Abstract
A potential strategy that can be used to combat the worldwide AIDS epidemic is the development of a vaginal microbicide that prevents the sexual transmission of human immunodeficiency virus type 1 (HIV-1). Certain CC chemokines, including RANTES, MIP-1α, and MIP-1β, might facilitate the development of such microbicides since they potently suppress HIV-1 infection by binding to CCR5, the viral coreceptor used by most sexually transmitted strains of HIV-1 to enter host cells. In this study, we evaluated whether a CCR5-specific fragment of RANTES that lacks two N-terminal residues (−2 RANTES) and possesses especially potent HIV-1 suppressive activity has toxicity profiles conducive to the advancement of testing in candidate microbicide formulations. Analyses were carried out with a synthetic version of the chemokine, which was formulated with either Novasomes 7474, a nonphospholipid liposome, or methylcellulose gel. Dialysis studies demonstrated that the formulated −2 RANTES was released from both vehicles and retained anti-HIV-1 activity. Preclinical toxicity studies carried out with Swiss Webster mouse and New Zealand White rabbit vaginal irritation models demonstrated minimal inflammation and minimal adverse changes in cervicovaginal tissue integrity after short-term (10 min) and long-term (24 h) exposure to formulations containing up to 1 mg/ml of −2 RANTES. Similarly, no toxicity was observed with formulations of bioactive murine RANTES in the Swiss Webster mouse vaginal irritation model. Overall, these preclinical studies suggest that −2 RANTES is suitable for further testing as a candidate anti-HIV-1 microbicide.
Heterosexual contact has emerged as the predominant mode of human immunodeficiency virus (HIV) type 1 (HIV-1) transmission worldwide, accounting for more than 90% of all adolescent and adult HIV-1 infections (27). Consequently, half of all individuals currently infected with HIV-1 are female (22). In the absence of an effective prophylactic anti-HIV vaccine, other preventative methods are urgently needed to control the spread of the virus (6, 13). Vaginal microbicides may provide an alternative, female-controlled method of protection against the sexual transmission of HIV-1. The ideal vaginal microbicide should be effective, safe, acceptable, affordable, colorless, odorless, stable, easy to store and use, available in contraceptive and noncontraceptive formulations, and available without a prescription (6). However, the immediate priority is the development of a microbicide that effectively blocks HIV-1 infection in a manner that is nontoxic to the genital tract.
Early attempts to develop vaginal microbicides focused on agents with nonspecific mechanisms of action, such as nonionic surfactant, nonoxynol-9 (N-9). N-9 has previously been demonstrated to possess in vitro activity against several sexually transmitted disease pathogens, including HIV-1 (24, 31). However, high concentrations and/or frequent exposures to these agents resulted in genital irritation and ulceration in clinical trials, which increased the risk for HIV-1 infection (35, 39). For these reasons, attention has turned toward the development of candidate microbicides with specific biological mechanisms of action.
In order to initiate replication, HIV-1 uses the host cell CD4 receptor for attachment and a coreceptor for viral entry. The vast majority of sexually transmitted HIV-1 strains use the CCR5 coreceptor, which naturally functions as a chemokine receptor (42, 47-49). Consequently, strong natural resistance to infection is associated with the genetic loss of CCR5 expression (14, 20, 23, 36). Notably, there are no apparent health consequences associated with this phenotype (14, 15, 20, 23, 25, 33, 36, 50). Taken together, these findings suggest that sexual transmission of HIV-1 infection may be safely blocked by agents that interfere with the coreceptor function of CCR5.
A growing body of evidence suggests that the natural chemokine ligands for CCR5, which include RANTES, MIP-1α, and MIP-β, may provide a means to block mucosal CCR5 use by HIV-1 (37). These chemokines suppress HIV-1 entry by competitively blocking HIV-CCR5 interactions and by down-regulating cell surface CCR5 (1, 3, 19, 29, 41, 46). A number of clinical studies have shown that an inborn capacity to produce high levels of HIV-suppressive CCR5 ligands is associated with persistent seronegative status, despite repeated exposure to HIV-1 (14, 20, 23, 25, 33, 36, 50). In agreement, primate vaccine studies have correlated increased chemokine production with protection from virus challenge (4).
Among CCR5 ligands, a truncated fragment of RANTES (termed −2 RANTES) exhibits qualities that are particularly conducive to microbicide development. Under natural conditions this fragment is generated by a dipeptidyl peptidase, CD26, which removes two N-terminal residues from the chemokine (21, 30, 32, 38). Although unprocessed RANTES binds to several chemokine receptors, −2 RANTES is a dedicated CCR5 ligand (40). This specificity appears to render −2 RANTES a more potent HIV-1 suppressive agent than the unprocessed chemokine (38). However, the N-terminal truncation also abrogates the ability of RANTES to stimulate calcium mobilization in monocytes and to chemoattract monocytes and eosinophils in vitro (21, 30, 32, 38). This reduced capacity to activate receptors could minimize the likelihood that −2 RANTES would cause local inflammation or other adverse side effects if it was used in a topical microbicide formulation.
In this study, standardized animal models were used to examine the safety and toxicity profiles of topical microbicide formulations containing −2 RANTES. Formulation vehicles providing sustained contact of the chemokine to the mucosal surface were selected. One formulation vehicle, methylcellulose, has previously been used for vaginal delivery of neutralizing antibodies (43) and has demonstrated excellent safety profiles in phase I trials (9). The second vehicle of choice, Novasomes 7474, a nonphospholipid liposome, has previously been tested as a vaginal vehicle in phase I trials and was shown to have acceptable safety profiles (Novavax investigational new drug permit 57,550). Here we report that both formulations release antiviral concentrations of −2 RANTES without resulting in detectable levels of inflammation or toxicity. These results indicate that microbicide formulations containing CCR5 ligands may represent candidate vaginal microbicides for the prevention of HIV-1 transmission.
MATERIALS AND METHODS
Reagents.
Synthetic −2 RANTES was prepared by solid-phase peptide synthesis in combination with native chemical ligation, according to previously published methods (11, 12). Briefly, the N-terminal peptide thioester RANTES (positions 3 to 33) αCOSR and the C-terminal peptide acid RANTES (positions 34 to 68) were synthesized on phenylacetamidomethyl resin by using the published in situ neutralization-HBTU [(1H-benzotriazoyl-1-yl)-1,1,3,3, tetramethyluroniumhexafluorophosphate] activation protocol for Boc chemistry solid-phase peptide synthesis (8). After hydrofluoric acid cleavage and deprotection from the resin, the crude peptides were purified by preparative reversed-phase high-pressure liquid chromatography and the molecular weights were verified by electrospray ionization mass spectrometry. Chemical ligation of RANTES (positions 3 to 33) αCOSR and RANTES (positions 34 to 68), purification of the ligation product (−2 RANTES), and protein refolding were as described previously (45). The folded protein was purified by fast-performance liquid chromatography and was characterized by analytical high-pressure liquid chromatography and electrospray ionization mass spectrometry to ascertain its quality. Before −2 RANTES was tested as a microbicide, the material was determined to be free of endotoxin (levels less than 0.5 endotoxin unit per ml) by kinetic Limulus amoebocyte lysate assay with Escherichia coli O55:B5 lipopolysaccharide (Biowhittaker/Cambrex, Baltimore, MD) as a standard. Additionally, the HIV-1 suppressive activity of synthetic −2 RANTES was verified against that of recombinant full-length RANTES (R&D Systems) in an in vitro peripheral blood mononuclear cell (PBMC) infection assay, as described below.
The vaginal surfactant N-9 was obtained from Aventis, Strasbourg, France. Novasomes 7474 was generously provided by Craig Wright; Novavax, Incorporated (Pacific Grove, CA). Novasomes 7474 structures are nonphospholipid liposomes made from amphiphiles. Drugs or other lipophilic, hydrophilic, or oil-soluble materials can be encapsulated in these structures for topical or oral delivery (44). Methylcellulose was a gift from Robin Maguire of The Population Council (Rockefeller University, New York, NY). Formulations of −2 RANTES were prepared as a 90% total volume of the respective vehicle and a 10% total volume of −2 RANTES resuspended in phosphate-buffered saline (PBS) at the appropriate final concentration.
