Abstract
A phase I study was conducted to formally evaluate the steady-state pharmacokinetics (PK) of tenofovir disoproxil fumarate (TDF) and ritonavir (RTV)-boosted saquinavir mesylate (SQV) when coadministered in healthy volunteers. Forty subjects received multiple doses of TDF (300 mg, once daily) and SQV/RTV (1,000 mg/100 mg, twice daily) alone and together under steady-state conditions in an open-label, fixed sequence design. Blood samples for tenofovir (TFV) and SQV/RTV PK were drawn over respective 24- and 12-h dosing intervals, and drug concentrations were measured by liquid chromatography-tandem mass spectrometry. Safety was assessed periodically by clinical and laboratory monitoring. Thirty-two subjects completed the study and were fully evaluable; three subjects discontinued participation in the study due to adverse events, three subjects withdrew for personal reasons, and two subjects withdrew because of inadequate venous access for blood sampling. Steady-state TFV PK were not significantly altered upon coadministration with SQV/RTV. Steady-state SQV (administered as SQV/RTV) AUCtau, Cmax, and Ctau increased 29, 22, and 47%, respectively, upon coadministration with TDF, and all subjects achieved a Ctau of >100 ng/ml. These modestly increased SQV exposures are not clinically meaningful given its clinical use with RTV already results in >10-fold-higher SQV levels. Steady-state RTV AUCtau and Cmax levels were not significantly altered, whereas Ctau was 23% higher upon coadministration of SQV/RTV and TDF. Thus, no clinically relevant interactions between TDF and RTV-boosted SQV were observed under conditions simulating clinical practice.
In the United States and Europe, the standard of care for the treatment of human immunodeficiency virus type 1 (HIV-1) infection uses a combination of antiretroviral drugs based on a backbone of two nucleoside or nucleotide reverse transcriptase inhibitors and either a non-nucleoside reverse transcriptase inhibitor or a protease inhibitor (http://aidsinfo.nih.gov/guidelines) (11).
While protease inhibitors have proven to be among the most potent antiretroviral drugs available to clinicians, because of their low and variable bioavailability and short plasma elimination half-lives most have required the administration of high doses two or three times a day. However, due to their metabolism in the gastrointestinal tract and liver by cytochrome P450 (CYP450), primarily the 3A4 isoenzyme (CYP3A4), these drugs may be combined with a subtherapeutic dose of ritonavir (RTV), a potent inhibitor of CYP3A4, to effectively increase their bioavailability and half-life (4). The use of ritonavir as a pharmacokinetic booster in combination antiretroviral therapies involving dual protease inhibitors has been so successful that the use of RTV is recommended with all of the currently approved protease inhibitors except for nelfinavir mesylate, for which boosting is unnecessary, due to its metabolism by CYP450 enzymes other than CYP3A4 (http://aidsinfo.nih.gov/guidelines). Therefore, with the increasing prevalence of antiretroviral regimens containing RTV-boosted protease inhibitors, it is appropriate to conduct prospective studies to evaluate the potential for drug-drug interactions between these agents and other antiretroviral drugs.
The nucleotide analogue, tenofovir DF is a recommended component of antiretroviral regimens (http://aidsinfo.nih.gov/guidelines) (11), hence the likelihood of concurrent administration of this drug with RTV-boosted protease inhibitors is high, and an understanding of the potential for drug-drug interaction between these agents is valuable. Saquinavir mesylate (SQV) is a commonly prescribed protease inhibitor that is recommended to be boosted with a subtherapeutic dose of RTV (according to the Invirase [saquinavir mesylate] capsule product summary [Roche Laboratories, Inc., Nutley, NY]), and we present here the results of a phase I study designed to evaluate the potential for a pharmacokinetic interaction between tenofovir, administered as tenofovir disoproxil fumarate (tenofovir DF [TDF]), and both ritonavir-boosted and unboosted saquinavir mesylate.
