Abstract
Use of indwelling catheters is often compromised as a result of biofilm formation. This study investigated if hydrogel-coated catheters pretreated with a coagulase-negative bacteriophage would reduce Staphylococcus epidermidis biofilm formation. Biofilms were developed on hydrogel-coated silicone catheters installed in a modified drip flow reactor. Catheter segments were pretreated with the lytic S. epidermidis bacteriophage 456 by exposing the catheter lumen to a 10-log-PFU/ml culture of the bacteriophage for 1 h at 37°C prior to biofilm formation. The untreated mean biofilm cell count was 7.01 ± 0.47 log CFU/cm2 of catheter. Bacteriophage treatment with and without supplemental divalent cations resulted in log-CFU/cm2 reductions of 4.47 (P < 0.0001) and 2.34 (P = 0.001), respectively. Divalent cation supplementation without bacteriophage treatment provided a 0.67-log-CFU/cm2 reduction (P = 0.053). Treatment of hydrogel-coated silicone catheters with an S. epidermidis bacteriophage in an in vitro model system significantly reduced viable biofilm formation by S. epidermidis over a 24-h exposure period, suggesting the potential of bacteriophage for mitigating biofilm formation on indwelling catheters and reducing the incidence of catheter-related infections.
One of the important challenges to inpatient medical care is the prevention of catheter-related infections. Between 1965 and 1991, more than half of the reported outbreaks of nosocomial bacteremia or candidemia were associated in some manner with intravascular access (37, 39). One-third to one-half of episodes of nosocomial endocarditis have been traced to infected intravascular catheters (21, 61). Approximately 250,000 cases of nosocomial intravascular catheter-related bloodstream infections (BSIs) occur in the United States each year, resulting in a mortality of between 12 to 25% with an estimated cost of treatment per episode of approximately $25,000 (35, 45). Urinary tract infections, which have an attributable mortality of less than 5%, are the second most common source of nosocomial bloodstream infections (41).
Many microorganisms colonize indwelling catheters, including central venous catheters (CVCs), forming biofilms (20, 49). These biofilms can result in BSIs and are often due to Staphylococcus epidermidis and Staphylococcus aureus (8, 65). A biofilm can be defined as a microbially derived, sessile community characterized by cells that are irreversibly attached to a living or nonliving substratum, interface, or each other. The organisms are embedded in a matrix of extracellular polymeric substances that they have produced and exhibit an altered phenotype with respect to growth rate and gene transcription (11, 17). Treatment of indwelling catheter-associated infections with conventional antimicrobial agents alone is frequently unsuccessful due to the extremely high tolerance of these agents in microorganisms comprising the biofilm (7, 66).
Several preventative measures and treatment strategies have been investigated for CVC-related infections, including silver-impregnated subcutaneous cuffs (22, 38), antimicrobial coating of catheters (33, 64), the use of antiseptic-impregnated catheter hubs (54), disinfection of the insertion site (40), and intraluminal antimicrobial therapy, or “antimicrobial locks” (3, 5, 13). While these approaches have met with some success, device-associated infections, including infections due to organisms exhibiting antimicrobial resistance, remain a concern for health care institutions worldwide. This has led to a renewed interest in bacteriophages (phages) for the treatment of device-associated infections. The use of phages has been the subject of considerable attention since they were first recognized early in the 20th century (15, 63).
Lytic phages have very effective bactericidal activity and have several advantages over antimicrobial agents. Most notably, phages replicate at the site of infection and are available in abundance where they are most required (58), and although no specific systematic studies have been carried out, no serious or irreversible side effects of phage therapy have yet been described. Phages have been used successfully to treat experimental infections, including BSIs and meningitis, in poultry and animals (2, 57, 59, 60) and antimicrobial-resistant infections in humans (9, 67-70). It is worth noting, however, that the majority of studies involving phage therapy in humans are mostly reports of the clinical treatment of infections that could not be treated successfully by other means and are not specific clinical trials with stringent negative controls.
Though evidence supporting the use of phages for the treatment of device-associated biofilms is lacking, recent studies involving the interaction of phage and biofilms have shown the ability of phage to degrade biofilm exopolysaccharide and infect biofilm cells (18, 19, 29, 30). The prevention of biofilm formation by Listeria monocytogenes by using phages has also been documented (28). While the number of publications regarding phage-biofilm interactions is limited, the current data suggest that further investigation is warranted.
This project investigated the use of phages for the prevention of biofilm formation on catheters. The purpose of this study was to determine whether pretreatment of a hydrogel-coated catheter with a particular phage would prevent or reduce biofilm formation by S. epidermidis in an in vitro system.
MATERIALS AND METHODS
Organisms and culture conditions.
S. epidermidis 414 (HER 1292—Félix d'Hérelle Reference Center for Bacterial Viruses) was selected for this study. The organism stock was maintained at −80°C. Coagulase-negative staphylococcus phage 456 (14) (Health Protection Agency, Colindale, United Kingdom) was also used in this study and was maintained as a lyophilized preparation stored at 4°C. The phage was propagated using the soft agar overlay technique (1, 27), and crude high-titer phage broth cultures were prepared as described by Adams (1) using S. epidermidis 414 as the host strain in Mueller-Hinton broth (MHB) (Difco, Becton Dickinson, Sparks, MD) containing 3 mM MgCl2 and 4 mM CaCl2 (added as MgCl2 · 6H2O and CaCl2 · 2H2O). One milliliter of an 18-h culture of S. epidermidis 414 in MHB (cultured at 37°C) was added to 49 ml of MHB containing 3 mM MgCl2 and 4 mM CaCl2. This was incubated at 37°C with shaking at 250 rpm, and the optical density at 600 nm (OD600) was monitored until an absorbance of 0.3 was reached (Spectronic 21D spectrophotometer; Spectronic Instruments Inc., Rochester, NY). Phage 456 was added to a final concentration of 106 to 107 PFU/ml. The culture was allowed to stand for 15 min at 37°C and then incubated for 18 h at 37°C with shaking at 100 rpm. Host cell debris was pelleted by centrifugation (4,000 × g for 20 min), and the supernatant containing phage was filter sterilized (Millipore, Billerica, MA; 0.22-μm pore size). The titer of the crude phage lysate was determined by plaque assay using the soft agar overlay technique on Mueller-Hinton agar (MHA) (1, 27), stored at 4°C, and used within a week.
