).
The role of lysosome destabilization in the initiation of the apoptotic process was further surmised from the fact that gentamicin (i) is able to permeabilize phospholipid bilayers under conditions that mimic the lysosomal environment (61) and (ii) has been shown, from about 10% of the total cell content, to quickly traffic through to the Golgi complex and the endoplasmic reticulum, from where it could be transported to the cytosol and reach mitochondria (56). The higher susceptibility of electroporated cells than incubated cells to apoptosis could therefore result from differences in the subcellular distribution of the bulk of the drug. Quantitative analysis based on the data presented in Fig. 3 and 4 supports this conclusion. Cells electroporated in the presence of gentamicin at 0.03 mM (13.9 mg/liter) showed an apparent drug concentration (measured after 1 h) of about 0.3 μg/mg protein, and these cells developed extensive apoptosis (16%) within the next 24 h. In this time frame, cells incubated for 24 h with 3 mM gentamicin develop about 11% apoptosis, for an apparent cell gentamicin content of about 5.4 μg/mg protein when the content is measured at 24 h. Only 5 to 6% of this amount would need to be delivered to the cytosol during the 24-h incubation period to create a condition similar to that which is probably taking place initially in electroporated cells. This value is close to that which has been suggested to be diverted from the lysosomes of cells incubated with gentamicin, based on a detailed morphological study of its disposition in LLC-PK1 cells (56).
Such a small proportion of the total amount of gentamicin taken up by cells could not be detected by the cell fractionation techniques previously used to assess the intracellular fate of gentamicin in cultured cells (68) or in the kidney cortex (18). Our study gives no explanation for the decrease in the gentamicin cell content from electroporated cells upon incubation, as seen in Fig. 4. A plausible explanation for the rapid loss of gentamicin from electroporated cells, however, could simply be the nonimmediate closure of the pores and, for cells electroporated with high concentrations of gentamicin and/or that have taken up a large amount of the drug, the onset of necrosis (as observed in Fig. 3). This should be studied in the future, but the limited size of the samples (due to technical constraints linked to the electroporation technique) will require the use of very sensitive and reliable techniques to examine in detail the disposition of the drug and its fate after electroporation.
With respect to the drug found to be associated with the cells after electroporation, we also ignored the subcellular distribution and the proportion that is free. Gentamicin is known to bind to a variety of acidic macromolecules and supramolecular structures, which, incidentally, would explain why its concentration may initially be higher in electroporated cells than in the extracellular fluid. However, binding to nontarget constituents should similarly affect the drug leaking from lysosomes and that entering cells by electroporation.
A direct effect of cytosolic gentamicin as a trigger of apoptosis (irrespective of its origin) is actually strongly suggested from the observation that the cell content of the proapoptotic protein Bax is increased in both electroporated and incubated cells when gentamicin is present. Bax has a pivotal role in controlling apoptosis; and an increase of its cellular content triggers apoptosis, as observed in both cancerous and noncancerous cell lines as well as in vivo, arguing for a non-cell-type-dependent effect (13, 23, 36).
During apoptosis, Bax is indeed known to translocate to the mitochondrial membrane (73) and to cause cytochrome c release, which amplifies the apoptotic signal (31). We see here that what differentiates electroporated cells from incubated cells is their greater sensitivity to the increase in the amount of Bax with respect to the gentamicin concentration. We also know that mitochondria are involved in gentamicin-induced apoptosis in LLC-PK1 cells when these cells are incubated with the drug (61). We may therefore reasonably assume that what triggers apoptosis in electroporated cells is also the increase in the Bax content. Unfortunately, the small amount of cells that could be subjected to electroporation has prevented us from performing fractionation studies to demonstrate directly the translocation of Bax and the release of cytochrome c.
As is the case for any protein, the increase in Bax content could result from an increase in its transcription or a decrease in its degradation, or both. The first mechanism is unlikely, since we did not observe significant changes in Bax mRNA levels. Conversely, we observed an increase in the amount of its ubiquitinated forms. Bax is degraded by the proteasome (8, 35) but, like other proteins, needs to be ubiquitinated before it can be processed (specific signaling [21]). Inhibition of the proteasome by specific inhibitors is known to cause an increase in ubiquitinated Bax, and this was shown to slow the rate at which Bax itself can be ubiquitinated (9, 30, 35, 50). Our observations are consistent with such a process but do not identify the nature of the proteasome inhibitor. Yet, a recent study showed that gentamicin may interact directly with the β type 9 subunit of the proteasome (22). The implications of this interaction in terms of modulation of proteolytic activity need, however, to be ascertained.