The primary objective of the study was to evaluate whether coadministration of tenofovir DF and ritonavir-boosted saquinavir mesylate would alter the steady-state pharmacokinetics of either tenofovir or saquinavir and whether coadministration of these drugs raised any safety concerns. A secondary objective was to investigate the effects of single and multiple (steady-state) doses of tenofovir DF on exposure to unboosted saquinavir mesylate and the effects of a single dose of ritonavir-boosted or unboosted saquinavir mesylate on exposure to tenofovir. These latter investigations were exploratory in nature and intended to provide additional information on the potential mechanisms of any drug-drug interactions that might be observed between TDF and protease inhibitors.
(This study was presented in part at the 44th International Conference on Antimicrobial Agents and Chemotherapy, Washington, D.C., 30 October to 2 November 2004.)
MATERIALS AND METHODS
Subjects.
Healthy male and female (nonpregnant, nonlactating) volunteers aged from 19 to 55 years, with no more than a 20% deviation from either extreme of the ideal body weight range for their frame size and gender, an estimated creatinine clearance of at least 75 ml/min (using the Cockcroft and Gault equation [3] and serum creatinine and actual body weight at screening), and confirmed negative serologies for HIV, hepatitis B virus (HBV), and hepatitis C virus (HCV) infection were eligible to participate in the present study. Subjects could be current smokers (maximum of 20 cigarettes/day) but were asked to keep their tobacco use consistent throughout the study.
Exclusion criteria included a history of clinically relevant disease, including prior relevant alcohol or drug abuse, and current illness or infection. Subjects were also ineligible if they needed treatment with drugs known to be competitors for renal excretion, nephrotoxic or potentially nephrotoxic, or if they had taken drugs known to induce or inhibit hepatic enzymes within 3 months prior to entry into the study. In general, other than the ongoing use of hormonal contraceptives, the use of all prescription and nonprescription medications (including herbal supplements) was discouraged during the study, with exceptions to be approved by the investigator and sponsor. Potentially hepatotoxic drugs and drugs contraindicated for either saquinavir or ritonavir were prohibited at all times, as were the consumption of grapefruit/grapefruit juice and the use of St. John's wort-containing products. Alcohol and caffeine were prohibited during each confinement for pharmacokinetic sampling. Subjects were required to avoid strenuous or prolonged exercise, saunas, steam baths, and sunbathing or other prolonged exposure to UV radiation.
This study was performed at a single study center, MDS Pharma Services (US), Inc., Lincoln, Nebraska, in the United States between December 2003 and May 2004 (first screening evaluation through last subject observation). Approval for the study was obtained from the MDS Pharma Services Institutional Review Board prior to initiation of the study, and all prospective subjects were required to provide written informed consent prior to their participation in the study.
Study design and procedures.
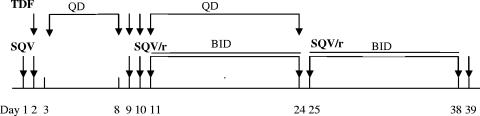
This was a 39-day, open-label, single- and multiple-dose, drug-drug interaction study. After screening procedures and baseline assessment (medical history, physical examination, and blood and urine laboratory tests) had confirmed study eligibility, each subject received the single- and multiple-dose treatments represented schematically in Fig. 1. All doses of tenofovir DF (one 300-mg tablet) were administered in the morning. Saquinavir mesylate (five 200-mg hard gelatin capsules), unboosted or boosted with ritonavir (one 100-mg soft gelatin capsule), was administered in the morning when given as a single dose and in the morning and evening when dosed twice daily. Each dose was to be taken at or close to the same time each day to maintain a 12- or 24-h dosing interval. When tenofovir DF and saquinavir or ritonavir-boosted saquinavir were given in combination, subjects took both study drugs together in the morning, with the evening dose of ritonavir-boosted saquinavir taken as close as possible to 12 h later. Study drugs given in combination were swallowed in the following order: tenofovir DF > saquinavir mesylate > ritonavir (where applicable).
FIG. 1.