In vitro cytotoxicity assays.
A colorimetric cell viability assay was used to assess whether the test microbicides exhibit toxic effects. HeLa cells (human cervical carcinoma cells; ATCC CCL-2) were maintained in Dulbecco's modified Eagle's medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), L-glutamine (0.3 mg/ml; Invitrogen), penicillin (0.04 mg/ml; Invitrogen), and streptomycin (0.04 mg/ml; Invitrogen). Cells were cultured overnight in a 96-well plate at a density of 4 × 104 cells per well. Fresh medium was then added along with the test microbicide in a 50% total volume per well. Following 30 min, 2 h, 4 h, 8 h, or 24 h of exposure to the microbicide, the cells were washed with fresh medium and assayed for cellular viability by using the CellTiter 96 AQueous nonradioactive cell proliferation assay (Promega, Madison, WI), according to the manufacturer's instructions. This assay uses a tetrazolium compound, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; MTS], and an electron-coupling reagent, phenazine methosulfate. MTS is bioreduced by cells into a soluble formazan product, which was measured directly by determination of the absorption at 490 nm (corrected for the background at 690 nm) from 96-well assay plates. The absorption of formazan is directly proportional to the number of viable cells. The viability index was then assigned on the basis of the percentage of viable cells in the microbicide-treated samples relative to the number of viable cells in the mock-infected controls. All tests were performed in triplicate in three independent experiments. The means and standard deviations of the viability index values for each concentration at each time point were calculated.
Mice.
Six- to 10-week-old female outbred Swiss Webster mice (CFW) were used for all experiments (Charles River Laboratories, Wilmington, MA). The cycles of the animals were synchronized 7 and 3 days prior to the start of each experiment with a 0.2-ml subcutaneous injection of Depo-Provera (Pharmacia and Upjohn Company, Peapack, NJ) diluted in lactated Ringer's saline solution (Baxter, Deerfield, IL), for a final concentration of 3 mg/ml per animal. All animal studies conformed to the Guiding Principals in the Care and Use of Animals, approved by the American Physiological Society (3a). Additionally, all animal studies were approved by the University of Maryland Biotechnology Institute's Institutional Animal Care and Use Committee (protocol 0009-02).
Murine in vivo toxicity studies.
The Swiss Webster mouse model was used as described previously to assess cellular damage and inflammation following microbicide exposure (5). Briefly, Depo-Provera-treated animals were anesthetized with a formulation of ketamine and xylazine (100 to 200 mg/kg of body weight and 5 to 10 ng/kg of body weight, respectively) and received an intravaginal inoculation (60 μl) of microbicide. The mice were treated with the following formulations: 1 mg/ml of −2 RANTES in PBS, 1% N-9 in PBS, 1 mg/ml of −2 RANTES formulated in Novasomes 7474, or 1 mg/ml of −2 RANTES formulated in methylcellulose. Untreated mice and mice treated with the PBS alone were used as controls to evaluate the normal tissue morphology and inflammation status in the cervicovaginal mucosal surface. Mice treated with 2 μg/ml of murine full-length RANTES (Peprotech, Inc., Rocky Hill, NJ) in PBS were also included as reference controls. The mice were killed at 30 min, 2 h, 4 h, 6 h, 8 h, and 24 h following compound application; and the entire reproductive tract was surgically excised. Tissues were formalin fixed and embedded in paraffin by standard procedures. Paraffin-embedded samples were cut to 4 μm thick, and sections were stained with hematoxylin and eosin for gross morphological analyses. Representative fields from the vaginal and cervical epithelium of each treatment group were photographed by using a high-resolution digital camera. Tissue toxicity was characterized in the following manner: mild disruption was described as a localized loss of tissue integrity and epithelial sloughing over less than 5% of the epithelial surface, which was otherwise contiguous and intact; moderate disruption was described as multiple areas of epithelial disturbance representing 5 to 25% of the total epithelial surface and small regions of sloughing that exposed the basal cell layer; and severe disruption was described as sloughing over large sections of the epithelial surface (>25%), which exposed the basal cell layer and which was generally apparent throughout the section.
Murine in vivo genital inflammation studies.
Tissue harvested from control and microbicide-treated mice were immediately processed for immunohistochemical analyses to determine the levels and distribution patterns of murine CD45 cells (leukocyte common antigen [Ly5]) within the cervicovaginal mucosa. The formalin-fixed, paraffin-embedded tissues were stained with a monoclonal rat anti-mouse antibody specific to murine CD45 cells (BD Pharmingen, San Diego, CA), according to the manufacturer's recommendations and as described previously (5). Briefly, baked tissue sections were dehydrated in xylene and rehydrated in alcohol gradients. Endogenous peroxidases were quenched with 3% hydrogen peroxide, and antigen retrieval was achieved with Retrievagen A solution, pH 6.0 (BD Biosciences, Franklin Lakes, NJ). Nonspecific activity was blocked by incubating the tissues with 20% horse serum (Vector Laboratories, Burlingame, CA). The tissues were then incubated with a 1:20 dilution of purified rat anti-mouse CD45 immunoglobulin G2b (IgG2b) diluted in antibody diluent (BD Biosciences) for 1 h at room temperature and then shifted to 4°C overnight. The tissues were washed with PBS and incubated for 30 min with biotinylated mouse anti-rat IgG2b secondary antibody (BD Biosciences). The tissue sections were again washed with PBS and stained with Vectastain ABC reagent (Vector Laboratories), according to the manufacturer's instructions. Streptavidin-peroxidase conjugate incubations were performed for 30 min at room temperature. Color visualization of the complex was achieved by incubating tissue sections with diaminobenzidine tetrahydrochloride containing Ni2+ (Pierce Biotechnology, Rockford, IL) and counterstaining with hematoxylin. Tissue sections stained with secondary antibody alone or a purified rat IgG2b immunoglobulin isotype (BD Biosciences) served as negatively staining controls. Representative tissue sections were photodocumented with a high-resolution digital camera. Inflammation was scored on the basis of the following criteria: mild inflammation resulted in slightly elevated levels of CD45-positive cells in isolated regions below the basal epithelium relative to the levels in the controls, and severe inflammation resulted in substantially elevated numbers of CD45-positive cells distributed across regions representing more than 10% of the epithelial tissue surface relative to the numbers of cells in the controls.
New Zealand White rabbit vaginal irritation study.
Twenty-four nulliparous and nonpregnant female New Zealand White rabbits were used to determine potential irritation effects following vaginal application of −2 RANTES formulations. All animals were acclimated for 5 days prior to the beginning of the experiment. The animals were categorized into four treatment groups, including six sham-treated animals, six Novasomes 7474-treated animals, six animals treated with −2 RANTES (500 μg/ml) formulated in Novosomes 7474, and six animals treated with −2 RANTES (50 μg/ml) formulated in Novasomes 7474. The animals received 1-ml vaginal doses of the respective compound daily for 10 consecutive days. The animals' body weights were measured daily; and clinical observations were recorded, including swollen vulva areas, blood-stained urine, and soft stools. On day 10, all animals were euthanized by intravenous injection of sodium pentobarbital, in accordance with the guidelines of the American Veterinary Medical Association Panel on Euthanasia (3b). The vaginal tracts were surgically excised, formalin fixed, and paraffin embedded by standard histological protocols. To assess gross tissue morphology, sections were stained with hematoxylin and eosin. A vaginal irritation grading system with scores from 0 (normal parameter or absent adverse effects) to 4 (most severe adverse findings) was used to score each formulation for epithelial integrity, epithelial vascular congestion, leukocyte infiltration, and edema. Composite average scores of 1 to 4 receive a vaginal irritation rating of “minimal,” scores of 5 to 8 receive a vaginal irritation rating of “mild,” scores of 9 to 11 receive a vaginal irritation rating of “borderline,” and scores of 12 to 16, receive a vaginal irritation rating of “unacceptable.” Formulations with vaginal irritation ratings between 1 and 8 are considered acceptable for vaginal application. All procedures for the rabbit irritation study were conducted at Springborn Laboratories, a division of Charles River Laboratories, Inc. (Spencerville, OH). This study was approved by the Springborn Laboratories Institutional Animal Care and Use Committee.