An apparent difficulty for giving to Bax the central role that we propose, however, is that maximum apoptosis is seen with 0.3 mM gentamicin, whereas the Bax content is maximal at a 10-fold lower gentamicin concentration (0.03 mM). This may result from the combination of three phenomena. First, apoptosis is not linearly related to the concentration of gentamicin. Second, the increase in the amount of Bax with respect to the concentration of an apoptosis-triggering agent is also not linear (a typical example has been presented elsewhere [20]). Third, an increase in the level of Bax precedes apoptosis, making the two processes not necessarily linearly related. Finally, we cannot exclude the possibility that gentamicin itself participates in the triggering of apoptosis (through a direct interaction with the mitochondria, once its concentration reaches a sufficient value, as has been observed in vitro [39]), causing an increase in the level of Bax and apoptosis to become dissociated with respect to the gentamicin concentration.
Many studies indicate that it is the ratio of the proapoptotic Bax and the antiapoptotic Bcl-2 that is critical in the initiation of drug-induced apoptotic cell death (75). We observed previously that Bcl-2 overexpression in LLC-PK1 cells (through transfection) prevents gentamicin-induced apoptosis (12). We did not examine this possibility here because (i) while Bcl-2 is abundant in embryonic kidney and is expressed in correlation with the apoptosis-related remodeling processes typical of this developmental stage, its expression in adult renal cells is low (63); and (ii) our attempts to detect Bcl-2 in the strain of LLC-PK1 cells used here revealed only minimal expression by Western blotting (12) or RT-PCR analysis (H. Servais, unpublished data). Yet, others have clearly observed such an expression in LLC-PK1 cells and have demonstrated its role in protecting the cell from apoptosis in at least two situations of drug-induced nephropathy (46, 74). The literature, however, suggests that rather than Bcl-2, it is its homologue, Bcl-xL, that may be controlling apoptosis in renal cells (37, 49). Future work is therefore needed to better ascertain the role of Bcl-2 and its homologues in gentamicin-induced apoptosis.
Our study also revealed that electroporation in the presence of gentamicin may cause necrosis if the drug concentration exceeds 0.3 mM. This toxic effect is not specifically linked to the electroporation since extensive necrosis is also observed for cells incubated with gentamicin if its extracellular concentration exceeds 3 mM (61). It illustrates the well-known fact that apoptosis and necrosis are two adverse effects that drugs cause at low and high concentrations, respectively (6, 19, 70). This explains why the cell Bax content decreases at high gentamicin concentrations through global, necrosis-related impairment of protein synthesis in association with the fast turnover (1 to 2 h) of that protein (35). In the case of aminoglycosides, apoptosis readily develops in the rat kidney cortex at low doses (11), together with other ultrastructural and functional alterations (for a review, see reference 69). Necrosis, however, becomes systematically predominant at high doses (for a review, see reference 40). The point of interest here is that necrosis could easily be obtained when cells were exposed to gentamicin during electroporation. As discussed earlier, this mode of delivery bypasses the lysosomal sequestration of gentamicin seen in incubated cells or in vivo as a result of its pinocytotic uptake. Thus, contrary to our previous hypotheses, it is possible that lysosomal damage (for a review, see reference 33) and necrosis are unrelated events. The former would be directly related to the local effects of gentamicin, such as phospholipase inhibition (32), while the latter would result from a direct effect of gentamicin on nonlysosomal constituents. The evidence presented here as well as in previous work may even suggest that gentamicin sequestration in lysosomes is, to some extent, protective against apoptosis and necrosis-related toxicities. The observation that polyaspartic acid protects against gentamicin-induced nephrotoxicity (16) by trapping the drug in lysosomes as a nondiffusible ionic complex (28) is also consistent with the view that the lysosomal sequestration of gentamicin is a favorable event as far as nonlysosomal effects are concerned. It remains true, however, that pinocytotic uptake of aminoglycosides by proximal tubular cells is the primary cause of nephrotoxicity, since (i) mice with genetic or functional megalin deficiency, which makes them unable to accumulate the drug in these cells by receptor-mediated pinocytosis, are protected against toxicity (59); and (ii) maneuvers that result in the decreased uptake of aminoglycosides by impairing or saturating their pinocytic uptake are associated with a reduced toxicity (for a review, see reference 40). The lysosomal accumulation of gentamicin would therefore appear to be a double-edged pharmacokinetic weapon for the cell. It would indeed afford protection from general cellular toxicity, as long as lysosomes remain intact. However, it would also precipitate cell death, once gentamicin, stored in large amounts in these organelles, is released as a consequence of drug and phospholipid overloading (33), direct permeabilization of the lysosomal membrane (61), interference with proteins that stabilize the lysosomal membrane (41), or retrograde trafficking trough the Golgi apparatus and the endoplasmic reticulum (55, 56). Protection against gentamicin-induced nephrotoxicity in vivo could therefore be envisaged not only by blocking its pinocytic uptake in cells (44) but also by preventing its release from lysosomes.