Dosing schema. Day 1, administration of a single 1,000-mg dose of saquinavir mesylate (SQV) in the morning; day 2, coadministration of a single 300-mg dose of tenofovir DF (TDF) with a single 1,000-mg dose of SQV in the morning; days 3 to 8, administration of a single 300-mg dose of TDF once daily (QD) in the morning; day 9, coadministration of a single 300-mg dose of TDF and a single 1,000-mg dose of SQV in the morning; day 10, coadministration of a single 300-mg dose of TDF and a single 1,000/100-mg dose of ritonavir-boosted saquinavir (SQV/r) in the morning; days 11 to 24, coadministration of 1,000/100 mg of SQV/r twice daily (BID) in the morning and evening with a 300-mg dose of TDF QD in the morning; days 25 to 38, administration of 1,000/100 mg of SQV/r BID in the morning and evening; day 39, administration of a single 1,000/100-mg dose of SQV/r in the morning.
Serial venous blood samples were collected to determine plasma drug concentrations on days 1, 2, 8, 9, 10, 24, and 39. Concentrations of tenofovir were measured over a 24-h period on days 2, 8, 9, 10, and 24, and concentrations of saquinavir and, where applicable, ritonavir were measured over a 12-h period on days 1, 2, 9, 10, 24, and 39. Blood samples were collected at the following time points: ≤5 min before dosing (predose) and at 0.25, 0.50, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, and 24 h postdose, as appropriate.
While sequestered at the study center, each study drug dosing was completed under the supervision of staff and no more than 30 min after the subject had consumed a standard meal (breakfast or dinner/supper) containing at least 20% of the total caloric content from fat, which is consistent with the original label recommendation for saquinavir to be taken with food. To minimize variation in pharmacokinetics due to food, on pharmacokinetic sampling days the same standard breakfast containing 20% of the total caloric content (373 kcal) from fat was consumed prior to administration of the morning dose of drug(s). Administration of tenofovir DF with a light meal has no significant effect on the pharmacokinetics of tenofovir compared to fasted administration of the drug (5). On the morning of the pharmacokinetic sampling days, other than the water (240 ml) provided with dosing, water was withheld for 1 h before dosing until 2 h after dosing. Subjects were required to remain in an upright position (sitting or semisupine) for 2 h after dosing.
When self-administered by the subjects outside of the clinic, the study drug(s) was to be taken with or within 30 min after the subject consumed a meal. Subjects were to complete a dosing diary recording the date and time of each self-administered dose of study drug, the amount of study drug(s) taken, and whether study drug(s) was taken with food.
Safety assessment.
The safety and tolerability of the study drugs were evaluated at each study visit on the basis of reported clinical adverse events, clinical laboratory test results, vital sign measurements, and physical examination findings. The severity of clinical adverse events and laboratory tests was graded by using modified NIH/DAIDS toxicity grading scales. The severity of adverse events and the investigator's assessment of their causality to study drugs were recorded.
Analytical methods.
Plasma concentrations of tenofovir, saquinavir, and ritonavir were determined by using validated liquid chromatography-tandem mass spectrometry (LC/MS/MS) bioanalytical methods. The assay for tenofovir concentrations was performed by Gilead Sciences Bioanalytical Laboratory (Durham, NC). In brief, the plasma sample (100 μl) was deproteinized by using methanol (400 μl) containing adefovir as internal standard. An aliquot (5 μl) of the extract was analyzed by the LC/MS/MS system. Chromatography was performed on a reversed-phase ThermoFinnigan Keystone Aquasil C18 column (100 by 2.1 mm, 3 μm) under isocratic conditions (0.1% formic acid in water-methanol, 85:15 [vol/vol]) at a flow rate of 125 μl/min. Tenofovir and the internal standard were detected by MS/MS in the selected reaction monitoring mode by using electrospray ionization with positive polarity. The calibration curve was validated and linear over the concentration range from 10 to 1,000 ng/ml, with the lower limit of quantification of 10 ng/ml. Interassay accuracy and precision ranged from −5.2 to 4.0% and from 4.4 to 7.9%, respectively.