In vitro chemokine release assays.
Formulations of −2 RANTES were injected into 100,000-molecular-weight-cutoff cellulose ester dialysis bags (Spectra/Por Biotech, Rancho Dominguez, CA) and placed in beakers containing 50 ml of PBS supplemented with 1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) and one complete protease inhibitor cocktail tablet (Roche Applied Science, Indianapolis, IN). The solutions were continuously stirred at room temperature; and at 0 min (immediately after the dialysis bag was inserted into the buffer), 30 min, 2 h, 4 h, 6 h, and 24 h, 3-ml aliquots were removed from the beaker for analyses. After each aliquot was removed, 3 ml of fresh PBS with 1% BSA was added back to the beaker. Vehicle control samples included vehicle alone diluted in PBS for a final formulation concentration of 90%. Dialysis sample buffer aliquots from the vehicle control were collected in the same manner used for the test formulations. The dialysis samples collected at the different time points were serially diluted and analyzed within 24 h by enzyme-linked immunosorbent assay (ELISA) to measure the concentrations of −2 RANTES that were released. Statistical analyses (t test) were performed to determine if the observed differences in the relative release of −2 RANTES from formulation vehicles (Novasomes 7474 versus methylcellulose) were statistically significant (P < 0.05).
RANTES ELISA.
Immulon plates were coated with 2 μg/ml of anti-RANTES antibody (R & D Systems, Minneapolis, MN) diluted in PBS and incubated at 4°C overnight. The plates were washed with a commercial wash buffer (R & D Systems), and nonspecific binding was blocked with 5% blocking solution (5% dry milk in wash buffer) at room temperature for 1 h. The plates were washed three times with wash buffer, and 100 μl of the diluted samples was transferred to the plates in triplicate wells. Following a 1-h incubation at room temperature, the wells were washed three times with wash buffer and filled with 100 μl of 5% blocking solution containing 100 ng/ml biotinylated anti-RANTES detection antibody (R & D Biosystems). After a 1-h incubation, the wells were washed with wash buffer and filled with 100 μl of 0.5 μg/ml of streptavidin peroxidase (Kirkegaard & Perry Laboratories, Incorporated, Gaithersburg, MD). After 30 min the wells were washed and filled with 200 μl of peroxidase substrate (BioFX Laboratories, Owings Mills, MD). The plates were incubated at room temperature, and the colorimetric reaction was stopped after 30 min by addition of 50 μl 2 N H2SO4. Absorption values were immediately read at 450 nm. The resultant absorption values were compared to the values on a standard curve, generated with serial concentrations of synthetic RANTES (starting at 500 ng). Only values that fell within the linear range of the standard curve were used to make the final determination of the RANTES concentration. The RANTES ELISAs demonstrated that no background was observed for the vehicle control aliquots at any of the time points at which the assays were performed. Each sample was performed in triplicate for each experiment, and each experiment was conducted a minimum of two times.
Human infectivity assays.
Human PBMCs obtained from healthy donors were separated by density gradient centrifugation of the buffy coat on Histopaque-1077, as recommended by the product protocol (Sigma). PMBCs were stimulated in vitro for 48 h with 5 μg/μl of phytohemagglutinin (Murex Biotech Ltd., Dartfort, United Kingdom) and 10 ng/μl of recombinant human interleukin-2 (rhIL-2; R & D Biosystems). Following 2 days of stimulation, the cells were washed in fresh medium and pelleted by use of a 10-min centrifugation at 1,000 rpm. Cells were reseeded into new flasks at a density of 5 × 104 PBMCs/ml and were restimulated for another 24 h with rhIL-2 alone. Following rhIL-2 stimulation, the cells were washed and resuspended in a 50-ml conical tube, for a final cell density of 100,000 cells per well in a total volume of 1 ml. The cells were incubated for 1 h with periodic mixing by using 50 50% tissue culture infective doses of HIV-1BaL stock virus. Mock-infected samples were included with each experiment. The cells were then washed three times in fresh medium to remove unbound virus and were recounted with a hemacytometer under containment. PBMCs were plated in a 96-well plate at a final density of 1 × 105 cells per well in a total volume of 100 μl of complete RPMI medium supplemented with 10 ng/μl of rhIL-2. Serial dilutions of −2 RANTES, full-length RANTES, and SDF-1β, as well as a buffer control (stock PBS and the 1% BSA solution used in the dialysis experiments), were added to three replica wells, for a total volume of 200 μl per well. The buffer control was added as an additional negative control in these experiments. To determine the biological activity of −2 RANTES that was released from each formulation, another aliquot from the original dialysis samples (used to quantify the amount of −2 RANTES released) was directly tested by the PBMC infectivity assay. After 3 days at 37°C (day 3 postinfection), a 100-μl aliquot of the supernatant was removed and assayed for viral p24 levels by ELISA on Nunc Maxisorp ELISA plates coated with a monoclonal α-p24 antibody mixture (NEN Life Science/Perkin-Elmer, Boston, MA). The wells were replenished with 100 μl of fresh RPMI medium supplemented with rhIL-2 and the appropriate chemokine or buffer dilution. After an additional 3 days of incubation (day 6 postinfection), 100 μl of the supernatant was assayed for viral p24 levels by ELISA. For each test sample, the percent inhibition of infection compared with the level of infection for the untreated control wells was determined. Replicate values were averaged and used to generate dose-effect curves. The curves were used to calculate 50% inhibitory concentrations (IC50) for each test chemokine. Statistical analysis (t test) was performed to determine if the averaged observed differences in anti-HIV activity of −2 RANTES released from the various formulation vehicles were statistically significant (P < 0.05).
RESULTS
High concentrations of −2 RANTES are not toxic in vitro.
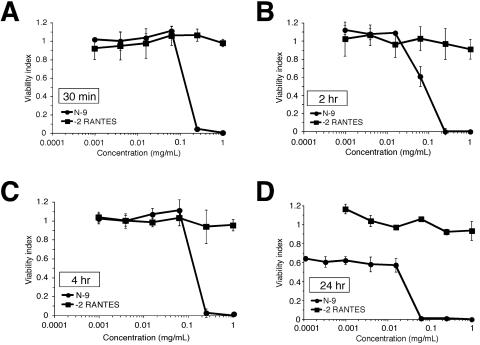
High concentrations of synthetic −2 RANTES may be needed to produce an effective microbicide formulation. Therefore, the intrinsic toxicity of synthetic chemokine concentrations up to 1 mg/ml was evaluated by using a colorimetric cell viability assay. HeLa cells were exposed to serial concentrations of −2 RANTES ranging from 0.1 μg to 1 mg/ml for 30 min, 2 h, 4 h, or 24 h. N-9 was used as a positive control for toxicity. The viability index, or the fraction of viable cells following microbicide treatment relative to the fraction of viable mock-exposed cells, was calculated. As shown in Fig. 1, −2 RANTES demonstrated viability indices of 0.9 to 1.2 at all concentrations tested, which indicated that it was nontoxic. Furthermore, prolonged exposure to high concentrations of chemokine did not alter the viability indices. In contrast, increasing amounts of N-9 were associated with significant toxicity, which was magnified by prolonged exposure (compare Fig. 1A and D).
FIG. 1.
Analysis of synthetic human −2 RANTES toxicity in vitro. HeLa cells were exposed to increasing concentrations of −2 RANTES or N-9 (positive control) for 30 min (A), 2 h (B), 4 h (C), and 24 h (D). Microbicide concentrations up to 1 mg/ml of −2 RANTES and N-9 were evaluated at the indicated time points. Cellular viability was measured immediately following microbicide exposure by using an MTS cellular viability assay. The results are expressed relative to those for mock-exposed cells. The data in each graph represent the average of three experiments, in which each concentration was tested in triplicate. Error bars indicate the standard deviations of the calculated mean values.