Acknowledgments
We thank V. Préat for giving us access to and providing guidance on the use of the electroporation apparatus and N. Mesaros for guidance with the competitive RT-PCR analyses. F. Andries-Renoird, M. C. Cambier, and M. Vergauwen provided skillful technical assistance.
H.S. was successively boursier of the Belgian Fonds pour la Formation à la Recherche dans l'Industrie et l'Agriculture and the recipient of a bourse de doctorat of the Université Catholique de Louvain. Y.J. is chargé de recherches and F.V.B. is chercheur qualifié of the Belgian Fonds National de la Recherche Scientifique. This work was supported by the Belgian Fonds de la Recherche Scientifique Medicale (grants 3.4549.00 and 9.4.549.04), the Fonds National de la Recherche Scientifique (grant 7.4573.03), and the Université Catholique de Louvain (Fonds Spécial de Recherches 2002).
REFERENCES
- 1.Appel, G. B., and H. C. Neu. 1978. Gentamicin in 1978. Ann. Intern. Med. 89:528-538. [DOI] [PubMed] [Google Scholar]
- 2.Aubert-Tulkens, G., F. Van Hoof, and P. Tulkens. 1979. Gentamicin-induced lysosomal phospholipidosis in cultured rat fibroblasts. Quantitative ultrastructural and biochemical study. Lab. Investig. 40:481-491. [PubMed] [Google Scholar]
- 3.Beebe, S. J., J. White, P. F. Blackmore, Y. Deng, K. Somers, and K. H. Schoenbach. 2003. Diverse effects of nanosecond pulsed electric fields on cells and tissues. DNA Cell Biol. 22:785-796. [DOI] [PubMed] [Google Scholar]
- 4.Bowen, J. M., R. J. Gibson, D. M. Keefe, and A. G. Cummins. 2005. Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 37:56-62. [DOI] [PubMed] [Google Scholar]
- 5.Buckmaster, M. J., B. D. Lo, Jr., A. L. Ferris, and B. Storrie. 1987. Retention of pinocytized solute by CHO cell lysosomes correlates with molecular weight. Cell Biol. Int. Rep. 11:501-507. [DOI] [PubMed] [Google Scholar]
- 6.Buttke, T. M., and P. A. Sandstrom. 1994. Oxidative stress as a mediator of apoptosis. Immunol. Today 15:7-10. [DOI] [PubMed] [Google Scholar]
- 7.Celi, F. S., M. E. Zenilman, and A. R. Shuldiner. 1993. A rapid and versatile method to synthesize internal standards for competitive PCR. Nucleic Acids Res. 21:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. C., Y. S. Lee, T. Tejima, K. Tanaka, S. Omura, N. H. Heintz, Y. Mitsui, and J. Magae. 1998. mdm2 and bax, downstream mediators of the p53 response, are degraded by the ubiquitin-proteasome pathway. Cell Growth Differ. 9:79-84. [PubMed] [Google Scholar]
- 9.Chen, W. J., and J. K. Lin. 2004. Induction of G1 arrest and apoptosis in human Jurkat T cells by pentagalloylglucose through inhibiting proteasome activity and elevating p27Kip1, p21Cip1/WAF1, and Bax proteins. J. Biol. Chem. 279:13496-13505. [DOI] [PubMed] [Google Scholar]
- 10.De Broe, M. E., G. J. Paulus, G. A. Verpooten, F. Roels, N. Buyssens, R. Wedeen, F. Van Hoof, and P. M. Tulkens. 1984. Early effects of gentamicin, tobramycin, and amikacin on the human kidney. Kidney Int. 25:643-652. [DOI] [PubMed] [Google Scholar]
- 11.El Mouedden, M., G. Laurent, M. P. Mingeot-Leclercq, H. S. Taper, J. Cumps, and P. M. Tulkens. 2000. Apoptosis in renal proximal tubules of rats treated with low doses of aminoglycosides. Antimicrob. Agents Chemother. 44:665-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Mouedden, M., G. Laurent, M. P. Mingeot-Leclercq, and P. M. Tulkens. 2000. Gentamicin-induced apoptosis in renal cell lines and embryonic rat fibroblasts. Toxicol. Sci. 56:229-239. [DOI] [PubMed] [Google Scholar]