The assay for simultaneous determination of saquinavir and ritonavir concentrations was performed by Quest Pharmaceutical Services, LLC (Newark, DE). In brief, plasma sample (50 μl) was mixed with an internal standard (diazepam, 50 μl) and phosphate buffer (0.1 M, 50 μl) and then extracted with methyl t-butyl ether (4 ml). The supernatant was evaporated to dryness and reconstituted with 0.5 ml of 0.1% acetic acid in water-methanol (50:50 [vol/vol]). An aliquot (2 μl) was injected onto a Phenomenex Luna C8 column (30 by 2 mm, 3 μm) and eluted under gradient conditions (A = H2O:NH4OH, 100:0.01 [vol/vol]; B = MeOH:NH4OH, 100:0.01 [vol/vol]; 0 min/40% B, 1 min/100% B, and 3.1 min/40% B) at a flow rate of 400 μl/min. Saquinavir, ritonavir, and the internal standard were detected by MS/MS in the multiple reaction monitoring mode using electrospray ionization with positive polarity. The calibration curve was validated and linear over the concentration range from 1 to 1,000 ng/ml with the lower limit of quantification of 1 ng/ml for both saquinavir and ritonavir. Interassay accuracy and precision ranged from −6.4 to 0.5% and 6.1 to 10.1% for saquinavir and from −7.7 to 1.6% and 6.4 to 7.4% for ritonavir, respectively.
Pharmacokinetic analysis.
Individual plasma concentration-time profiles were analyzed by application of a nonlinear curve-fitting software package (WinNonlin, Professional Edition, version 3.3; Pharsight Corp., Mountain View, CA) using noncompartmental methods.
Single-dose pharmacokinetic parameters determined after administration of a single dose of tenofovir DF (day 2), saquinavir mesylate (days 1, 2, and 9), or ritonavir-boosted saquinavir mesylate (day 10) included the following: maximum observed concentration of drug (Cmax), time (observed time point) of Cmax (Tmax), estimate of the terminal elimination half-life of the drug (t1/2), and area under the concentration versus time curve extrapolated to infinite time (AUCinf). Parameters estimated after multiple dosing of tenofovir DF (days 8, 9, 10, and 24) and ritonavir-boosted saquinavir mesylate (days 24 and 39) included: Cmax, the observed drug concentration at the end of the dosing interval (Ctau), Tmax, t1/2, and the area under the concentration versus time curve over the dosing interval (AUCtau).
Statistical analysis.
All statistical analyses were performed by using SAS, version 8.02 (SAS Institute, Cary, NC). The critical pharmacokinetic parameters for assessing the potential interaction between tenofovir DF and ritonavir-boosted saquinavir were AUCtau, Cmax, and Ctau using the parameters for tenofovir or ritonavir-boosted saquinavir administered alone as a reference. These parameters were log transformed and compared by analysis of variance using a mixed-effects linear model. Ninety percent confidence intervals (CIs) were constructed for the ratio of geometric least-squares means of AUCtau, Cmax, and Ctau for each drug when dosed in combination relative to alone. The CIs were obtained by analyzing the logarithms of the pharmacokinetic parameters and computing 90% CIs for the difference in least-squares means on a logarithmic scale with or without the other drug. The resulting CIs were then transformed back to and reported on the original measurement scale. The study was powered to detect a 30% difference in steady-state pharmacokinetics, and each drug's pharmacokinetics were considered to be not significantly altered if the 90% CI about the ratio of geometric least-squares means fell within the range of 70 to 143% (0.7 to 1.43). A maximum change of 30% was selected, based on the demonstrated higher tenofovir exposures (+34% in terms of AUC) when it is used with lopinavir/ritonavir, a combination with proven safety and efficacy in HIV-infected patients (J. M. Molina, A. Wilkin, P. Domingo, R. Myers, J. Hairrell, C. Naylor, T. Podsadecki, M. King, and G. Hanna, Abstr. Third IAS Conf. HIV Pathog. Treatment, abstr. WePe12.3C12, 2005).
RESULTS
Demographics.
A total of 40 healthy subjects were enrolled in the study; most (90%) were white, with approximately equal numbers of male (45%) and female (55%) subjects. The mean (range) age was 31 (19 to 55) years, the mean (range) weight was 72 (58 to 91) kg, and most (80%) subjects had a medium frame size.
Pharmacokinetics.