Catheters.
Lubri-sil all-silicone 16 French Foley catheters were used (C. R. Bard, Covington, GA). These catheters are coated with a neutral hydrogel on both the inner and outer surfaces. Foley catheters were used in place of CVCs, as no commercial source of neutral hydrogel-coated CVCs could be found. Hydrogel-coated catheters were used in order to adsorb and help maintain the phage culture in the catheter lumen long enough for their effect on viable S. epidermidis cells to be determined.
In vitro model of biofilm development on hydrogel-coated catheters.
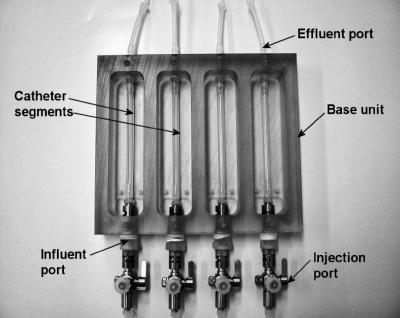
Biofilms were grown using a modified drip flow biofilm reactor (mDFR; Biosurface Technologies Corp., Bozeman, MT). The device was composed of four separate chambers, each with a sealing lid. The original device was modified to allow the connection of catheter segments of any lumen size to influent and effluent ports within the device (Fig. 1). Before each experiment, the device containing the catheters was sterilized using ethylene oxide gas. The mDFR was coupled to a batch culture of S. epidermidis 414 in MHB and a sterile medium reservoir containing half-strength MHB (Fig. 2). The culture (mid-exponential phase) was pumped through the mDFR for 2 h (1 ml/min), irrigating the catheter segments attached inside. The mean CFU per ml of the batch culture ranged from 108 to 109 during this 2-h period. This was followed by irrigation for 22 h with sterile half-strength MHB (0.5 ml/min) to establish a biofilm. All mDFR experiments were carried out at 37°C in triplicate.
FIG. 1.
Modified drip flow reactor, showing catheter segments in each of the four chambers.
FIG. 2.
Model system used for evaluating the effect of phage treatment on biofilm formation. The various parts are labeled as follows: 1, magnetic stirrer; 2, batch culture; 3, sterile medium reservoir; 4, peristaltic pump; 5, mDFR; 6, waste reservoir.
Phage pretreatment of catheter surfaces.
Phage pretreatment involved exposing the catheter lumen to a phage culture for a defined period of time. For phage pretreatment experiments, a crude MHB culture of phage 456 with a titer between 1 × 1010 to 2.2 × 1010 PFU/ml was used. Prior to biofilm formation, each catheter segment in the mDFR was filled with the phage culture. The phage culture was incubated at 37°C for 1 h within the catheter lumens before removal. Experiments were also conducted using MHB containing 3 mM MgCl2 and 4 mM CaCl2. Many phages, including staphylococcal phages, require divalent cations for efficient growth and multiplication (34, 53); thus, divalent cations were added to the media to assess their effect on phage activity. Biofilms were grown using the supplemented media with and without phage pretreatment of the catheter surface. Heat-inactivated phages (80°C for 3 h) were used as a control pretreatment using both divalent cation supplement and nonsupplemented MHB for biofilm growth. Biofilm formation took place as previously described over 24 h.
Enumeration of viable adherent organisms.
During the course of experiments, single catheter segments were removed aseptically from the device, and the fluid contained within the lumen was collected at 2, 6, and 24 h. The catheter was cut into smaller sections, each with an internal curved surface area of 1 cm2. Three of these sections were sliced vertically into two halves, and each halved section washed gently in 5 ml phosphate-buffered saline (PBS) (7.2 pH) to remove planktonic and loosely adherent cells. Individual sections were subjected to high-speed vortexing in 5 ml PBS for 15 s, followed by sonication for 10 min at 42 kHz (Branson 2510; Branson, Danbury, CT), further vortexing for 15 s, sonication for 5 min, and a final vortexing for 15 s. Earlier studies indicated that the process removed essentially all of the viable S. epidermidis 414 cells from the surface of the catheter and that sonication was not associated with loss of viability of the cells in suspension (data not shown). In each experiment, the viable bacterial counts for the three 1-cm2 sections of catheter were established and the mean viable count, expressed as CFU/cm2, was determined. In addition to determining viable bacterial counts, the titer of intraluminal fluid from the catheter was determined for phage 456 by the soft agar overlay method (1, 27) where appropriate. The tubes containing the PBS and catheter sections were kept on ice as much as possible throughout the procedure to prevent further phage action on viable cells. All experiments were performed in triplicate.
Organisms recovered from the biofilms were subcultured on MHA and preserved at −80°C. The organisms were confirmed as S. epidermidis using a Vitek GPI card (biomerieux, Durham, NC). S. epidermidis cells that were still viable after exposure to phage 456 were tested to determine if their survival in the presence of the phages was due to acquired resistance. A fresh culture of phage 456 was grown to a high titer. A new stock of S. epidermidis 414 not previously exposed to the phage and S. epidermidis 414 cultures recovered from each of the three separate phage coating experiments (three cultures from each experiment) were revived. The titer of phage 456 was determined by plaque assay (1, 27) using each S. epidermidis culture, and each was plated in triplicate. Plaques were counted and log transformed, and the titers of phage cultures were determined, with each S. epidermidis culture previously exposed to the phage compared to the phage titer for the S. epidermidis 414 culture that was not exposed to the phage (freshly revived from −80°C stocks) by using a two-tailed t test. A P value of <0.05 was considered significant.
Analysis of phage effect on S. epidermidis viability during the sampling procedure.
