Abstract
Transposon Tn2610, found in a conjugative plasmid from an Escherichia coli isolate recovered at a hospital in Chiba, Japan, in 1975, was completely sequenced. Tn2610 is 23,883 bp long and is bracketed by two transposition modules, a Tn1721-like module and a Tn21-derived module, which correspond, respectively, to the long inverted repeats IRa and IRb previously described for this transposon. Although both tnpA genes are intact, only that in the Tn21-derived module (IRb) functions in the transposition, while that in the Tn1721-derived module (IRa) cannot recognize the 38-bp imperfect repeat at the end of the IRb element. Both tnpR and res are present in IRa, while the tnpR gene of IRb is interrupted by the insertion of an IS26 insertion element. The intervening region, between the res site of the Tn1721 module and IS26, carries multiple integron-associated resistance genes within a Tn21 backbone, including a region identical to that found in the genome of Salmonella enterica serovar Typhimurium DT104. These findings suggest that Tn2610 originated from Tn1721 and Tn21, with extensive recombination events with other elements which have resulted in a complex mosaic structure.
Tn2610 is a multidrug resistance transposon that was originally identified in 1983 on a self-transmissible plasmid, pCS200, originating from an Escherichia coli strain isolated in 1975 at a hospital in Chiba, Japan (20). Tn2610 is 24 kb long and is flanked by 3-kb inverted-repeat (IR) sequences. The intervening nonrepeated region was shown to include genes for resistance to ampicillin, streptomycin, and sulfonamide. Preliminary analysis revealed that Tn2610 carries two copies of the transposition genes tnpA and tnpR and that these regions form a stable heteroduplex (19).
Several large transposons conferring resistance to more than one antibiotic have been identified. Among these, Tn21, Tn1691, Tn2603, and Tn2424, which are classified as class II transposons, seem to be evolutionarily related (11, 13, 18). On the basis of restriction maps and heteroduplex analyses, we originally proposed that the Tn21-related transposons had descended from an ancestral mercury resistance transposon, resembling Tn2613, by subsequent insertions of antibiotic resistance genes and/or insertion sequences (18). This hypothesis has been supported by sequence data from a large group of these transposons (11). Current knowledge on Tn2610 suggests that it may also have evolved from an ancestral mercury resistance transposon via a series of recombination events resulting in a complex configuration.
To confirm this hypothesis, we determined the complete sequence of Tn2610 and compared its structure to that of other known elements.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used were E. coli DH5α (supE44 ΔlacU169 [φ80 lacZ ΔM15] hsdR17 recA1 endA1 gyrA96 thi-1 relA1), AB2463 (recA thr leu thi lac gal ara xyl mtl pro his arg str tsx sup), and P3478Rif, a rifampin-resistant mutant of P3478 (thy polA). The Tn2610-containing plasmid used in this study was pTKY170, formerly termed pMK1::Tn2610#4 (20). Subcloning for DNA sequencing was performed in pUC18 (17). Plasmids pTKY171 and pTKY172 are pAO3 derivatives carrying Tn1722 and Tn1722 with a kanamycin-resistant determinant, respectively. Plasmid pAO3 is a small derivative of plasmid ColE1 (21). Plasmid pTKY173 is a pTKY172 derivative defective in the tnpA gene. Plasmids pTKY174 and pTKY175 are pACYC184-based plasmids loaded with tnpA genes from Tn2610 and Tn1722, respectively. Bacterial cells were routinely cultured at 37°C in Luria-Bertani (LB) medium or on LB agar. For selection with trimethoprim, Mueller-Hinton agar (Difco Laboratories) was used with 0.5% (vol/vol) lysed horse blood. Antibiotics were added at the following concentrations: ampicillin, 50 μg ml−1; chloramphenicol, 25 μg ml−1; kanamycin, 50 μg ml−1; tetracycline, 25 μg ml−1; trimethoprim, 50 μg ml−1; rifampin, 100 μg ml−1.
Construction of plasmids.