Synthetic −2 RANTES does not elicit changes in cervicovaginal epithelial surfaces.
The Swiss Webster mouse vaginal irritation model, which was developed for the preclinical evaluation of candidate vaginal microbicides (5), was used to determine whether the topical application of −2 RANTES results in epithelial tissue damage. This model is relevant for these analyses, as human RANTES is biologically active in mice (7, 26). Initial experiments assessed a single intravaginal application of 1 mg/ml of human −2 RANTES suspended in PBS. The reproductive tracts of the mice were harvested at 30 min, 2 h, 4 h, 6 h, 8 h, and 24 h postapplication. Untreated animals and animals receiving PBS alone did not exhibit signs of changes in cervical or vaginal mucosal tissue at any time point at which the tissues were assessed. Specifically, the exocervical and vaginal mucosas presented intact, differentiated, squamous epithelia (representative PBS-treated tissue is shown in Fig. 2A). A continuous layer of mucus-secreting columnar epithelial cells lining the endocervical region was observed above the lamina propria (representative PBS-treated tissue is shown in Fig. 3A).
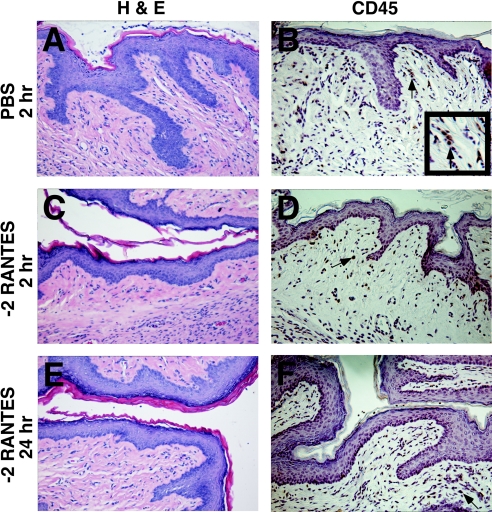
FIG. 2.
Effects of synthetic human −2 RANTES on the vaginal mucosa in the Swiss Webster mouse model. Swiss Webster mice were inoculated intravaginally with 60 μl of −2 RANTES (1 mg/ml). The genital tracts were harvested from the mice at 30 min, 2 h (C and D), 4 h, 8 h, and 24 h (E and F) following application. Formalin-fixed, paraffin-embedded tissue sections were stained with hematoxylin and eosin (H & E) for gross morphological analyses of the surface epithelium (A, C, and E). Immunohistochemical analyses were also performed for the detection of CD45-positive stained cells, as indicated by arrows (B, D, and F). The inset in panel B magnifies cells staining positive for CD45. Two independent experiments were performed with three mice per time point, and representative tissue sections are presented. PBS-treated mice (2 h) (A and B) and 1% N-9-treated (data not shown) mice were included in each experiment as reference controls.
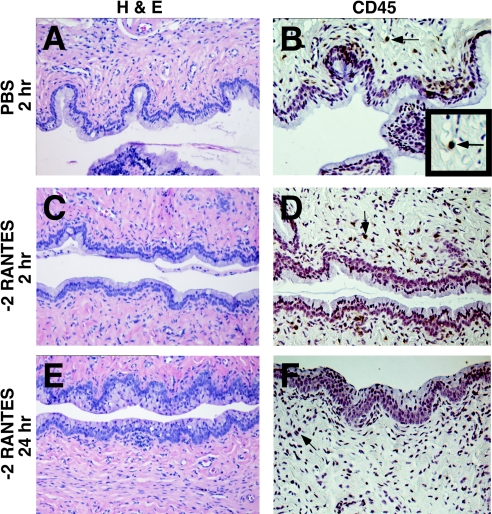
FIG. 3.
Effects of synthetic human −2 RANTES on the cervical mucosa in the Swiss Webster mouse model. Swiss Webster mice were inoculated intravaginally with 60 μl of −2 RANTES (1 mg/ml). The genital tracts were harvested from the mice at 30 min, 2 h (C and D) 4 h, 8 h, and 24 h (E and F) following application. Formalin-fixed, paraffin-embedded tissue sections were stained with hematoxylin and eosin (H & E) for gross morphological analyses of the surface epithelium (A, C, and E). Immunohistochemical analyses were also performed for the detection of CD45-positive stained cells, as indicated by arrows (B, D, and F). The inset in panel B magnifies cells staining positive for CD45. Two independent experiments were performed with three mice per time point, and representative tissue sections are presented. PBS-treated mice (2 h) (A and B) and 1% N-9-treated (data not shown) mice were included in each experiment as reference controls.
Histological analyses of tissues treated with 1 mg/ml of −2 RANTES showed that the tissue resembled the control tissues at every time point at which they were assessed during the study (Fig. 2A, C, and E and Fig. 3A, C, and E). Similarly, treatment of mice with 2 μg of full-length murine RANTES did not result in apparent toxicity to the cervicovaginal mucosa at any time point at which it was assessed (Fig. 4). In contrast, animals treated with 1% N-9 exhibited severe morphological tissue damage to the endocervical region from 2 h to 8 h following application (data not shown) (5). Tissue regeneration restored the N-9-treated columnar epithelium to the condition of that for the control animals by 24 h postapplication. Such results are consistent with previously published observations (5).
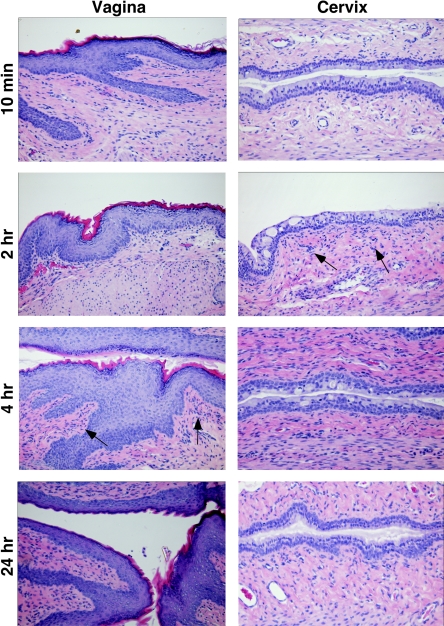
FIG. 4.
Effects of recombinant murine RANTES on cervicovaginal mucosa in the Swiss Webster mouse model. Murine cervicovaginal epithelium was treated with 2 μg/ml of murine RANTES, and tissues were assessed by hematoxylin and eosin staining for evidence of gross morphological damage or evidence of inflammation. Representative vaginal and cervical tissues collected at 30 min, 2 h, 4 h, and 24 h are displayed in the columns labeled vagina and cervix, respectively. Arrows indicate the initial points when increased cell infiltrate was observed below the epithelium. Three independent experiments were performed with at least three mice per time point.
The degree of mucosal inflammation within the cervicovaginal tissues following treatment with −2 RANTES in PBS was assessed immediately following application and at 30 min, 2 h, 4 h, 8 h, and 24 h postapplication. The number and distribution patterns of CD45-positive cells, which include all cells of hematopoietic origin with the exception of erythrocytes, were determined by immunohistochemical analyses. In these studies, infiltration of CD45-positive cells into the cervicovaginal mucosa following microbicide exposure was indicative of an inflammatory response.
PBS-treated tissues collected at 30 min, 2 h, 4 h, 8 h, and 24 h postapplication displayed the expected dispersion pattern of CD45-positive cells in the epithelial and basal layers of the vaginal mucosa. CD45-positive stained cells were also identified in the lamina propria and in the stroma of the cervicovaginal tissue (representative PBS-treated tissue [Fig. 2B and Fig. 3B]). The numbers and the distribution patterns of the CD45-positive cells in the cervicovaginal tissue sections were consistent at all time points after PBS application.