- 13.Fortuno, M. A., S. Ravassa, J. C. Etayo, and J. Diez. 1998. Overexpression of Bax protein and enhanced apoptosis in the left ventricle of spontaneously hypertensive rats: effects of AT1 blockade with losartan. Hypertension 32:280-286. [DOI] [PubMed] [Google Scholar]
- 14.Gehl, J. 2003. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 177:437-447. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert, D. N. 2005. Aminoglycosides, p. 328-356. In G. L. Mandell, J. Bennet, and R. Dolin (ed.), Principles and practice of infectious diseases. Elsevier/Churchill Livingstone, Philadelphia, Pa.
- 16.Gilbert, D. N., C. A. Wood, S. J. Kohlhepp, P. W. Kohnen, D. C. Houghton, H. C. Finkbeiner, J. Lindsley, and W. M. Bennett. 1989. Polyaspartic acid prevents experimental aminoglycoside nephrotoxicity. J. Infect. Dis. 159:945-953. [DOI] [PubMed] [Google Scholar]
- 17.Girton, R. A., D. P. Sundin, and M. E. Rosenberg. 2002. Clustering protects renal tubular epithelial cells from gentamicin-mediated cytotoxicity. Am. J. Physiol. Renal Physiol. 282:F703-F709. [DOI] [PubMed] [Google Scholar]
- 18.Giurgea-Marion, L., G. Toubeau, G. Laurent, J. A. Heuson-Stiennon, and P. M. Tulkens. 1986. Impairment of lysosome-pinocytic vesicle fusion in rat kidney proximal tubules after treatment with gentamicin at low doses. Toxicol. Appl. Pharmacol. 86:271-285. [DOI] [PubMed] [Google Scholar]
- 19.He, L., and M. H. Fox. 1997. Variation of heat shock protein 70 through the cell cycle in HL-60 cells and its relationship to apoptosis. Exp. Cell Res. 232:64-71. [DOI] [PubMed] [Google Scholar]
- 20.He, Z., W. Y. Ma, T. Hashimoto, A. M. Bode, C. S. Yang, and Z. Dong. 2003. Induction of apoptosis by caffeine is mediated by the p53, Bax, and caspase 3 pathways. Cancer Res. 63:4396-4401. [PubMed] [Google Scholar]
- 21.Hershko, A. 2005. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 12:1191-1197. [DOI] [PubMed] [Google Scholar]
- 22.Horibe, T., H. Matsui, M. Tanaka, H. Nagai, Y. Yamaguchi, K. Kato, and M. Kikuchi. 2004. Gentamicin binds to the lectin site of calreticulin and inhibits its chaperone activity. Biochem. Biophys. Res. Commun. 323:281-287. [DOI] [PubMed] [Google Scholar]
- 23.Hunter, J. J., and T. G. Parslow. 1996. A peptide sequence from Bax that converts Bcl-2 into an activator of apoptosis. J. Biol. Chem. 271:8521-8524. [DOI] [PubMed] [Google Scholar]
- 24.Inui, K., H. Saito, and R. Hori. 1986. The use of kidney epithelial cell line (LLC-PK1) to study aminoglycoside nephrotoxicity. Dev. Toxicol. Environ. Sci. 14:217-226. [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Jaroszeski, M. J., R. A. Gilbert, and R. Heller. 1997. In vivo antitumor effects of electrochemotherapy in a hepatoma model. Biochim. Biophys. Acta 1334:15-18. [DOI] [PubMed] [Google Scholar]
- 27.Just, M., G. Erdmann, and E. Habermann. 1977. The renal handling of polybasic drugs. 1. Gentamicin and aprotinin in intact animals. Naunyn Schmiedebergs Arch. Pharmacol. 300:57-66. [DOI] [PubMed] [Google Scholar]