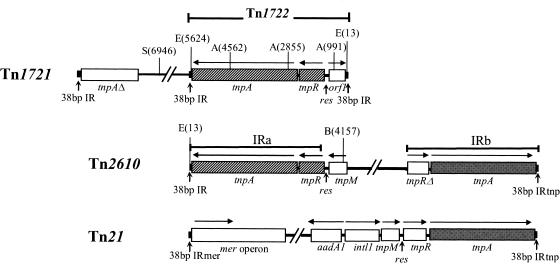
Plasmid pTKY171 (pAO3::Tn1722) was derived from plasmid pAO3::Tn1721 by deletion of the DNA fragment between the SalI site at nucleotide (nt) 6946 in Tn1721 (Fig. 1) and the BstEII site in pAO3. The pUC4K-derived kanamycin resistance determinant was inserted into the AvaI site at nt 991 in Tn1722 (Fig. 1), resulting in pTKY172. Plasmid pTKY173 is an AvaI-generated deletion of pTKY172 which was cleaved at nt 2855 and nt 4562 in Tn1722 and self-ligated. The DNA fragment between the EcoRI site at nt 13 and the BamHI site at nt 4157 in Tn2610 from pTKY170 was cloned into BamHI-digested pACYC184, resulting in pTKY174. Plasmid pTKY175 was constructed by cloning of the DNA fragment between nt 13 and nt 5624 in Tn1722 into EcoRI-digested pACYC184.
FIG. 1.
Structure of the transposition modules forming the backbone of Tn2610. Arrows above open bars indicate the transcription orientations of the ORFs. Restriction cleavage sites shown are those used for the construction of plasmid derivatives described in Materials and Methods. The structures and restriction sites are based on GenBank sequences for Tn2610 (accession no. AB207867) and Tn1721 (X61367). Abbreviations: A, AvaI; B, BamHI; E, EcoRI; S, SalI.
Determination of transposition proficiency.
The mating-out assay was used to determine transposition frequency (19). The pACYC184-based plasmid loaded with a relevant tnpA gene was introduced into AB2463 containing R388, a conjugative plasmid devoid of transposable elements, and pTKY173. The resulting strain was used as a donor to mate with the recipient strain P3478Rif. Donor and recipient strains were mixed in a 1:5 ratio and passed through a Millipore filter, and then the filters were incubated on the agar plates at 37°C for 6 h. Transconjugants receiving R388::Tn1722 were selected with kanamycin and rifampin. Transconjugants receiving R388 were selected with trimethoprim and rifampin. The transposition frequency was expressed as the ratio of the number of kanamycin-resistant transconjugants to the number of R388 transconjugants.
DNA isolation and restriction mapping.
Plasmid DNA for restriction analysis and cloning was isolated by the alkaline lysis method (3). Restriction enzymes (TaKaRa Bio Inc., TOYOBO) were used in accordance with the manufacturer's instructions. DNA fragments were separated by electrophoresis on 1% (wt/vol) agarose gels, and individual fragments were isolated from the gels using a QIAEX II gel extraction kit (QIAGEN). HindIII-digested lambda phage DNA fragments and HinfII-digested pBR322 plasmid DNA fragments were used as size markers.
Sequence analysis.
Sequencing was performed in the facility at QIAGEN, Japan, on an ABI PRISM model 3100 sequencer. DNA sequences were assembled using the GENETYX version 10.1 software package. PCR was used to amplify the pTKY170 fragments to confirm the boundaries between the cloned fragments predicted by mapping and to obtain sequences. The sequence obtained was used to query the GenBank database in order to identify putative genes by using the BLAST program via the World Wide Web interface of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih/BLAST).
Nucleotide sequence accession number.
The 23,883-bp sequence of Tn2610 has been submitted to the DDBJ/EMBL/GenBank databases under accession no. AB207867.
RESULTS AND DISCUSSION
General features of the Tn2610 sequence.
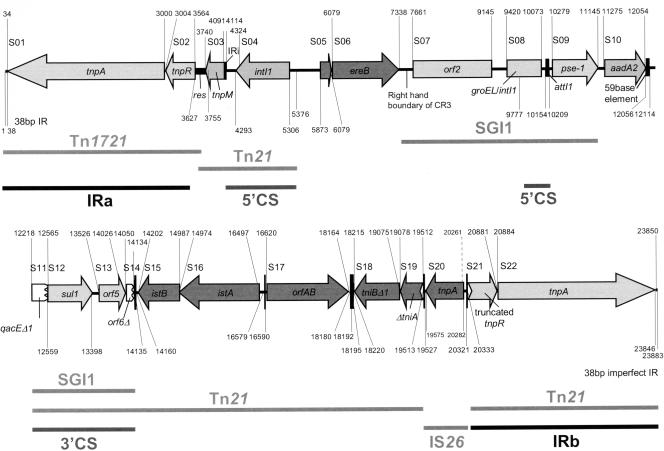
To complete the sequence of Tn2610, plasmid pTKY170 (formerly named pMK1::Tn2610#4 [20]), containing a complete copy of the transposon, was initially subjected to restriction analysis, and relevant fragments were subcloned for sequencing. The complete sequence of the 23,883-bp region bracketed by the previously characterized inverted repeats IRa (formerly named IR-R) and IRb (formerly named IR-L), which are defined by 5-bp direct repeats at their outer ends, was determined. A total of 22 open reading frames (ORFs) were identified and are listed in Table 1. The putative products encoded by these ORFs either were identical to or exhibited significant homology to protein sequences available in GenBank. The nature and positions of relevant features (even in the noncoding regions) are also shown in Table 1. Figure 2 is a linear map of Tn2610 showing the transposon structure.