The vaginal and cervical epithelia of −2 RANTES-treated tissues remained intact throughout the duration of the study. The overall levels and distribution patterns of the CD45-positive cells within the cervicovaginal mucosa were similar to those of the cells in PBS-treated tissues (Fig. 2B, D, and F and Fig. 3B, D, and F). Occasionally, animals in the −2 RANTES-treated group demonstrated a mild increase in CD45-positive cells below the basal layer of the vaginal and cervical epithelia by 8 h to 24 h postapplication. However, these observations were recorded for less than 20% of the −2 RANTES-treated animals. Furthermore, all −2 RANTES-treated mice retained intact vaginal and cervical epithelia at every time point at which the epithelia were assessed during this study.
Additional analyses were conducted with full-length murine RANTES, the only murine variant of RANTES available. These studies revealed that the cervical and vaginal epithelia of mice treated with 2 μg/ml of murine RANTES remained morphologically intact at every time point at which they were assessed. However, hematoxylin and eosin staining of these samples indicated minor cell infiltrate below the basal layer in the cervicovaginal mucosa (Fig. 4, arrows). Such staining was evident below the basal layer of the cervical and vaginal mucosa at 2 to 4 h postapplication, increased during the next 4 to 6 h, but then diminished by 24 h postapplication. Overall, the numbers of stained cells were minimal at all time points, whereas severe cell infiltration was observed in 1% N-9-treated tissues (data not shown) (5).
Human −2 RANTES is released from formulation vehicles and retains its HIV-1 suppressive activity.
Before the −2 RANTES formulations were evaluated in animal models, it was important to verify that carrier vehicles release the chemokine in an antiviral form. Two vehicles, Novasomes 7474 and methylcellulose, were selected for testing. Test formulations were prepared with serial amounts of chemokine in each vehicle, and control formulations were prepared with the corresponding concentrations of chemokine in PBS. Each formulation was then placed in a dialysis bag and dialyzed against PBS containing 1% BSA (buffer) for 24 h. The molecular-weight-cutoff size of the dialysis tubing was selected to permit any RANTES aggregates, which may form at high chemokine concentrations, to diffuse into the buffer (10) while still retaining the vehicles within the bags. Samples were collected from the buffer at 30, 120, 240, 360, and 1,140 min and assayed by ELISA to quantify the amount of −2 RANTES that was released from the carrier vehicle into the buffer. Control experiments demonstrated that both vehicles were retained in the dialysis bag and did not interfere with the RANTES ELISA (data not shown).
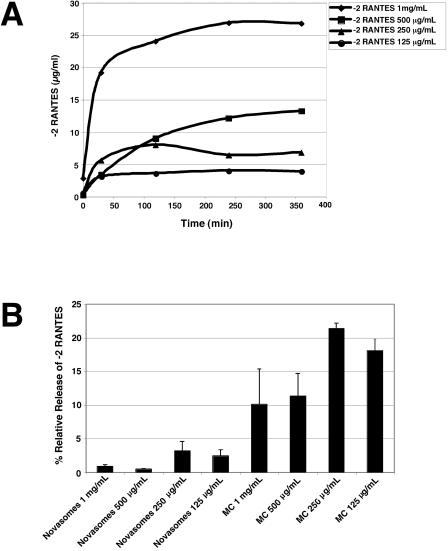
As shown in Fig. 5, −2 RANTES formulated in PBS dialyzed into the buffer over time (Fig. 5A) and reached an equilibrium concentration within 360 min. At 360 min, the chemokine concentration in the buffer was always in accordance with the expected amount that accounted for the volume change (50-fold). In comparison, the two vehicles allowed less extensive release of −2 RANTES into the buffer. In the case of the Novasomes 7474 formulations, the amount of −2 RANTES in the buffer at 360 min was less than 4% of the amount determined for the corresponding PBS formulation (Fig. 5B). In the case of the methylcellulose formulations, the concentration of −2 RANTES in the buffer at 360 min was 10 to 22% of the amount released by the PBS control formulations (Fig. 5B). However, the methylcellulose formulations released at least sixfold more chemokine than the Novasomes 7474 formulations containing the corresponding amounts of chemokine. Statistical analyses indicated that the percentage of −2 RANTES released from methylcellulose (relative to the amount released from the PBS control) was significantly higher than that released from Novasomes 7474 (P < 0.008).
FIG. 5.
Release of −2 RANTES from carrier vehicles. Dialysis experiments (see Materials and Methods) were used to evaluate the release of chemokine from methylcellulose (MC) and Novasomes 7474 carrier vehicles. (A) PBS formulations containing the indicated concentrations of −2 RANTES were placed in dialysis bags and dialyzed against PBS. Samples of the dialysis buffer were collected at the indicated times and assayed for −2 RANTES by ELISA. (B) Various concentrations of −2 RANTES were formulated in Novasomes 7474 or methylcellulose vehicles and placed in dialysis bags. The amount of chemokine detected in the buffer after 360 min of dialysis is presented as the percentage of that released from the corresponding −2 RANTES formulations in PBS. The data shown represent the average of values obtained from at least two independent experiments. Error bars indicate standard deviations of the calculated mean values.
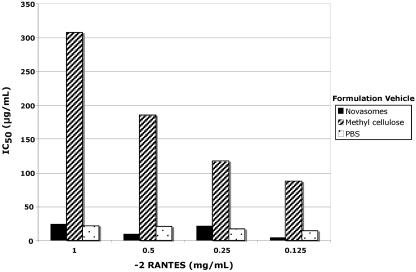
To determine if the −2 RANTES released from the vehicle formulations retained its anti-HIV activity, samples collected from the dialysis experiment at 120 min were tested in HIV-1 infectivity assays by using PBMCs as target cells. This time frame represents the period during which there is the highest likelihood of potential exposure to the virus following microbicide application. HIV-1 p24 values were determined at 6 days after infection with HIV-1BaL. The IC50 values, which reflect the chemokine concentration that reduces infection by ≥50% relative to the level of infection in the untreated controls, were then determined for each sample.
As shown in Fig. 6, the HIV-1 suppressive activity of −2 RANTES was retained after −2 RANTES release from PBS, as well as after −2 RANTES release from both test vehicles. Importantly, the anti-HIV activity of the −2 RANTES concentrations released from Novasomes 7474 was similar (P > 0.5, which is not statistically significant) to that observed for the PBS formulations (Fig. 6). In contrast, the HIV suppressive activity of the −2 RANTES released from the methylcellulose formulations was significantly lower than that of the −2 RANTES released from either −2 RANTES formulated in PBS (P < 0.05) or −2 RANTES formulated in Novasomes 7474 (P < 0.05) (Fig. 6).
FIG. 6.
Anti-HIV activity of −2 RANTES released from vehicle formulations. Samples collected after 120 min of dialysis were tested in infectivity assays with HIV-1BaL (see Materials and Methods). After 6 days, infection was determined as a function of HIV p24 levels in the culture supernatants. Replicate values were averaged and were used to generate dose-effect curves. The curves were used to calculate the IC50 of each test formulation. An average value from two independent experiments is presented.
Microbicide formulations containing −2 RANTES do not cause cervicovaginal inflammation or general toxic effects.