- 28.Kishore, B. K., Z. Kallay, P. Lambricht, G. Laurent, and P. M. Tulkens. 1990. Mechanism of protection afforded by polyaspartic acid against gentamicin-induced phospholipidosis. I. Polyaspartic acid binds gentamicin and displaces it from negatively charged phospholipid layers in vitro. J. Pharmacol. Exp. Ther. 255:867-874. [PubMed] [Google Scholar]
- 29.Kosek, J. C., R. I. Mazze, and M. J. Cousins. 1974. Nephrotoxicity of gentamicin. Lab. Investig. 30:48-57. [PubMed] [Google Scholar]
- 30.Kuhn, D. J., A. C. Burns, A. Kazi, and Q. P. Dou. 2004. Direct inhibition of the ubiquitin-proteasome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2 and LDL receptor. Biochim. Biophys. Acta 1682:1-10. [DOI] [PubMed] [Google Scholar]
- 31.Kuwana, T., J. J. Smith, M. Muzio, V. Dixit, D. D. Newmeyer, and S. Kornbluth. 1998. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J. Biol. Chem. 273:16589-16594. [DOI] [PubMed] [Google Scholar]
- 32.Laurent, G., M. B. Carlier, B. Rollman, F. Van Hoof, and P. Tulkens. 1982. Mechanism of aminoglycoside-induced lysosomal phospholipidosis: in vitro and in vivo studies with gentamicin and amikacin. Biochem. Pharmacol. 31:3861-3870. [DOI] [PubMed] [Google Scholar]
- 33.Laurent, G., B. K. Kishore, and P. M. Tulkens. 1990. Aminoglycoside-induced renal phospholipidosis and nephrotoxicity. Biochem. Pharmacol. 40:2383-2392. [DOI] [PubMed] [Google Scholar]
- 34.Laurent, G., P. Maldague, M. B. Carlier, and P. M. Tulkens. 1983. Increased renal DNA synthesis in vivo after administration of low doses of gentamicin to rats. Antimicrob. Agents Chemother. 24:586-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, B., and Q. P. Dou. 2000. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc. Natl. Acad. Sci. USA 97:3850-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, X., M. Marani, J. Yu, B. Nan, J. A. Roth, S. Kagawa, B. Fang, L. Denner, and M. Marcelli. 2001. Adenovirus-mediated Bax overexpression for the induction of therapeutic apoptosis in prostate cancer. Cancer Res. 61:186-191. [PubMed] [Google Scholar]
- 37.Lorz, C., P. Justo, A. B. Sanz, J. Egido, and A. Ortiz. 2005. Role of Bcl-xL in paracetamol-induced tubular epithelial cell death. Kidney Int. 67:592-601. [DOI] [PubMed] [Google Scholar]
- 38.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 39.Mather, M., and H. Rottenberg. 2001. Polycations induce the release of soluble intermembrane mitochondrial proteins. Biochim. Biophys. Acta 1503:357-368. [DOI] [PubMed] [Google Scholar]
- 40.Mingeot-Leclercq, M. P., and P. M. Tulkens. 1999. Aminoglycosides: nephrotoxicity. Antimicrob. Agents Chemother. 43:1003-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyazaki, T., R. Sagawa, T. Honma, S. Noguchi, T. Harada, A. Komatsuda, H. Ohtani, H. Wakui, K. Sawada, M. Otaka, S. Watanabe, M. Jikei, N. Ogawa, F. Hamada, and H. Itoh. 2004. 73-kDa molecular chaperone HSP73 is a direct target of antibiotic gentamicin. J. Biol. Chem. 279:17295-17300. [DOI] [PubMed] [Google Scholar]
- 42.Moestrup, S. K., S. Cui, H. Vorum, C. Bregengard, S. E. Bjorn, K. Norris, J. Gliemann, and E. I. Christensen. 1995. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J. Clin. Investig. 96:1404-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montenez, J. P., F. Van Bambeke, J. Piret, R. Brasseur, P. M. Tulkens, and M. P. Mingeot-Leclercq. 1999. Interactions of macrolide antibiotics (erythromycin A, roxithromycin, erythromycylamine [dirithromycin], and azithromycin) with phospholipids: computer-aided conformational analysis and studies on acellular and cell culture models. Toxicol. Appl. Pharmacol. 156:129-140. [DOI] [PubMed] [Google Scholar]