TABLE 1.
Features of ORFs and discrete DNA segments in Tn2610
| Locationa (5′-3′) | DNA segment | ORFb
|
% Homology (DNA/aa) | Description of gene or gene product (accession no.) | ||
|---|---|---|---|---|---|---|
| No. | Name | Sizec | ||||
| 1-38 | IR | 38-bp IR in 1Ra of Tn2610 | ||||
| 34-3000 | S01 | tnpA | 988 | 99.7/99.2 | Transposase from Tn1721 (X61367) | |
| 3004-3564 | S02 | tnpR | 186 | 99.8/100 | Resolvase from Tn1721 (X61367) | |
| 3627-3755 | res | resIII (3627-3656), resII (3661-3703), resI (3717-3755) | ||||
| 3740-4324 | S03 | tnpM | 194 | 100/100 | TnpM on Tn21 (AF071413) | |
| 4091-5376 | 5′-CS | Partial 5′-CS of class 1 integron (AF261825) | ||||
| 4091-4114 | IRi | IRi of class 1 integron (AF261825) | ||||
| 4293-5306 | S04 | intI1 | 337 | 100/100 | Integrase of class 1 integron (AY214164) | |
| 5873-6079 | S05 | small ORF | 68 | 100/100 | Small ORF from pIP1527 (X03988) | |
| 6079-7338 | S06 | ereB | 419 | 100/100 | Erythromycin esterase type II (X03988) | |
| 7661-9145 | S07 | orf2 | 494 | 100/100 | Putative OrfA from SGI1 (AF261825) | |
| 9420-10073 | S08 | groEL/intI1 | 217 | 100/100 | GroEl/integrase fusion protein from SGI1 | |
| 9777-10209 | 5′-CS | Partial 5′-CS of class 1 integron (AF261825) | ||||
| 10154-10209 | attI | attI of class 1 integron (AF261825) | ||||
| 10279-11145 | S09 | pse-1 | 288 | 100/100 | PSE-1 β-lactamase from SGI (AF261825) | |
| 11275-12054 | S10 | aadA2 | 259 | 100/100 | Streptomycin resistance protein (AF164956) | |
| 12056-12114 | 59 bp | 59-bp element of class 1 integron (AF261825) | ||||
| 12114-14134 | 3′-CS | 3′-CS of class 1 integron (AF261825) | ||||
| 12218-12565 | S11 | qacEΔ1 | Quaternary ammonium compound and disinfectant resistance partial protein on SGI | |||
| 12559-13398 | S12 | sul1 | 279 | 100/100 | Sulfonamide resistance protein on SGI | |
| 13526-14026 | S13 | orf5 | 166 | 100/100 | Putative acetyltransferase in Tn21 (AF071413) | |
| 14050-14134 | S14 | orf6Δ | Hypothetical partial protein (AF261825) | |||
| 14135-14160 | IR | Terminal IR of IS1326 (AY123253.3) | ||||
| 14202-14987 | S15 | istB | 261 | 100/100 | Unknown function of IS1326 (AY123253.3) | |
| 14974-16497 | S16 | istA | 507 | 100/100 | Possible transposase of IS1326 (AY123253.3) | |
| 16579-16590 | IR | Terminal IR of IS1353 (AY123523) | ||||
| 16620-18164 | S17 | OrfAB | 516 | 100/100 | OrfAB from IS1353 (AY123523) | |
| 18180-18192 | IR | Terminal IR of IS1353 (AY123253.3) | ||||
| 18195-18280 | IR | Terminal IR of IS1326 (AY123253.3) | ||||
| 18215-19075 | S18 | tniBΔ1 | Truncated TniB from In2 (U42226) | |||
| 19078-19512 | S19 | tniA | Partial TniA of In2 (U42226) | |||
| 19513-19527 | IR | IR of IS26 (AY123523) | ||||
| 19575-20282 | S20 | tnpA | 235 | 100/100 | Transposase of IS26 (AY123523) | |
| 20321-20333 | IR | IR of IS26 (AY123523) | ||||
| 20334-20881 | S21 | tnpR | Truncated resolvase of Tn21 (AF071413) | |||
| 20884-23850 | S22 | tnpA | 988 | 100/100 | Transposase of Tn21 (AF071413) | |
| 23846-23883 | IR | 38-bp IR in IRb of Tn2610 | ||||
Nucleotide position in the sequence deposited under accession no. AB207867.