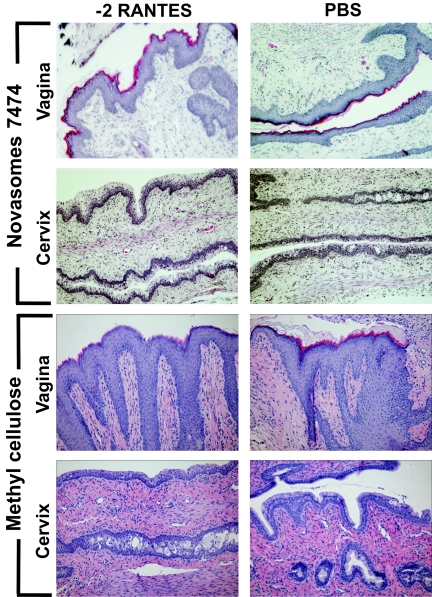
The Swiss Webster mouse vaginal irritation model was further used to assess tissue damage following cervicovaginal exposure to −2 RANTES formulated in carrier vehicles. The two vehicles selected for these experiments, Novasomes 7474 and methylcellulose gel, were previously tested in vaginal applications and demonstrated acceptable safety profiles (9, 43, 44). Furthermore, −2 RANTES alone did not appear to result in significant toxicity when it was administered to the cervicovaginal mucosa (Fig. 2 to 4). Therefore, −2 RANTES was mixed with each vehicle to a final concentration of 1 mg/ml and was applied intravaginally. The reproductive tracts of the mice were harvested at 30 min, 2 h, 4 h, 6 h, 8 h, and 24 h after treatment. Tissues treated with Novasomes 7474 alone, methylcellulose alone, or formulations containing 1 mg/ml −2 RANTES all exhibited minimal morphological tissue toxicity. Specifically, the vaginal and cervical epithelia retained intact, continuous surfaces, similar to that of PBS-treated tissues, at all time points assessed (representative tissue sections obtained at 2 h are shown in Fig. 7).
FIG. 7.
−2 RANTES formulations are nontoxic to the murine cervicovaginal mucosa. Swiss Webster mice were anesthetized and intravaginally inoculated with 60 μl of −2 RANTES (1 mg/ml) formulated in Novasomes 7474 and methylcellulose carrier vehicles. The results for controls treated with vehicle alone are also presented. The genital tracts were harvested from the mice at 30 min, 2 h, 4 h, 8 h, and 24 h following application. Formalin-fixed, paraffin-embedded tissue sections were stained with standard hematoxylin and eosin for gross morphological analyses of the surface epithelium. Representative tissue sections harvested at 2 h are presented here. Three independent experiments were performed with at least two mice per time point.
The New Zealand White rabbit model was used to assess whether repeated applications of formulated −2 RANTES resulted in vaginal irritation. Animals were treated with 1-ml doses of Novasomes 7474 containing either 50 μg/ml or 500 μg/ml of −2 RANTES. Animals treated with Novasomes 7474 alone were included as a reference control. Sham-treated animals that did not receive compound were subjected to the same technical application protocol as the treated animals. All animals received daily intravaginal doses of the test articles for 10 consecutive days.
Twenty-four hours after the last application of the test articles, the vaginal tracts were excised from all animals and processed for histopathological evaluation. As expected, sham-treated animals exhibited normal tissue morphology and staining profiles. The vaginal tissues taken from animals treated with Novasomes 7474 showed some polymorphonuclear cell infiltration in the epithelial and subepithelial connective tissue. However, the infiltration observed was graded as “moderate,” and vascular congestion and edema were judged to be “mild to moderate.” The vaginal epithelium remained intact, and only minor morphological changes were noted. Comparable determinations were made for samples taken from the animals given 50 μg/ml −2 RANTES or 500 μg/ml −2 RANTES in Novasomes 7474. In both of these −2 RANTES-treated groups, the vaginal epithelium demonstrated slightly less polymorphonuclear cell infiltration compared with that in the Novasomes 7474-treated group. Squamous metaplasia and focal erosion were occasionally observed with a degree of leukocyte infiltration primarily in the subepithelial connective tissues. Minimum to mild vascular congestion was noted in most rabbits in the −2 RANTES-treated groups, and edema was observed only occasionally. No necrosis or vascular thrombi were observed in any animals during this study. Standardized microscopic evaluation criteria (see Materials and Methods) were used to assign a composite average score for each test formulation. The composite average scores for each of the test groups are as follows: sham-treated control, 1.1; Novasomes 7474-treated group, 6.7; the group treated with 50 μg/ml −2 RANTES in Novasomes 7474, 7.3; and the group treated with 500 μg/ml −2 RANTES in Novasomes 7474, 7.1. In conclusion, both −2 RANTES formulations received a “mild” vaginal irritation rating, indicating that they are acceptable for vaginal use (16, 17). In comparison, 2% N-9 formulated in carboxymethyl cellulose scored 12.8, which is considered unacceptable by the standard scoring method (18).
DISCUSSION
The development of topical microbicides has gained increasing attention as a means of containing sexually transmitted disease epidemics, including the HIV and AIDS epidemic. Over thirty candidate compounds are being evaluated for their potential use as topical microbicides (Alliance for Microbicide Development [www.microbicide.org]). Each class of compounds demands specific types of analyses to assess potential safety and efficacy. In the case of biological antiviral molecules formulated in carrier vehicles, it is necessary to evaluate whether a candidate compound is nontoxic, whether it is released from the vehicle in an antiviral form and concentrations, and whether biological activity causes inflammation or irritation which could subsequently promote infection.
In this study, we evaluated whether −2 RANTES formulated in a carrier vehicle warrants testing for efficacy as a vaginal anti-HIV-1 microbicide. Since RANTES is a chemoattractant cytokine that plays a role in inflammatory responses, our analytical models were selected and configured to determine whether the test formulations caused significant inflammation or cell infiltration of vaginal tissues. The nonionic surfactant N-9 was used as a positive control in these experiments for severe cervicovaginal toxicity. N-9 has repeatedly been demonstrated to cause gross disturbances in the cervicovaginal mucosa, subsequently increasing the risk of acquiring HIV-1 infection through sexual intercourse (28, 34, 35, 39).
Four of our analytical parameters indicated that vehicles deliver and release −2 RANTES in an active form and without evidence of toxicity. First, concentrations of −2 RANTES up to 2,000-fold higher than what is necessary for blocking HIV-1 infection in vitro (1 mg/ml) were nontoxic, as determined in MTS cytotoxicity assays (Fig. 1). Thus, it was reasonable to expect that relatively high concentrations of −2 RANTES can be incorporated into carrier vehicles with a minimal risk of causing general toxic effects in vivo.
Second, analyses in the Swiss Webster mouse vaginal toxicity model demonstrated that −2 RANTES, either alone (Fig. 2 and 3) or in a formulation with methylcellulose or Novasomes 7474 (Fig. 7), causes no morphological damage to the cervical or vaginal epithelium at concentrations up to 1 mg/ml. Furthermore, neither vehicle alone caused detectable damage, in accordance with the findings of clinical trials that used these compounds as placebo controls (Novavax investigational new drug permit 57,550) (9). It is particularly noteworthy that no damage was evident between 30 min and 4 h after microbicide application, which corresponds to the period when the maximum risk of potential exposure to HIV-1 is likely to occur. Previous studies with this model have also demonstrated that these are the time points when the greatest amount of tissue toxicity and inflammation occurred following 1% N-9 application. Immunohistochemical analyses of murine CD45-positive cells further demonstrated that up to 1 mg/ml −2 RANTES (Fig. 2 and 3) caused minimal infiltration of cells over the 24-h time course of the experiment. Collectively, these findings suggest that vaginal application of −2 RANTES is unlikely to cause recruitment of potential host cells to HIV-1-exposed areas of the vaginal epithelium or to induce major changes in tissue architecture that might promote mucosal HIV-1 infection. It is reasonable to use these findings to predict the safety of −2 RANTES in humans, since studies demonstrate that the human chemokine is biologically active in mice (7, 26).
Analyses of 2 μg/ml of full-length murine RANTES in the Swiss Webster mouse model revealed signs of more significant cell infiltrate in the animals compared to that observed with human −2 RANTES. However, the murine chemokine did not result in gross visible signs of inflammation or morphological damage to the cervicovaginal mucosa (Fig. 4). These differences could be due to the higher activity of the murine chemokine in the mouse or to the generally lower chemotactic activity of −2 RANTES compared with that of the unprocessed form (38). Recent studies in our laboratory demonstrated that the vaginal application of formulated −2 RANTES was nontoxic to the cervicovaginal epithelium of the cynomolgus macaque model (T. M. Kish-Catalone et al., unpublished data).