To demonstrate that phage 456 was affecting the viability of S. epidermidis 414 cells on the catheter surface rather than during the biofilm sampling procedure, a suspension of S. epidermidis 414 was prepared in 5 ml PBS at 4°C to the turbidity of a 0.5 McFarland standard. A 1-ml aliquot was removed for viable cell count, and 1 ml of a 1010-PFU/ml phage 456 culture in MHB was added to the remaining 4-ml suspension. The tube was subjected to the same biofilm removal procedure used during mDFR experiments: i.e., sonication and vortexing, followed by chilling on ice for 2 h (twice the length of time required for sampling during biofilm/phage experiments). The number of viable cells was then determined by plate count.
In addition, the remaining biofilm suspensions (containing the catheter segment and suspended biofilm cells in PBS) from several phage-treated catheter segments were stored at 4°C for 2, 6, and 24 h, the number of viable bacterial cells and phage were determined again, and the results were compared to biofilm cell counts on the same samples prior to 4°C storage.
Developing biofilms on hydrogel catheters pretreated with phages and human serum.
A conditioning film was simulated on the catheter lumen to assess its affect on biofilm formation and phage 456 effectiveness. Filter-sterilized whole human serum (complement inactivated at 56°C for 30 min) was instilled into both phage-pretreated and untreated control catheter segment lumens in the mDFR and incubated for 2 h at 37°C. The presence of the serum conditioning film was confirmed by fluorescent microscopy. Catheter segments incubated with serum were cut into segments as before. The segments were washed with sterile PBS three times (1 min per wash), incubated with 3% bovine serum albumin at 37°C for 1 h to prevent nonspecific binding, and rewashed three times in PBS. The segments were then incubated with goat anti-human immunoglobulin G (H+L)-fluorescein isothiocyanate (FITC) conjugate (Zymed Laboratories, San Francisco, CA) for 1 h at room temperature and washed three times in sterile PBS. Catheter segments not coated with serum were used as controls. All catheter segments were sliced lengthwise into 1-mm sections and examined using a Zeiss Axioplan 2 imaging fluorescent microscope (10×, 20×, and 100× objective lenses), (Carl Zeiss Light Microscopy, Göttingen, Germany) with an FITC filter (filter set 41001; excitation filter, 480/40×; emission filter, 535/50m; dichroic mirror, 505 nm; Chroma Technology Corp., Rockingham, VT). Samples were photographed using a Zeiss Axiocam high-resolution digital camera (Carl Zeiss Light Microscopy, Göttingen, Germany). Images were analyzed using Axiovision 4.0 software (Carl Zeiss Vision, München-Hallbergmoos, Germany). Both test catheter segments and control segments were photographed using the same exposure time. Biofilms were developed on serum-coated catheters either with or without phage pretreatment as previously described (with divalent cation supplementation).
SEM.
The biofilm was visualized by scanning electron microscopy (SEM). Following the washing step, the biofilm was fixed with 5% glutaraldehyde (Ted Pella Inc., Redding, CA) in cacodylate buffer (0.67 M, pH 6.2) for 1 h and dehydrated through a series of aqueous ethanol washes (30 to 100%) for durations of 10 to 15 min. Specimens were treated with 1 drop of hexamethyldisilazane (Polysciences Inc., Warrington, PA), mounted on aluminum stubs with quick-drying silver paint (Ted Pella Inc., Redding, CA), coated in gold (Polaron SC7640 sputter coater; Thermo VG Scientific, United Kingdom), and viewed and photographed with a scanning electron microscope (Philips XL30 ESEM, FEI Co., Hillsboro, OR). The entire surfaces of 1-cm2 sections of catheter were examined, and images were chosen that represented the typical field of view.
Statistical analysis.
Bacterial and phage count data were log transformed, and differences in microbial recovery were analyzed using the two-tailed t test and Excel 2003 (Microsoft Corporation, Redmond, WA). P values of <0.05 were considered significant.
RESULTS
In vitro model of biofilm development on catheters after phage pretreatment.
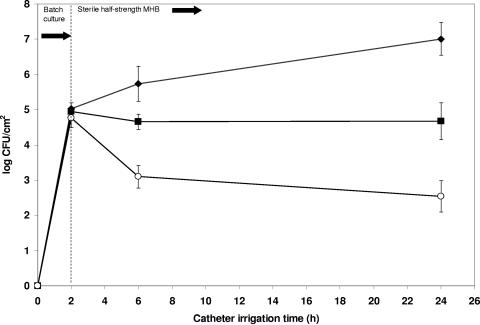
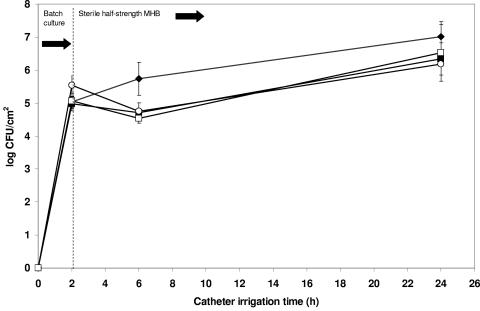
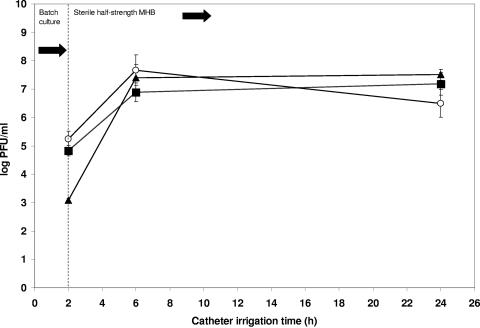
The mean viable count of S. epidermidis after 24-h biofilm formation was 7.01 ± 0.47 log CFU/cm2 of catheter. Pretreatment of catheters with phage 456, with and without supplemental divalent cations, resulted in significant log reductions of 4.47 (P < 0.0001) and 2.34 (P = 0.001) staphylococcal CFU/cm2, respectively (Fig. 3). Divalent cation supplementation of the growth medium without phage pretreatment resulted in a nonsignificant reduction in bacterial viability (log 0.67 CFU/cm2, P = 0.053), as did pretreatment with heat-inactivated phage both with (log 0.48 CFU/cm2, P = 0.149) and without (log 0.83 CFU/cm2, P = 0.065) divalent cations (Fig. 4). The presence of phage 456 in the intraluminal media was confirmed at all time points throughout the phage pretreatment and subsequent biofilm growth experiments (Fig. 5). The presence of a biofilm on catheter surfaces was confirmed by SEM (Fig. 6). After phage 456 pretreatment using supplemental divalent cations, the number of visibly attached S. epidermidis cells was considerably smaller in all fields of view (Fig. 6). S. epidermidis 414 cells recovered from biofilms that had been previously exposed to phage and S. epidermidis 414 cells that had not been grown in a biofilm and exposed to the phage were equally susceptible to the phage, as determined by plaque assay (P values ranged from 0.0567 to 0.4455 using a two-tailed t test), demonstrating that S. epidermidis 414 cells that survived exposure to phage 456 did not exhibit phage resistance.