Named ORFs are based on those previously characterized.
Expressed as the number of amino acid residues.
FIG. 2.
Genetic organization of Tn2610 based on complete nucleotide sequence analysis. Labeled lines represent the regions in which sequences exhibit significant homology to extant sequences on various genetic elements. The accession numbers of the sequences used for comparative analysis are given in Table 1.
Tn2610 contains two modules for transposition.
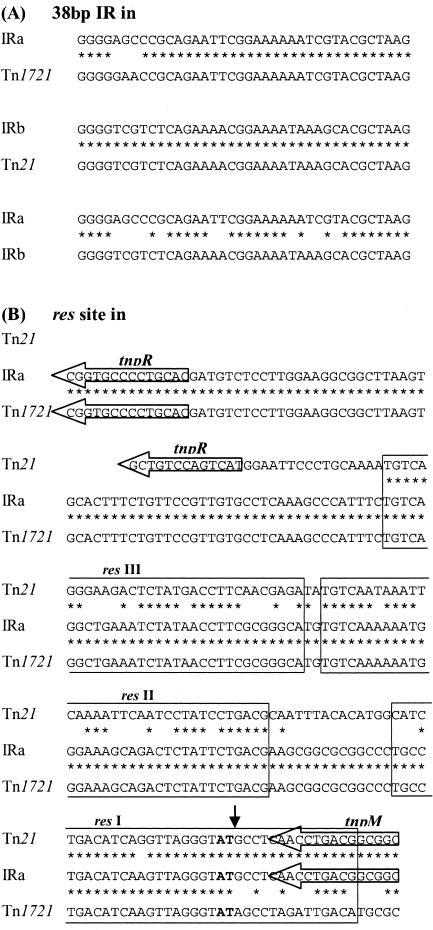
Analysis of the sequence showed that Tn2610 is composed of two transposition modules, a Tn1721-like module and a Tn21-derived module, which correspond, respectively, to IRa and IRb (Fig. 2). Within the Tn1721-like (IRa) module, the transposase gene tnpA, the resolvase gene tnpR, and the res site show strong homology to the corresponding regions of Tn1721 (Table 1; Fig. 2 and 3) (1). A 38-bp sequence was identified at one end of IRa, differing in 3 bases from that in Tn1721 (Fig. 3A). The 129-bp res site of IRa is identical to that of Tn1721 as far as resI, where recombination has occurred with the res site of Tn21 (Fig. 3B) (16).
FIG. 3.
Comparison of the 38-bp inverted repeats (A) and res sites (B). The sequences of the 38-bp inverted repeats and res sites in Tn2610 IRa (this study), Tn1721 (accession no. X61367), and Tn21 (accession no. AF071413) are aligned, with asterisks indicating identical bases. The res subsites (9) are boxed, and the AT site at which resolvase-mediated recombination takes place is boldfaced. The recombination crossover point to generate the hybrid res is indicated by a vertical arrow. tnpR and tnpM, regions adjacent to each end of the res sites.
In contrast, IRb is a Tn21 remnant including a partially deleted tnpR gene, tnpA, and a 38-bp IR identical to those in Tn21 (Fig. 2). The tnpR gene is interrupted by the insertion of an IS26 insertion element, leading to the loss of 12 bp including the ATG start codon of the gene and the res site. Taking all these findings together, we conclude that Tn2610 is bracketed by 38-bp imperfect IRs (10-bp differences) (Fig. 3) at both ends and carries two intact tnpA genes, one intact tnpR gene, and one res site as a transposition module.