Third, toxicity studies with the standardized New Zealand White rabbit vaginal irritation model emphasize that minimal tissue damage resulted following 10 consecutive daily applications of the −2 RANTES formulations. Specifically, the Novasomes 7474 formulations containing 50 μg/ml or 500 μg/ml of −2 RANTES received an acceptable vaginal irritation rating, according to established scoring methodologies for assessment of the toxicities of vaginal products. Notably, this model system is a required in vivo assay necessary for all candidate vaginal microbicides advancing into clinical trials.
Fourth, the dialysis experiments demonstrated that −2 RANTES was released from formulation vehicles with retention of antiviral activity. However, more −2 RANTES was released from methylcellulose than from Novasomes 7474. These experiments were not expected to predict precisely how much −2 RANTES would be released in the cervicovaginal environment in situ, since many factors (e.g., pH, temperature, enzymatic activities, and the presence of vaginal microflora and semen) are not represented. However, the system allowed us to compare different formulations for chemokine release. In general, the vehicles impeded the dialysis of −2 RANTES into the buffer compared to that for −2 RANTES in PBS (Fig. 5). Between the two vehicles, more chemokine was released from methylcellulose than from Novasomes 7474. The physical and/or chemical basis for this difference is unclear. An important consideration is that methylcellulose is a mixture of heterogeneous polymers, with the smallest having a molecular weight of 90,000. Therefore, a minor portion of the vehicle might have passed through the dialysis bag along with the chemokine. Such an effect could explain why methylcellulose appeared to release more −2 RANTES than the corresponding Novasomes 7474 formulations. Notably, our control assays determined that methylcellulose did not interfere in the ELISA or inhibit HIV infection when it was tested alone (data not shown). Therefore, we do not believe that the quantitative assays were influenced by methylcellulose contamination. However, the antiviral potency of the −2 RANTES released from methylcellulose was significantly reduced compared to that of the −2 RANTES released from PBS or Novasomes 7474 (Fig. 6). These results collectively emphasize the importance of formulation design in the development of a vaginal microbicide based on biological antiviral molecules.
In conclusion, the excellent safety profile of −2 RANTES provides strong support for the advancement of −2 RANTES as a vaginal microbicide. The ideal formulation for any vaginal microbicide requires a balance between minimizing the toxicity of the microbicide to the cervicovaginal mucosa and maintaining maximal activity against HIV-1. This report on the preclinical evaluation of −2 RANTES demonstrates that −2 RANTES is favorable for topical vaginal application for prevention of the sexual transmission of R5 tropic strains of HIV-1.
Acknowledgments
We acknowledge D. Craig Wright from Novavax, Incorporated, for the generous gift of Novasomes 7474. We also gratefully acknowledge Robin Maguire of The Population Council (Rockefeller University) for the gift of methylcellulose. We acknowledge Abigail Bowlsbey for technical support.
These studies were supported by Public Health Service grant PO1 AI52050 from NIAID.
REFERENCES
- 1.Alkhatib, G., M. Locati, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1997. HIV-1 coreceptor activity of CCR5 and its inhibition by chemokines: independence from G protein signaling and importance of coreceptor downmodulation. Virology 234:340-348. [DOI] [PubMed] [Google Scholar]
- 2.Reference deleted.
- 3.Amara, A., S. L. Gall, O. Schwartz, J. Salamero, M. Montes, P. Loetscher, M. Baggiolini, J. L. Virelizier, and F. Arenzana-Seisdedos. 1997. HIV coreceptor downregulation as antiviral principle: SDF-1alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 186:139-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.American Physiological Society. 1996. Guiding principles in the care and use of animals. National Academy Press, Washington, D.C.
- 3b.AVMA Panel on Euthanasia. 2001. 2000 report of the AVMA Panel on Euthanasia. J. Am. Vet. Med. Assoc. 218:669-696. [DOI] [PubMed] [Google Scholar]
- 4.Bogers, W. M., L. A. Bergmeier, J. Ma, H. Oostermeijer, Y. Wang, C. G. Kelly, P. Ten Haaft, M. Singh, J. L. Heeney, and T. Lehner. 2004. A novel HIV-CCR5 receptor vaccine strategy in the control of mucosal SIV/HIV infection. AIDS 18:25-36. [DOI] [PubMed] [Google Scholar]
- 5.Catalone, B. J., T. M. Kish-Catalone, L. R. Budgeon, E. B. Neely, M. Ferguson, F. C. Krebs, M. K. Howett, M. Labib, R. Rando, and B. Wigdahl. 2004. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob. Agents Chemother. 48:1837-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catalone, B. J., F. C. Krebs, M. Labib, and B. Wigdahl. 2001. Broad-spectrum microbicides as a strategy for reducing sexually transmitted disease prevalence and HIV-1 transmission. Res. Adv. Antimicrob. Agents Chemother. 2:55-80. [Google Scholar]
- 7.Chvatchko, Y., A. E. Proudfoot, R. Buser, P. Juillard, S. Alouani, M. Kosco-Vilbois, A. J. Coyle, R. J. Nibbs, G. Graham, R. E. Offord, and T. N. Wells. 2003. Inhibition of airway inflammation by amino-terminally modified RANTES/CC chemokine ligand 5 analogues is not mediated through CCR3. J. Immunol. 171:5498-5506. [DOI] [PubMed] [Google Scholar]
- 8.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 9.Coetzee, N., K. Blanchard, C. Ellertson, A. A. Hoosen, and B. Friedland. 2001. Acceptability and feasibility of Micralax applicators and of methyl cellulose gel placebo for large-scale clinical trials of vaginal microbicides. AIDS 15:1837-1842. [DOI] [PubMed] [Google Scholar]
- 10.Czaplewski, L. G., J. McKeating, C. J. Craven, L. D. Higgins, V. Appay, A. Brown, T. Dudgeon, L. A. Howard, T. Meyers, J. Owen, S. R. Palan, P. Tan, G. Wilson, N. R. Woods, C. M. Heyworth, B. I. Lord, D. Brotherton, R. Christison, S. Craig, S. Cribbes, R. M. Edwards, S. J. Evans, R. Gilbert, P. Morgan, E. Randle, N. Schofield, P. G. Varley, J. Fisher, J. P. Waltho, and M. G. Hunter. 1999. Identification of amino acid residues critical for aggregation of human CC chemokines macrophage inflammatory protein (MIP)-1alpha, MIP-1beta, and RANTES. Characterization of active disaggregated chemokine variants. J. Biol. Chem. 274:16077-16084. [DOI] [PubMed] [Google Scholar]
- 11.Dawson, P. E. 1997. Synthesis of chemokines by native chemical ligation. Methods Enzymol. 287:34-45. [DOI] [PubMed] [Google Scholar]
- 12.Dawson, P. E., T. W. Muir, I. Clark-Lewis, and S. B. Kent. 1994. Synthesis of proteins by native chemical ligation. Science 266:776-779. [DOI] [PubMed] [Google Scholar]
- 13.D'Cruz, O. J., and F. M. Uckun. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 10:315-336. [DOI] [PubMed] [Google Scholar]
- 14.Dean, M., M. Carrington, C. Winkler, G. A. Huttley, M. W. Smith, R. Allikmets, J. J. Goedert, S. P. Buchbinder, E. Vittinghoff, E. Gomperts, S. Donfield, D. Vlahov, R. Kaslow, A. Saah, C. Rinaldo, R. Detels, and S. J. O'Brien. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 273:1856-1862. [DOI] [PubMed] [Google Scholar]
- 15.De Clercq, E., and D. Schols. 2001. Inhibition of HIV infection by CXCR4 and CCR5 chemokine receptor antagonists. Antivir. Chem. Chemother. 12(Suppl. 1):19-31. [PubMed] [Google Scholar]
- 16.Draize, J. H., G. Woodard, and O. H. Calvery. 1944. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 82:377-390. [Google Scholar]
- 17.Eckstein, P., M. C. Jackson, N. Millman, and A. J. Sobrero. 1969. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J. Reprod. Fertil. 20:85-93. [DOI] [PubMed] [Google Scholar]
- 18.Fichorova, R. N., M. Bajpai, N. Chandra, J. G. Hsiu, M. Spangler, V. Ratnam, and G. F. Doncel. 2004. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol. Reprod. 71:761-769. [DOI] [PubMed] [Google Scholar]
- 19.Forster, R., E. Kremmer, A. Schubel, D. Breitfeld, A. Kleinschmidt, C. Nerl, G. Bernhardt, and M. Lipp. 1998. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J. Immunol. 160:1522-1531. [PubMed] [Google Scholar]
- 20.Huang, Y., W. A. Paxton, S. M. Wolinsky, A. U. Neumann, L. Zhang, T. He, S. Kang, D. Ceradini, Z. Jin, K. Yazdanbakhsh, K. Kunstman, D. Erickson, E. Dragon, N. R. Landau, J. Phair, D. D. Ho, and R. A. Koup. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat. Med. 2:1240-1243. [DOI] [PubMed] [Google Scholar]
- 21.Iwata, S., N. Yamaguchi, Y. Munakata, H. Ikushima, J. F. Lee, O. Hosono, S. F. Schlossman, and C. Morimoto. 1999. CD26/dipeptidyl peptidase IV differentially regulates the chemotaxis of T cells and monocytes toward RANTES: possible mechanism for the switch from innate to acquired immune response. Int. Immunol. 11:417-426. [DOI] [PubMed] [Google Scholar]
- 22.Joint United Nations Programme on HIV/AIDS (UNAIDS)/World Health Organization (WHO). 2004. Executive summary. In UNAIDS 2004 report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS, Geneva, Switzerland.