FIG. 3.
Effect of phage pretreatment of catheter surface on biofilm formation. ♦, mean log CFU/cm2 of viable S. epidermidis 414 recovered from hydrogel-coated catheters; ▪, mean log CFU/cm2 of viable S. epidermidis 414 recovered from hydrogel-coated catheters pretreated with phage 456; ○, mean log CFU/cm2 of viable S. epidermidis 414 recovered from hydrogel-coated catheters pretreated with phage 456 (MHB supplemented with divalent cations). Error bars represent ±standard deviation (n = 3).
FIG. 4.
Effect of heat-inactivated phage and divalent cations on biofilm formation on catheter surface. ♦, mean log CFU/cm2 of viable S. epidermidis 414 recovered from hydrogel-coated catheters; ▪, mean log CFU/cm2 of viable S. epidermidis 414 recovered from hydrogel-coated catheters (MHB supplemented with divalent cations); ○, mean log CFU/cm2 of viable S. epidermidis 414 recovered from hydrogel-coated catheters pretreated with heat-inactivated phage 456; □, mean log CFU/cm2 of viable S. epidermidis 414 recovered from hydrogel-coated catheters pretreated with heat-inactivated phage 456 (MHB supplemented with divalent cations). Error bars represent ±standard deviation (n = 3).
FIG. 5.
Viable phage recovered from catheter lumens. ▪, mean log PFU/ml of viable phage 456 recovered from phage- pretreated hydrogel-coated catheters during biofilm formation; ○, mean log PFU/ml of viable phage 456 recovered from phage-pretreated hydrogel-coated catheters during biofilm formation (MHB supplemented with divalent cations); ▴, mean log PFU/ml of viable phage 456 recovered from phage-pretreated and subsequently serum-coated, hydrogel-coated catheters during biofilm formation (MHB supplemented with divalent cations). Error bars represent ±standard deviation (n = 3).
FIG. 6.
(A) Scanning electron micrograph of the surface of a section of a Lubri-sil hydrogel-coated all-silicone Foley catheter after biofilm formation by S. epidermidis 414 for 24 h (×3,000 magnification). Attached cells are clearly visible in large quantities on the surface. (B) Surface of a section of a Lubri-sil hydrogel-coated all-silicone Foley catheter pretreated with phage 456 after biofilm formation by S. epidermidis 414 for 24 h (×3,000 magnification) with divalent cation-supplemented MHB. No biofilm matrix or clusters of attached cells are visible (images represent typical fields of view).
Effect of phages on S. epidermidis viability during sampling procedure.
The standardized 4-ml PBS suspension of S. epidermidis 414 inoculated with 1 ml of a 1010-PFU/ml culture of phage 456 and then subjected to the same biofilm removal and sampling procedure described previously did not result in a decrease in viable cell numbers as a result of phage action. An increase in mean viable S. epidermidis cell numbers of log 0.04 CFU/ml was recorded, which was not statistically significant (P = 0.586). This indicated that there was no effect of phage on biofilm cells during the recovery process.
After storage at 4°C for 2 h, the log-CFU/ml cell count in the PBS biofilm suspension increased 0.02 (P = 0.943). After 6 and 24 h, the log-CFU/ml cell counts decreased 0.28 (P = 0.593) and 0.01 (P = 0.968), respectively. These data demonstrated that there was no significant effect on S. epidermidis 414 viability by phage 456 in PBS at 4°C.
Effect of human serum and phage pretreatment on biofilm development.
When serum-coated catheters treated with goat anti-human immunoglobulin G (H+L)-FITC conjugate were examined using fluorescent microscopy, a distinct, easily visible coating could be seen on the catheter surface. No such fluorescent coating could be seen on the control catheter samples (data not shown). Following biofilm formation for 24 h in the presence of a serum conditioning film, the mean viable count of S. epidermidis was 7.76 log CFU/cm2 of catheter. The presence of a serum conditioning film resulted in a 0.75-log increase in CFU/cm2 compared to the level in catheters without the conditioning film after 24 h (P = 0.022). Serum conditioning of phage-pretreated catheters did not significantly affect the ability of the phages to prevent biofilm formation. The log-CFU/cm2 reduction in viable S. epidermidis cells was 4.61 (P < 0.0001), compared to 4.47 (P < 0.0001) for catheters not conditioned with serum (MHB supplemented with divalent cations).