Our earlier analysis indicated that tnpA in IRb is functional, while tnpA in IRa is not functional, in the transposition of Tn2610 (19). Although the IRa module shows strong homology to that of Tn1721, the corresponding genes are not identical. The tnpA gene in IRa differs from the Tn1721 sequence at nine positions, leading to the alteration of 7 amino acid residues. Furthermore, a 3-base difference is found in the 38-bp IR sequences between IRa and Tn1721 (Fig. 3A). Therefore, the inability of tnpA in IRa to promote the transposition of Tn2610 may be due to a mutation. To determine whether the tnpA gene of IRa is active, the ability of the product to promote the transposition of Tn1721 was examined by complementation analysis of a Tn1722 tnpA-defective mutant as described in Materials and Methods. As shown in Table 2, the tnpA gene in IRa complemented the tnpA defect in Tn1722, suggesting that it is active even though it cannot promote the transposition of Tn2610. The Tn1721-like tnpA product of IRa probably cannot recognize the 38-bp element at the end of IRb, while the Tn21-like tnpA product of IRb recognizes both 38-bp elements, even though they are imperfect. This hypothesis is supported by a report showing that the Tn21 tnpA products can act on the IR of Tn501 (which is identical to the IR of Tn1721) but the Tn501 tnpA product cannot promote the transposition of Tn21 (8).
TABLE 2.
Complementation of a Tn1722ΔtnpA mutant
| Complementing plasmid (tnpA) | Transposition frequencya |
|---|---|
| pTKY175 (Tn1722) | 2.3 × 10−2 |
| pTKY174 (Tn2610 IRa) | 3.0 × 10−2 |
| pACYC184 | <10−4 |
Determined as described in Materials and Methods.
The intervening nonrepeated region of Tn2610.
Analysis of the intervening nonrepeated region from nt 3514 to nt 20333 reveals the presence of discrete DNA regions carried between the two transposition modules in Tn2610 (Fig. 2).
The Tn1721-like transposition module merges (at the res site) with a Tn21-derived sequence (nt 3565 to 5376) including tnpM and the 5′ conserved segment (5′-CS) of the class 1 integron (the insertion site of the integron IRi into tnpM is identical to that in Tn21). The intI1 gene, in this case, is not preceded by an attI1 site (14) with inserted gene cassettes but by a segment containing the ereB gene (2) with part of CR3 (15). The right-hand boundary of CR3 merges with a long region (nt 7339 to 14134) identical to a part of Salmonella genomic island I (SGI1) (5) of Salmonella enterica serovar Typhimurium phage type DT104 except for the presence of an additional aadA2 cassette in Tn2610. This region includes orf2 (5, 11), the remnant of the 5′-CS of a class 1 integron containing an intI1-groEL hybrid, two gene cassettes (blaPSE-1 and aadA2), and the 3′-CS of a class 1 integron including qacEΔ1, sul1, orf5, and orf6Δ (Fig. 2), which is also identical to that found in In2 carried on Tn21 (11, 15). The homology with In2 continues down to the tniA gene, which is interrupted by the IS26 insertion element (at nt 20333) located between this region and IRb (Fig. 2).
This complex mosaic structure is likely derived by multiple recombination events which involved Tn21-like and SGI1-like sequences, as well as other sequences.
Concluding remarks.
The present study has shown that Tn2610 is a composite transposon comprising two transposition modules, Tn1721-like IRa and Tn21-derived IRb, surrounding a central region containing the drug resistance genes ereB, pse-1, aadA2, and sul1. It is proposed that the ancestors of Tn1721 and Tn21 were independently inserted into a plasmid or genome via transposition events catalyzed by their own transposition modules, leading to the backbone of Tn2610. Later, genes could have been lost by deletion during or after the acquisition of the regions that include the integrons. This seems plausible, since a transposon carrying mphB with an organization very similar to that of the transposition modules in Tn2610 has been found in E. coli (12).
SGI1 has been identified in DT104, whose prevalence increased dramatically in the 1990s (4, 5, 7, 10). DT104 isolates have been reported to be resistant to a core group of antibiotics including ampicillin, chloramphenicol, streptomycin, sulfonamide, and tetracycline (commonly abbreviated ACSSuT). Furthermore, a number of variants of SGI1 that are associated with different resistance phenotypes (e.g., ACSSuS plus trimethoprim, SSu, ASu, and ASSuT) have been identified, suggesting that the multidrug resistance region of SGI1 was subject to recombination events that generated variants (4, 6). Since Tn2610 was found in a plasmid from a strain isolated before SGI1-containing strains, it could also have been involved in the generation of SGI1 structures.
Acknowledgments
We thank T. Tomoyasu and N. Kon for technical assistance.
This work was supported in part by a grant from the Research Project for Emerging and Reemerging Infectious Diseases (H15-Shinkou-9) from the Ministry of Health, Labor and Welfare of Japan.
