Carbapenem resistance mediated by acquired carbapenemases is a growing concern worldwide. Acquired class A carbapenemases of the NMC/IMI, SME, and KPC types remain infrequent in Enterobacteriaceae (2, 6-8), but some of them have increasingly been reported recently (10). IMI-1 was first described in 1996 in clinical isolates of Enterobacter cloacae (9). Recently, IMI-2, a variant that differs from IMI-1 by 2 amino acid residues, was found in a strain of Enterobacter asburiae isolates in U.S. rivers (1). In this study, we report on the detection of IMI-2 in a clinical isolate of Enterobacter cloacae from China.
An imipenem-resistant E. cloacae isolate (isolate 8) was obtained from the blood of a patient in our hospital in August 2001. The patient had previously been treated with ampicillin-sulbactam and cefotaxime. E. cloacae isolate 8 was resistant to imipenem, meropenem, and ertapenem; had intermediate resistance to cefotaxime and ceftazidime; and was susceptible to cefepime (Table 1). Conjugation was carried out by a broth method as previously described (11). Transconjugant clones were selected on Mueller-Hinton agar containing rifampin (256 mg/liter) and imipenem (4 mg/liter). Imipenem resistance was transferred to a transconjugant along with a plasmid with a size of about 80 kb. The MIC for the transconjugant (Escherichia coli C600E8) showed that it has resistance to carbapenems but not to expanded-spectrum cephalosporins (Table 1). The β-lactamase activity was determined by UV spectrophotometry with imipenem as a substrate (1, 5). In E. coli C600E8, expression of carbapenemase activity increased from 643 to 1,352 U/mg of protein upon exposure to imipenem (4 mg/liter) as an inducer. These results demonstrated that the β-lactamase was produced at a high basal level and that expression was very poorly inducible. Isoelectric focusing was performed according to a published protocol (3). The result demonstrated that E. cloacae 8 had 5 pI bands at pI 5.1, 5.4, 6.9, 8.1, and 8.6, while E. coli C600E8 had only one band at pI 8.1, which was inhibited by clavulanic acid.
TABLE 1.
MICs of selected antimicrobial agents for various strains determined by Etest
| β-Lactam | MIC (mg/liter)
|
||
|---|---|---|---|
| E. cloacae 8 |
E. coli
|
||
| C600 | C600E8 | ||
| Imipenem | 64 | 0.38 | 64 |
| Meropenem | 32 | 0.08 | 16 |
| Ertapenem | 32 | 0.08 | 16 |
| Cefepime | 2 | 0.064 | 0.064 |
| Ceftazidime | 16 | 0.5 | 0.38 |
| Cefotaxime | 12 | 0.05 | 0.125 |
| Cefoperazone | ≥256 | 0.5 | 1.5 |
| Cefoperazone-sulbactam | 128 | 0.25 | 1 |
| Piperacillin | ≥256 | 32 | 64 |
| Piperacillin-tazobactam | ≥256 | 2 | 4 |
| Ampicillin | ≥256 | 16 | ≥256 |
| Ampicillin-sulbactam | ≥256 | 8 | 64 |
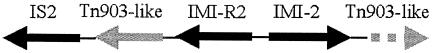
The imipenem resistance gene was cloned into pGEM-T Easy (Promega, Madison, Wis.) as a 10.6-kb EcoRI fragment. A 10,629-bp stretch of DNA sequence was obtained by nucleotide sequencing on both strands (GenBank accession no. AY780889). In the cloned fragment, blaIMI-2 is preceded by a gene encoding a protein 97% identical to ImiR (9). The regions flanking the blaIMI-R2-blaIMI-2 gene contain sequences related to Tn903 (4) and IS2 (Fig. 1). Their structure apparently differs from that previously reported to be flanking the blaIMI-R2-blaIMI-2 gene complex from E. asburiae (1). The transposable elements flanking the blaIMI-R2-blaIMI-2 gene complex could be involved in mobilization of the carbapenemase gene to enterobacterial plasmids.
FIG. 1.
Schematic representation of the structure of the region containing the blaIMI-R2-blaIMI-2 gene complex from the E. cloacae 8 plasmid. The two sides of the blaIMI-R2-blaIMI-2 gene are inverted repeat sequences. “Tn903-like” downstream is an incomplete open reading frame that is part of “Tn903-like” upstream.
Acknowledgments
This work was supported in part by the National Basic Research Program 973 of China (no. 2005CB523101) and the Program for New Century Excellent Talents in University (no. NCET-04-0552).
REFERENCES
- 1.Aubron, C., L. Poirel, R. J. Ash, and P. Nordmann. 2005. Carbapenemase-producing Enterobacteriaceae, U.S. rivers. Emerg. Infect. Dis. 11:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coudron, P. E., E. S. Moland, and K. S. Thomson. 2000. Occurrence and detection of AmpC β-lactamases among Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates at a veterans medical center. J. Clin. Microbiol. 38:1791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grindley, N. D., and C. M. Joyce. 1980. Genetic and DNA sequence analysis of the kanamycin resistance transposon Tn903. Proc. Natl. Acad. Sci. USA 77:7176-7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent, P., T. Naas, M. Guibert, E. B. Chaibi, R. Labia, and P. Nordmann. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naas, T., and N. Patrice. 1994. Analysis of a carbapenem-hydrolyzing class A β-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc. Natl. Acad. Sci. USA 91:7693-7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordmann, P., and L. Poirel. 2002. Emerging carbapenemases in Gram-negative aerobes. Clin. Microbiol. Infect. 8:321-331. [DOI] [PubMed] [Google Scholar]
- 8.Nordmann, P., and L. Poirel. 2002. Acquired carbapenem-hydrolyzing β-lactamases and their genetic support. Curr. Pharm. Biotechnol. 3:117-127. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen, B. A., K. Bush, D. Keeney, Y. Yang, R. Hare, C. O'Gara, and A. A. Medeiros. 1996. Characterization of IMI-1 β-lactamase, a class A carbapenem-hydrolyzing enzyme from Enterobacter cloacae. Antimicrob. Agents Chemother. 40:2080-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodford, N., P. M. Tierno, Jr., K. Young, L. Tysall, M.-F. I. Palepou, E. Ward, R. E. Painter, D. F. Suber, D. Shungu, L. L. Silver, K. Inglima, J. Kornblum, and D. M. Livermore. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan, J.-J., W. C. Ko, and J.-J. Wu. 2001. Identification of a plasmid encoding SHV-12, TEM-1, and a variant of IMP-2 metallo-β-lactamase, IMP-8, from a clinical isolate of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:2368-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]