DISCUSSION
Catheter-related infections due to biofilms will remain a major challenge in health care for the foreseeable future. Current preventive measures to decrease the risk related to catheter-related infections include both bactericidial and bacteriostatic approaches, such as antimicrobial agent-impregnated catheters and cuffs, in-line filters, and antimicrobial lock therapy (45). However, while such approaches appear to reduce the risk of catheter-related bloodstream infections (43, 48), they do not universally prevent catheter-associated biofilm formation, particularly those caused by antimicrobial-resistant strains. This study investigated a novel approach to controlling biofilms on catheter surfaces by using a phage pretreatment step. Many studies have shown the potential of phage for the treatment of infectious diseases in plants (23, 62) and animals (2, 57, 58), including infections caused by antimicrobial-resistant strains of bacteria (4, 51, 60). Similarly, phage therapy has been shown to have potential for the treatment of infections in humans (56), including those caused by multidrug-resistant bacteria (67). Certain infections now thought to be associated with biofilms, including otitis media, urinary tract infections, periodontitis (12, 17), and burn infections (52), have also been effectively treated with phage therapy (56, 67).
Despite the potential advantages of phage therapy, only a few studies have concentrated on its direct application toward biofilm control and treatment. Sillankorva et al. (55) demonstrated the ability of phage ΦS1 to reduce Pseudomonas fluorescens biofilm biomass by 85%. The biofilms treated with phage ΦSI were more efficiently controlled than by using traditional chemical biocides. Additionally, the disruption of Pantoea agglomerans biofilms by a phage in combination with degradation of the exopolysaccharide matrix and subsequent lysis of cells has been reported (30). Doolittle et al. (18) observed that the extracellular matrix of Escherichia coli biofilms did not protect the bacterial cells from infection with phage T4 and that phage-infected cells were associated with the biofilm surface (19). Infecting phage titer may also be an important factor; it has been previously reported that a multiplicity of infection of 100 resulted in a greater disruption than an multiplicity of infection of 10 at 90 min post-T4 phage infection of an E. coli biofilm (10). Recently, a nontoxic, biodegradable polymer film impregnated with phages, ciprofloxacin, and benzocaine was developed. This technology, named PhagoBioDerm, has been used successfully to treat patients with ulcers (42) and infected burns (32) that were unresponsive to conventional therapies. It is important to note that there is a distinct lack of stringently controlled, blinded studies on the efficacy of phage therapy for the treatment of human infections. This notwithstanding, however, these studies suggest that there is a clear potential for the development of phage-impregnated or coated materials for the prevention of bacterial infection.
The results from our study suggest that phages could be used to reduce growth of S. epidermidis biofilms. Biofilm formation on both hydrogel-coated and serum/hydrogel-coated silicone catheters was significantly reduced over 24 h in the presence of phage 456. Not only did the phage reduce the number of viable cells recovered but also reduced the number of cells adhering to the catheter surface as determined by SEM (Fig. 6). The presence of divalent cations in the growth medium (MgCl2 and CaCl2) appeared to increase the effectiveness of phage 456 at reducing biofilm formation. Previous studies have demonstrated that many phages, including staphylococcal phages, require divalent cations for efficient growth and multiplication (34, 53). However, as shown in Fig. 5, the phage titer within the lumen was similar regardless of the presence or absence of additional divalent cations. It is not known why there was no increase in phage numbers as a result of increased phage activity against S. epidermidis cells. In this study, the levels of divalent cations required for optimal phage activity (3 mM MgCl2 and 4 mM CaCl2) were low, and many intravenous fluids include similar levels of magnesium and calcium. The addition of divalent cations to the media without phage pretreatment of the catheter surface did not result in a significant increase or decrease in viable biofilm cells.
While the present study utilized phages to reduce initial biofilm formation, it did not assess the ability of the phages to infect and lyse cells in an established biofilm. Biofilm formation by S. epidermidis takes place in two phases, the first being primary attachment of staphylococcal cells on a biomaterial surface followed by the second, accumulation of bacteria in multiple layers and glycocalyx formation (26). It is not possible to draw any conclusion from this study as to the effect of the phage on established multilayered biofilms; however, it is possible that the phages could infect and lyse cells associated with a more established biofilm. We speculate that while many of the S. epidermidis cells entering the catheter lumen may be infected by the phage before any substantial adherence to the surface takes place, phage infection also occurs after bacterial cells have attached to the surface. Attachment of coagulase-negative staphylococci to catheter materials has been shown to occur rapidly, with adherent microorganisms visible on the surface by electron microscopy within minutes of exposure to the bacterial culture (24, 47). No demonstrable effect of the phage on S. epidermidis adherence to the catheter material at 2 h was recorded. In fact, there was no significant difference between the number of cells recovered at 2 h from the non-phage-pretreated controls and the phage 456-pretreated catheters (in the presence of divalent cations). This may account for the time needed for the phage to successfully infect and begin to lyse the S. epidermidis cells.
Upon insertion, central venous catheters quickly become coated with platelets, plasma, and host proteins such as albumin, fibrinogen, fibronectin, and laminin (50). Many authors have shown that the adhesion of staphylococci, including S. epidermidis, to polymer surfaces is not enhanced (44) and, in certain cases, is reduced or impaired by the presence of such proteins (6, 25, 36, 46). In the present study, catheter surfaces were coated with human serum after phage pretreatment and prior to biofilm formation. This was carried out mainly to assess what effect the presence of blood proteins would have on phage effectiveness and biofilm formation. Surprisingly, a significant increase in the number of viable biofilm cells at 2 h (P = 0.0136) and 24 h (P = 0.0221) was recorded in the presence of the serum conditioning film (compared with the control). However, it did not affect the ability of the phage to reduce the number of viable cells recovered from the surface when compared with non-serum-coated catheters pretreated with phage 456 (in the presence of divalent cations). We cannot definitely state that phage pretreatment would be effective on CVCs since Foley catheters were used in our model system; however, it is possible that a similar effect would be observed using neutral hydrogel-coated CVCs. At the time of this study, no commercial source of such intravascular devices was available.
While new technologies, such as pretreatment of catheter surfaces with furanones (31) or liposomal/ciprofloxacin hydrogel coatings (16), show promise as prophylaxis/treatment, this study has highlighted the potential of phages for the reduction of biofilm development on biomedical implant surfaces. While phages are not the only option for novel antibacterial/antibiofilm treatment of catheters, our findings have demonstrated that further research is needed, particularly as the problem of antibiotic resistance continues to grow and traditional antimicrobial agents become less effective. Future work is planned using a model that more closely correlates with the in vivo situation over longer periods of time. This in vitro work will help to define the parameters that should be addressed in future in vitro and in vivo studies.