- 23.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 24.Malkovsky, M., A. Newell, and A. G. Dalgleish. 1988. Inactivation of HIV by nonoxynol-9. Lancet i:645. [DOI] [PubMed] [Google Scholar]
- 25.Michael, N. L., G. Chang, L. G. Louie, J. R. Mascola, D. Dondero, D. L. Birx, and H. W. Sheppard. 1997. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat. Med. 3:338-340. [DOI] [PubMed] [Google Scholar]
- 26.Mrowietz, U., U. Schwenk, S. Maune, J. Bartels, M. Kupper, I. Fichtner, J. M. Schroder, and D. Schadendorf. 1999. The chemokine RANTES is secreted by human melanoma cells and is associated with enhanced tumour formation in nude mice. Br. J. Cancer 79:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institutes of Health. 2004. HIV infection in women. National Institute of Allergy and Infectious Diseases, Bethesda, Md.
- 28.Niruthisard, S., R. E. Roddy, and S. Chutivongse. 1991. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex. Transm. Dis. 18:176-179. [DOI] [PubMed] [Google Scholar]
- 29.Oravecz, T., M. Pall, and M. A. Norcross. 1996. Beta-chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J. Immunol. 157:1329-1332. [PubMed] [Google Scholar]
- 30.Oravecz, T., M. Pall, G. Roderiquez, M. D. Gorrell, M. Ditto, N. Y. Nguyen, R. Boykins, E. Unsworth, and M. A. Norcross. 1997. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J. Exp. Med. 186:1865-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polsky, B., P. A. Baron, J. W. Gold, J. L. Smith, R. H. Jensen, and D. Armstrong. 1988. In vitro inactivation of HIV-1 by contraceptive sponge containing nonoxynol-9. Lancet i:1456. [DOI] [PubMed] [Google Scholar]
- 32.Proost, P., S. Struyf, A. Wuyts, P. Menten, I. De Meester, A. M. Lambeir, S. Scharpe, D. Schols, E. De Clercq, and J. Van Damme. 1998. Isolation and identification of naturally modified C-C chemokines MCP-1, MCP-2 and RANTES: effects of posttranslational modifications on receptor usage, chemotactic and anti-HIV-1 activity. Eur. Cytokine Network 9:73-75. [PubMed] [Google Scholar]
- 33.Quillent, C., E. Oberlin, J. Braun, D. Rousset, G. Gonzalez-Canali, P. Metais, L. Montagnier, J. L. Virelizier, F. Arenzana-Seisdedos, and A. Beretta. 1998. HIV-1-resistance phenotype conferred by combination of two separate inherited mutations of CCR5 gene. Lancet 351:14-18. [DOI] [PubMed] [Google Scholar]
- 34.Roddy, R. E., M. Cordero, C. Cordero, and J. A. Fortney. 1993. A dosing study of nonoxynol-9 and genital irritation. Int. J. STD AIDS 4:165-170. [DOI] [PubMed] [Google Scholar]
- 35.Rustomjee, R., Q. Abdool Karim, S. S. Abdool Karim, M. Laga, and Z. Stein. 1999. Phase 1 trial of nonoxynol-9 film among sex workers in South Africa. AIDS 13:1511-1515. [DOI] [PubMed] [Google Scholar]
- 36.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 37.Schols, D. 2004. HIV co-receptors as targets for antiviral therapy. Curr. Top. Med. Chem. 4:883-893. [DOI] [PubMed] [Google Scholar]
- 38.Schols, D., P. Proost, S. Struyf, A. Wuyts, I. De Meester, S. Scharpe, J. Van Damme, and E. De Clercq. 1998. CD26-processed RANTES(3-68), but not intact RANTES, has potent anti-HIV-1 activity. Antivir. Res. 39:175-187. [DOI] [PubMed] [Google Scholar]
- 39.Stafford, M. K., H. Ward, A. Flanagan, I. J. Rosenstein, D. Taylor-Robinson, J. R. Smith, J. Weber, and V. S. Kitchen. 1998. Safety study of nonoxynol-9 as a vaginal microbicide: evidence of adverse effects. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:327-331. [DOI] [PubMed] [Google Scholar]
- 40.Struyf, S., I. De Meester, S. Scharpe, J. P. Lenaerts, P. Menten, J. M. Wang, P. Proost, and J. Van Damme. 1998. Natural truncation of RANTES abolishes signaling through the CC chemokine receptors CCR1 and CCR3, impairs its chemotactic potency and generates a CC chemokine inhibitor. Eur. J. Immunol. 28:1262-1271. [DOI] [PubMed] [Google Scholar]
- 41.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 42.van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrecht-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Coutinho, F. Miedema, and H. Schuitemaker. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Investig. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veazey, R. S., P. J. Klasse, T. J. Ketas, J. D. Reeves, M. Piatak, Jr., K. Kunstman, S. E. Kuhmann, P. A. Marx, J. D. Lifson, J. Dufour, M. Mefford, I. Pandrea, S. M. Wolinsky, R. W. Doms, J. A. DeMartino, S. J. Siciliano, K. Lyons, M. S. Springer, and J. P. Moore. 2003. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J. Exp. Med. 198:1551-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallach, D. F. H., and J. Phillipot. 1992. New type of lipid visicle: novasome. CRC Press, Inc., Boca Raton, Fla.
- 45.Wilken, J., D. Hoover, D. A. Thompson, P. N. Barlow, H. McSparron, L. Picard, A. Wlodawer, J. Lubkowski, and S. B. Kent. 1999. Total chemical synthesis and high-resolution crystal structure of the potent anti-HIV protein AOP-RANTES. Chem. Biol. 6:43-51. [DOI] [PubMed] [Google Scholar]
- 46.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 47.Zhang, L. Q., P. MacKenzie, A. Cleland, E. C. Holmes, A. J. Brown, and P. Simmonds. 1993. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J. Virol. 67:3345-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]
- 49.Zhu, T., N. Wang, A. Carr, D. S. Nam, R. Moor-Jankowski, D. A. Cooper, and D. D. Ho. 1996. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J. Virol. 70:3098-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmerman, P. A., A. Buckler-White, G. Alkhatib, T. Spalding, J. Kubofcik, C. Combadiere, D. Weissman, O. Cohen, A. Rubbert, G. Lam, M. Vaccarezza, P. E. Kennedy, V. Kumaraswami, J. V. Giorgi, R. Detels, J. Hunter, M. Chopek, E. A. Berger, A. S. Fauci, T. B. Nutman, and P. M. Murphy. 1997. Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol. Med. 3:23-36. [PMC free article] [PubMed] [Google Scholar]