REFERENCES
- 1.Allmeier, H., B. Cresnar, M. Greck, and R. Schmitt. 1992. Complete nucleotide sequence of Tn1721: gene organization and a novel gene product with features of a chemotaxis protein. Gene 111:11-20. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, M., D. Autissier, and P. Courvalin. 1986. Analysis of the nucleotide sequence of the ereB gene encoding the erythromycin esterase type II. Nucleic Acids Res. 14:4987-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, D., A. Cloeckaert, E. Chaslus-Dancla, and M. R. Mulvey. 2002. Characterization of variant Salmonella genomic island 1 multidrug resistance regions from serovars Typhimurium DT104 and Agona. Antimicrob. Agents Chemother. 46:1714-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd, D. A., G. A. Peters, A. Cloeckaert, K. S. Boumedine, E. Chaslus-Dancla, H. Imberechts, and M. R. Mulvey. 2001. Complete nucleotide sequence of a 43-kilobase genomic island associated with the multidrug resistance region of Salmonella enterica serovar Typhimurium DT104 and its identification in phage type DT120 and serovar Agona. J. Bacteriol. 183:5725-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doublet, B., D. Boyd, M. R. Mulvey, and A. Cloeckaert. 2005. The Salmonella genomic island 1 is an integrative mobilizable element. Mol. Microbiol. 55:1911-1924. [DOI] [PubMed] [Google Scholar]
- 7.Glynn, M. K., C. Bopp, W. Dewitt, P. Dabney, M. Mokhtar, and F. J. Angulo. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338:1333-1338. [DOI] [PubMed] [Google Scholar]
- 8.Grinsted, J., F. de la Cruz, and R. Schmitt. 1990. The Tn21 subgroup of bacterial transposable elements. Plasmid 24:163-189. [DOI] [PubMed] [Google Scholar]
- 9.Hall, S. C., and S. E. Halford. 1993. Specificity of DNA recognition in the nucleoprotein complex for site-specific recombination by Tn21 resolvase. Nucleic Acids Res. 21:5712-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izumiya, H., J. Terajima, S. Matsushita, K. Tanuma, and H. Watanabe. 2001. Characterization of multidrug-resistant Salmonella enterica serovar Typhimuirum isolated in Japan. J. Clin. Microbiol. 39:2700-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liebert, C. A., R. M. Hall, and A. O. Summers. 1999. Transposon Tn21, flagship of the floating genome. Microbiol. Mol. Biol. Rev. 63:507-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noguchi, N., J. Katayama, and M. Sasatsu. 2000. A transposon carrying the gene mphB for macrolide 2′-phosphotransferase II. FEMS Microbiol. Lett. 192:175-178. [DOI] [PubMed] [Google Scholar]
- 13.Ouellette, M., L. Bissonnette, and P. H. Roy. 1987. Precise insertion of antibiotic resistance determinants into Tn21-like transposons: nucleotide sequence of the OXA-1 β-lactamase gene. Proc. Natl. Acad. Sci. USA 84:7378-7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Partridge, S. R., G. D. Recchia, C. Scaramuzzi, C. M. Collis, H. W. Stokes, and R. M. Hall. 2000. Definition of the attI1 site of class 1 integrons. Microbiology 146:2855-2864. [DOI] [PubMed] [Google Scholar]
- 15.Partridge, S. R., H. J. Brown, H. W. Stokes, and R. M. Hall. 2001. Transposons Tn1696 and Tn21 and their integrons In4 and In2 have independent origins. Antimicrob. Agents Chemother. 45:1263-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogowsky, P., S. E. Halford, and R. Schmitt. 1985. Definition of three resolvase binding sites at the res loci of Tn21 and Tn1721. EMBO J. 4:2135-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 18.Tanaka, M., T. Yamamoto, and T. Sawai. 1983. Evolution of complex resistance transposons from an ancestral mercury transposon. J. Bacteriol. 153:1432-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto, T. 1989. Organization of complex transposon Tn2610 carrying two copies of tnpA and tnpR. Antimicrob. Agents Chemother. 33:746-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto, T., M. Watanabe, K. Matsumoto, and T. Sawai. 1983. Tn2610, a transposon involved in the spread of the carbenicillin-hydrolysing β-lactamase gene. Mol. Gen. Genet. 189:282-288. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto, T., S. Yamagata, Y. Hashimoto, and S. Yamagishi. 1980. Restriction endonuclease cleavage maps of the ampicillin transposons Tn2601 and Tn2602. Microbiol. Immunol. 24:1139-1149. [DOI] [PubMed] [Google Scholar]