Acknowledgments
The authors wish to acknowledge the financial support of the ASM/NCID Postdoctoral Research Fellowship program.
In addition, we wish to thank the following for their help and contributions: Janice Carr, Sigrid McAllister, Wayne Kirby, and Samantha Broaders (CDC, GA); Vincent Fischetti and Mattias Collin (Rockefeller University, NY); Ronnie Bracken and Michele Davis (CR Bard, GA); and finally Tyrone Pitt and Mark Ganner (Health Protection Agency, United Kingdom).
Use of trade names is for identification only and does not constitute endorsement by the Public Health Service or by the U.S. Department of Health and Human Services.
REFERENCES
- 1.Adams, M. 1959. Bacteriophages. Interscience Publishers, London, United Kingdom.
- 2.Barrow, P., M. Lovell, and A. Berchieri, Jr. 1998. Use of lytic bacteriophage for control of experimental Escherichia coli septicemia and meningitis in chickens and calves. Clin. Diagn. Lab. Immunol. 5:294-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit, J. L., G. Carandang, M. Sitrin, and P. M. Arnow. 1995. Intraluminal antibiotic treatment of central venous catheter infections in patients receiving parenteral nutrition at home. Clin. Infect. Dis. 21:1286-1288. [DOI] [PubMed] [Google Scholar]
- 4.Biswas, B., S. Adhya, P. Washart, B. Paul, A. N. Trostel, B. Powell, R. Carlton, and C. R. Merril. 2002. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 70:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capdevila, J. A., J. Gavalda, and A. Pahissa. 1996. Antibiotic-lock technique: usefulness and controversies. Antimicrobics Infect. Dis. Newsl. 15:9-13. [Google Scholar]
- 6.Carballo, J., C. M. Ferreiros, and M. T. Criado. 1991. Influence of blood proteins in the in vitro adhesion of Staphylococcus epidermidis to teflon, polycarbonate, polyethylene and bovine pericardium. Rev. Esp. Fisiol. 47:201-208. [PubMed] [Google Scholar]
- 7.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37:1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen, G. D., L. M. Baddour, D. L. Hasty, J. H. Lowrance, and A. W. Simpson. 1989. Microbial and foreign body factors in the pathogenesis of medical device infections, p. 27-59. In A. L. Bisno and F. A. Waldvogel (ed.), Infections associated with indwelling medical devices. American Society for Microbiology, Washington, D.C.
- 9.Cislo, M., M. Dabrowski, B. Weber-Dabrowska, and A. Woyton. 1987. Bacteriophage treatment of suppurative skin infections. Arch. Immunol. Ther. Exp. 35:175-183. [PubMed] [Google Scholar]
- 10.Corbin, B. D., R. J. McLean, and G. M. Aron. 2001. Bacteriophage T4 multiplication in a glucose-limited Escherichia coli biofilm. Can. J. Microbiol. 47:680-684. [PubMed] [Google Scholar]
- 11.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 12.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 13.Curtin, J., M. Cormican, G. Fleming, J. Keelehan, and E. Colleran. 2003. Linezolid compared with eperezolid, vancomycin, and gentamicin in an in vitro model of antimicrobial lock therapy for Staphylococcus epidermidis central venous catheter-related biofilm infections. Antimicrob. Agents Chemother. 47:3145-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dean, B. A., R. E. Williams, F. Hall, and J. Corse. 1973. Phage typing of coagulase-negative staphylococci and micrococci. J. Hyg. 71:261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Herelle, F. 1917. Sur un microbe invisible antagoniste des bacilles dysentériques. C. R. Acad. Sci. (Paris) 165:373-375. [Google Scholar]
- 16.DiTizio, V., G. W. Ferguson, M. W. Mittelman, A. E. Khoury, A. W. Bruce, and F. DiCosmo. 1998. A liposomal hydrogel for the prevention of bacterial adhesion to catheters. Biomaterials 19:1877-1884. [DOI] [PubMed] [Google Scholar]
- 17.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doolittle, M. M., J. J. Cooney, and D. E. Caldwell. 1995. Lytic infection of Escherichia coli biofilms by bacteriophage T4. Can. J. Microbiol. 41:12-18. [DOI] [PubMed] [Google Scholar]
- 19.Doolittle, M. M., J. J. Cooney, and D. E. Caldwell. 1996. Tracing the interaction of bacteriophage with bacterial biofilms using fluorescent and chromogenic probes. J. Ind. Microbiol. 16:331-341. [DOI] [PubMed] [Google Scholar]
- 20.Elliott, T. S., H. A. Moss, S. E. Tebbs, I. C. Wilson, R. S. Bonser, T. R. Graham, L. P. Burke, and M. H. Faroqui. 1997. Novel approach to investigate a source of microbial contamination of central venous catheters. Eur. J. Clin. Microbiol. Infect. Dis. 16:210-213. [DOI] [PubMed] [Google Scholar]