- 44.Nagai, J., and M. Takano. 2004. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab. Pharmacokinet. 19:159-170. [DOI] [PubMed] [Google Scholar]
- 45.Nagai, J., H. Tanaka, N. Nakanishi, T. Murakami, and M. Takano. 2001. Role of megalin in renal handling of aminoglycosides. Am. J. Physiol. Renal Physiol. 281:F337-F344. [DOI] [PubMed] [Google Scholar]
- 46.Nagothu, K. K., R. Bhatt, G. P. Kaushal, and D. Portilla. 2005. Fibrate prevents cisplatin-induced proximal tubule cell death. Kidney Int. 68:2680-2693. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen, R., H. Birn, S. K. Moestrup, M. Nielsen, P. Verroust, and E. I. Christensen. 1998. Characterization of a kidney proximal tubule cell line, LLC-PK1, expressing endocytotic active megalin. J. Am. Soc. Nephrol. 9:1767-1776. [DOI] [PubMed] [Google Scholar]
- 48.Oltvai, Z. N., C. L. Milliman, and S. J. Korsmeyer. 1993. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609-619. [DOI] [PubMed] [Google Scholar]
- 49.Ortiz, A. 2000. Renal cell loss through cell suicide. Kidney Int. 58:2235-2236. [DOI] [PubMed] [Google Scholar]
- 50.Powell, S. R., P. Wang, A. Divald, S. Teichberg, V. Haridas, T. W. McCloskey, K. J. Davies, and H. Katzeff. 2005. Aggregates of oxidized proteins (lipofuscin) induce apoptosis through proteasome inhibition and dysregulation of proapoptotic proteins. Free Radic. Biol. Med. 38:1093-1101. [DOI] [PubMed] [Google Scholar]
- 51.Ramagli, L. S. 1999. Quantifying protein in 2-D PAGE solubilization buffers. Methods Mol. Biol. 112:99-103. [DOI] [PubMed] [Google Scholar]
- 52.Robertson, J. D., and S. Orrenius. 2000. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit. Rev. Toxicol. 30:609-627. [DOI] [PubMed] [Google Scholar]
- 53.Rols, M. P., and J. Teissie. 1998. Flow cytometry quantification of electropermeabilization. Methods Mol. Biol. 91:141-147. [DOI] [PubMed] [Google Scholar]
- 54.Saikumar, P., and M. A. Venkatachalam. 2003. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin. Nephrol. 23:511-521. [DOI] [PubMed] [Google Scholar]
- 55.Sandoval, R., J. Leiser, and B. A. Molitoris. 1998. Aminoglycoside antibiotics traffic to the Golgi complex in LLC-PK1 cells. J. Am. Soc. Nephrol. 9:167-174. [DOI] [PubMed] [Google Scholar]
- 56.Sandoval, R. M., and B. A. Molitoris. 2004. Gentamicin traffics retrograde through the secretory pathway and is released in the cytosol via the endoplasmic reticulum. Am. J. Physiol. Renal Physiol. 286:F617-F624. [DOI] [PubMed] [Google Scholar]
- 57.Sarkar, F. H., K. M. Rahman, and Y. Li. 2003. Bax translocation to mitochondria is an important event in inducing apoptotic cell death by indole-3-carbinol (I3C) treatment of breast cancer cells. J. Nutr. 133:2434S-2439S. [DOI] [PubMed] [Google Scholar]
- 58.Sastrasinh, M., T. C. Knauss, J. M. Weinberg, and H. D. Humes. 1982. Identification of the aminoglycoside binding site in rat renal brush border membranes. J. Pharmacol. Exp. Ther. 222:350-358. [PubMed] [Google Scholar]