- 21.Fang, G., T. F. Keys, L. O. Gentry, A. A. Harris, N. Rivera, K. Getz, P. C. Fuchs, M. Gustafson, E. S. Wong, A. Goetz et al. 1993. Prosthetic valve endocarditis resulting from nosocomial bacteremia. A prospective, multicenter study. Ann. Intern. Med. 119:560-567. [DOI] [PubMed] [Google Scholar]
- 22.Flowers, R. H., III, K. J. Schwenzer, R. F. Kopel, M. J. Fisch, S. I. Tucker, and B. M. Farr. 1989. Efficacy of an attachable subcutaneous cuff for the prevention of intravascular catheter-related infection. A randomized, controlled trial. JAMA 261:878-883. [PubMed] [Google Scholar]
- 23.Fox, J. 2000. Phage treatments yield healthier tomato, pepper plants. ASM News 66:455-456. [Google Scholar]
- 24.Franson, T. R., N. K. Sheth, H. D. Rose, and P. G. Sohnle. 1984. Scanning electron microscopy of bacteria adherent to intravascular catheters. J. Clin. Microbiol. 20:500-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galliani, S., M. Viot, A. Cremieux, and P. Van der Auwera. 1994. Early adhesion of bacteremic strains of Staphylococcus epidermidis to polystyrene: influence of hydrophobicity, slime production, plasma, albumin, fibrinogen, and fibronectin. J. Lab. Clin. Med. 123:685-692. [PubMed] [Google Scholar]
- 26.Gotz, F. 2002. Staphylococcus and biofilms. Mol. Microbiol. 43:1367-1378. [DOI] [PubMed] [Google Scholar]
- 27.Gratia, A. 1936. Des relations numériques entre bactéries lysogènes et particules de bactériophagie. C. R. Soc. Biol. 122:812. [Google Scholar]
- 28.Hibma, A. M., S. A. Jassim, and M. W. Griffiths. 1997. Infection and removal of L-forms of Listeria monocytogenes with bred bacteriophage. Int. J. Food Microbiol. 34:197-207. [DOI] [PubMed] [Google Scholar]
- 29.Hughes, K. A., I. W. Sutherland, J. Clark, and M. V. Jones. 1998. Bacteriophage and associated polysaccharide depolymerases—novel tools for study of bacterial biofilms. J. Appl. Microbiol. 85:583-590. [DOI] [PubMed] [Google Scholar]
- 30.Hughes, K. A., I. W. Sutherland, and M. V. Jones. 1998. Biofilm susceptibility to bacteriophage attack: the role of phage-borne polysaccharide depolymerase. Microbiology 144:3039-3047. [DOI] [PubMed] [Google Scholar]
- 31.Hume, E. B., J. Baveja, B. Muir, T. L. Schubert, N. Kumar, S. Kjelleberg, H. J. Griesser, H. Thissen, R. Read, L. A. Poole-Warren, K. Schindhelm, and M. D. Willcox. 2004. The control of Staphylococcus epidermidis biofilm formation and in vivo infection rates by covalently bound furanones. Biomaterials 25:5023-5030. [DOI] [PubMed] [Google Scholar]
- 32.Jikia, D., N. Chkhaidze, E. Imedashvili, I. Mgaloblishvili, G. Tsitlanadze, R. Katsarava, J. G. Morris, Jr., and A. Sulakvelidze. 2005. The use of a novel biodegradable preparation capable of the sustained release of bacteriophages and ciprofloxacin, in the complex treatment of multidrug-resistant Staphylococcus aureus-infected local radiation injuries caused by exposure to Sr90. Clin. Exp. Dermatol. 30:23-26. [DOI] [PubMed] [Google Scholar]
- 33.Kamal, G. D., D. Divishek, G. C. Kumar, B. R. Porter, D. J. Tatman, and J. R. Adams. 1998. Reduced intravascular catheter-related infection by routine use of antibiotic-bonded catheters in a surgical intensive care unit. Diagn. Microbiol. Infect. Dis. 30:145-152. [DOI] [PubMed] [Google Scholar]
- 34.Kay, D. 1952. The effect of divalent metals on the multiplication of coli bacteriophage T 5st. Br. J. Exp. Pathol. 33:228-235. [PMC free article] [PubMed] [Google Scholar]
- 35.Kluger, D., and D. Maki. 1999. The relative risk of intravascular device related bloodstream infections in adults, p. 514. 39th Intersci. Conf. Antimicrob. Agents Chemother.
- 36.Kristinsson, K. G. 1989. Adherence of staphylococci to intravascular catheters. J. Med. Microbiol. 28:249-257. [DOI] [PubMed] [Google Scholar]
- 37.Maki, D. G. 1981. Nosocomial bacteremia. An epidemiologic overview. Am. J. Med. 70:719-732. [DOI] [PubMed] [Google Scholar]
- 38.Maki, D. G., L. Cobb, J. K. Garman, J. M. Shapiro, M. Ringer, and R. B. Helgerson. 1988. An attachable silver-impregnated cuff for prevention of infection with central venous catheters: a prospective randomized multicenter trial. Am. J. Med. 85:307-314. [DOI] [PubMed] [Google Scholar]
- 39.Maki, D. G., and L. A. Mermel. 1998. Infections due to infusion therapy, p. 689-724. In J. V. Bennett and P. S. Brachman (ed.), Hospital infections. Lippincott-Raven, Philadelphia, Pa.
- 40.Maki, D. G., M. Ringer, and C. J. Alvarado. 1991. Prospective randomised trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 338:339-343. [DOI] [PubMed] [Google Scholar]
- 41.Maki, D. G., and P. A. Tambyah. 2001. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 7:342-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markoishvili, K., G. Tsitlanadze, R. Katsarava, J. G. Morris, Jr., and A. Sulakvelidze. 2002. A novel sustained-release matrix based on biodegradable poly(ester amide)s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int. J. Dermatol. 41:453-458. [DOI] [PubMed] [Google Scholar]
- 43.Mermel, L. A. 2000. Prevention of intravascular catheter-related infections. Ann. Intern. Med. 132:391-402. [DOI] [PubMed] [Google Scholar]
- 44.Muller, E., S. Takeda, D. A. Goldmann, and G. B. Pier. 1991. Blood proteins do not promote adherence of coagulase-negative staphylococci to biomaterials. Infect. Immun. 59:3323-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Grady, N. P., M. Alexander, E. P. Dellinger, J. L. Gerberding, S. O. Heard, D. G. Maki, H. Masur, R. D. McCormick, L. A. Mermel, M. L. Pearson, I. I. Raad, A. Randolph, and R. A. Weinstein. 2002. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. Morb. Mortal. Wkly. Rep. Recomm. Rep. 51:1-29. [PubMed] [Google Scholar]
- 46.Pascual, A., A. Fleer, N. A. Westerdaal, and J. Verhoef. 1986. Modulation of adherence of coagulase-negative staphylococci to Teflon catheters in vitro. Eur. J. Clin. Microbiol. 5:518-522. [DOI] [PubMed] [Google Scholar]
- 47.Peters, G., R. Locci, and G. Pulverer. 1982. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J. Infect. Dis. 146:479-482. [DOI] [PubMed] [Google Scholar]
- 48.Raad, I., R. Darouiche, J. Dupuis, D. Abi-Said, A. Gabrielli, R. Hachem, M. Wall, R. Harris, J. Jones, A. Buzaid, C. Robertson, S. Shenaq, P. Curling, T. Burke, C. Ericsson, et al. 1997. Central venous catheters coated with minocycline and rifampin for the prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. Ann. Intern. Med. 127:267-274. [DOI] [PubMed] [Google Scholar]
- 49.Raad, I. I., M. F. Sabbagh, K. H. Rand, and R. J. Sherertz. 1992. Quantitative tip culture methods and the diagnosis of central venous catheter-related infections. Diagn. Microbiol. Infect. Dis. 15:13-20. [DOI] [PubMed] [Google Scholar]
- 50.Raad, I. I. 1998. Intravascular-catheter-related infections. Lancet 351:893-898. [DOI] [PubMed] [Google Scholar]
- 51.Ramesh, V., J. Fralick, and R. Rolfe. 1999. Prevention of Clostridium difficile induced ileocecitis with bacteriophage. Anaerobe 5:69-78. [Google Scholar]
- 52.Richard, P., R. Le Floch, C. Chamoux, M. Pannier, E. Espaze, and H. Richet. 1994. Pseudomonas aeruginosa outbreak in a burn unit: role of antimicrobials in the emergence of multiply resistant strains. J. Infect. Dis. 170:377-383. [DOI] [PubMed] [Google Scholar]
- 53.Rountree, P. M. 1955. The role of divalent cations in the multiplication of staphylococcal bacteriophages. J. Gen. Microbiol. 12:275-287. [DOI] [PubMed] [Google Scholar]
- 54.Segura, M., F. Alvarez-Lerma, J. M. Tellado, J. Jimenez-Ferreres, L. Oms, J. Rello, T. Baro, R. Sanchez, A. Morera, D. Mariscal, J. Marrugat, and A. Sitges-Serra. 1996. A clinical trial on the prevention of catheter-related sepsis using a new hub model. Ann. Surg. 223:363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sillankorva, S., R. Oliveira, M. J. Vieira, I. W. Sutherland, and J. Azeredo. 2004. Bacteriophage Phi S1 infection of Pseudomonas fluorescens planktonic cells versus biofilms. Biofouling 20:133-138. [DOI] [PubMed] [Google Scholar]
- 56.Slopek, S., B. Weber-Dabrowska, M. Dabrowski, and A. Kucharewicz-Krukowska. 1987. Results of bacteriophage treatment of suppurative bacterial infections in the years 1981-1986. Arch. Immunol. Ther. Exp. 35:569-583. [PubMed] [Google Scholar]
- 57.Smith, H. W., and M. B. Huggins. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J. Gen. Microbiol. 129:2659-2675. [DOI] [PubMed] [Google Scholar]
- 58.Smith, H. W., and M. B. Huggins. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J. Gen. Microbiol. 128:307-318. [DOI] [PubMed] [Google Scholar]
- 59.Smith, H. W., M. B. Huggins, and K. M. Shaw. 1987. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J. Gen. Microbiol. 133:1111-1126. [DOI] [PubMed] [Google Scholar]
- 60.Soothill, J. S. 1992. Treatment of experimental infections of mice with bacteriophages. J. Med. Microbiol. 37:258-261. [DOI] [PubMed] [Google Scholar]
- 61.Terpenning, M. S., B. P. Buggy, and C. A. Kauffman. 1988. Hospital-acquired infective endocarditis. Arch. Intern. Med. 148:1601-1603. [PubMed] [Google Scholar]
- 62.Thomas, R. 1935. A bacteriophage in relation to Stewart's disease of corn. Phytopathology 25:371-372. [Google Scholar]
- 63.Twort, F. W. 1915. An investigation on the nature of ultramicroscopic viruses. Lancet ii:1241. [Google Scholar]
- 64.Veenstra, D. L., S. Saint, S. Saha, T. Lumley, and S. D. Sullivan. 1999. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA 281:261-267. [DOI] [PubMed] [Google Scholar]
- 65.von Eiff, C., C. Heilmann, and G. Peters. 1998. Staphylococcus epidermidis: why is it so successful? Clin. Microbiol. Infect. 4:207-300. [Google Scholar]
- 66.Walters, M. C., III, F. Roe, A. Bugnicourt, M. J. Franklin, and P. S. Stewart. 2003. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weber-Dabrowska, B., M. Mulczyk, and A. Gorski. 2000. Bacteriophage therapy of bacterial infections: an update of our institute's experience. Arch. Immunol. Ther. Exp. 48:547-551. [PubMed] [Google Scholar]
- 68.Weber-Dabrowska, B., M. Mulczyk, and A. Gorski. 2003. Bacteriophages as an efficient therapy for antibiotic-resistant septicemia in man. Transplant. Proc. 35:1385-1386. [DOI] [PubMed] [Google Scholar]
- 69.Weber-Dabrowska, B., M. Mulczyk, and A. Górski. 2001. Bacteriophage therapy for infections in cancer patients. Clin. Appl. Immunol. Rev. 1:131-134. [Google Scholar]
- 70.Wood, H. L., S. R. Holden, and R. Bayston. 2001. Susceptibility of Staphylococcus epidermidis biofilm in CSF shunts to bacteriophage attack. Eur. J. Pediatr. Surg. 11(Suppl. 1):S56-S57. [PubMed] [Google Scholar]