- 59.Schmitz, C., J. Hilpert, C. Jacobsen, C. Boensch, E. I. Christensen, F. C. Luft, and T. E. Willnow. 2002. Megalin deficiency offers protection from renal aminoglycoside accumulation. J. Biol. Chem. 277:618-622. [DOI] [PubMed] [Google Scholar]
- 60.Sepulveda, F. V., K. A. Burton, and J. D. Pearson. 1982. The development of gamma-glutamyltransferase in a pig renal-epithelial-cell line in vitro. Relationship to amino acid transport. Biochem. J. 208:509-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Servais, H., P. Van Der Smissen, G. Thirion, G. Van der Essen, F. Van Bambeke, P. M. Tulkens, and M. P. Mingeot-Leclercq. 2005. Gentamicin-induced apoptosis in LLC-PK1 cells: involvement of lysosomes and mitochondria. Toxicol. Appl. Pharmacol. 206:321-333. [DOI] [PubMed] [Google Scholar]
- 62.Silverblatt, F. J., and C. Kuehn. 1979. Autoradiography of gentamicin uptake by the rat proximal tubule cell. Kidney Int. 15:335-345. [DOI] [PubMed] [Google Scholar]
- 63.Sorenson, C. M. 2004. Bcl-2 family members and disease. Biochim. Biophys. Acta 1644:169-177. [DOI] [PubMed] [Google Scholar]
- 64.Steinmassl, D., W. Pfaller, G. Gstraunthaler, and W. Hoffmann. 1995. LLC-PK1 epithelia as a model for in vitro assessment of proximal tubular nephrotoxicity. In Vitro Cell Dev. Biol. Anim. 31:94-106. [DOI] [PubMed] [Google Scholar]
- 65.Sweetman, S. C. (ed.). 2005. Martindale: the complete drug reference. Pharmaceutical Press, London, United Kingdom.
- 66.Takamoto, K., M. Kawada, T. Usui, D. Ikeda, M. Ishizuka, and T. Takeuchi. 2002. Inhibitory activity of dome formation in LLC-PK1 cells is a selective index of aminoglycoside nephrotoxicity. J. Antibiot. (Tokyo) 55:605-606. [DOI] [PubMed] [Google Scholar]
- 67.Toutain, H., and J. P. Morin. 1992. Renal proximal tubule cell cultures for studying drug-induced nephrotoxicity and modulation of phenotype expression by medium components. Renal Failure 14:371-383. [DOI] [PubMed] [Google Scholar]
- 68.Tulkens, P., and A. Trouet. 1978. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem. Pharmacol. 27:415-424. [DOI] [PubMed] [Google Scholar]
- 69.Tulkens, P. M. 1986. Experimental studies on nephrotoxicity of aminoglycosides at low doses. Mechanisms and perspectives. Am. J. Med. 80:105-114. [DOI] [PubMed] [Google Scholar]
- 70.Vayssier, M., N. Banzet, D. Francois, K. Bellmann, and B. S. Polla. 1998. Tobacco smoke induces both apoptosis and necrosis in mammalian cells: differential effects of HSP70. Am. J. Physiol. 275:L771-L779. [DOI] [PubMed] [Google Scholar]
- 71.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams, P. D. 1989. The application of renal cells in culture in studying drug-induced nephrotoxicity. In Vitro Cell Dev. Biol. 25:800-805. [DOI] [PubMed] [Google Scholar]
- 73.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yano, T., Y. Itoh, T. Kubota, T. Sendo, T. Koyama, T. Fujita, K. Saeki, A. Yuo, and R. Oishi. 2005. A prostacyclin analog prevents radiocontrast nephropathy via phosphorylation of cyclic AMP response element binding protein. Am. J. Pathol. 166:1333-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, X., Q. Xu, and I. Saiki. 2000. Quercetin inhibits the invasion and mobility of murine melanoma B16-BL6 cells through inducing apoptosis via decreasing Bcl-2 expression. Clin. Exp. Metastasis 18:415-421. [DOI] [PubMed] [Google Scholar]