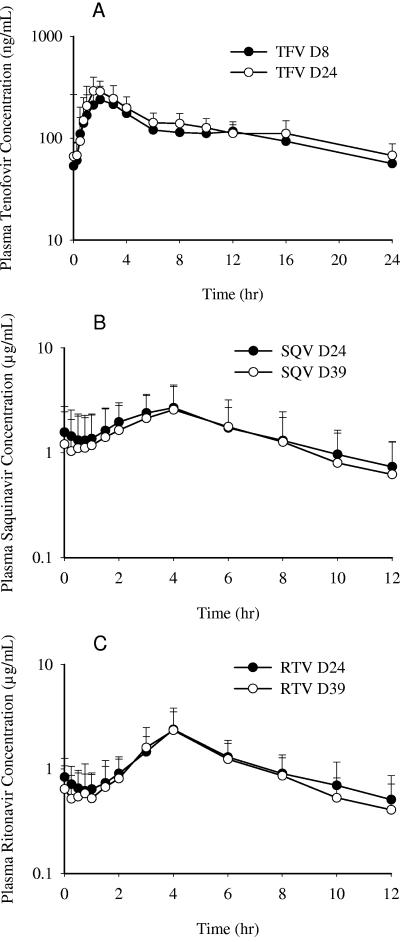
Thirty-five subjects completed pharmacokinetic evaluations for tenofovir, and thirty-two subjects completed pharmacokinetic evaluations for saquinavir and ritonavir. Figure 2A shows the mean (plus the standard deviation [SD]) steady-state plasma concentration-versus-time profiles for tenofovir after administration of multiple doses of tenofovir DF alone (day 8) or with multiple doses of ritonavir-boosted saquinavir mesylate (day 24). Tenofovir was absorbed after oral administration of tenofovir DF alone or coadministration with ritonavir-boosted saquinavir mesylate with a median Tmax value of 2 h after dosing (Table 1). All subjects had measurable plasma concentrations at 24 h after study drug administration (Ctau), except one subject, who had one value below the limit of quantitation (mean ± SD Ctau on day 24 of 67.6 ± 20.5 ng/ml). Steady-state Cmax, Ctau, and AUCtau values increased by 15, 23, and 14%, respectively, for tenofovir when coadministered with ritonavir-boosted saquinavir; however, the associated 90% confidence intervals were contained within the range of 70 to 143%, suggesting that the steady-state plasma PK of tenofovir were not significantly altered after coadministration with ritonavir-boosted saquinavir mesylate compared to tenofovir DF alone (Table 2).
FIG. 2.
(A) Steady-state plasma tenofovir (TFV) concentration versus time profiles after administration of tenofovir DF alone (day 8, solid circle) or with multiple doses of ritonavir-boosted saquinavir mesylate (day 24, open circle). (B) Steady-state saquinavir (SQV) concentration versus time profiles after administration of ritonavir-boosted saquinavir mesylate alone (day 39, open circle) or with multiple doses of tenofovir DF (day 24, solid circle). (C) Steady-state ritonavir (RTV) concentration versus time profiles after administration of ritonavir-boosted saquinavir mesylate alone (day 39, open circle) or with multiple doses of tenofovir DF (day 24, solid circle). Values are expressed as means plus the SD (error bars).
TABLE 1.
Mean (CV%) steady-state PK parameters for tenofovir, saquinavir, and ritonavir after administration of tenofovir DF and ritonavir-boosted saquinavir alone and in combinationa
| Parameterb | TFV PK
|
SQV PK
|
RTV PK
|
|||
|---|---|---|---|---|---|---|
| TDF at 300 mg QD (n = 35) | TDF at 300 mg QD + SQV/r at 1,000/100 mg BID (n = 35) | SQV/r at 1,000/100 mg BID (n = 32) | SQV/r at 1,000/100 mg BID + TDF at 300 mg QD (n = 32) | SQV/r at 1,000/100 mg BID (n = 32) | SQV/r at 1,000/100 mg BID + TDF at 300 mg QD (n = 32) | |
| Cmax (ng/ml or μg/ml)* | 293 (24) | 338 (27) | 2.700 (68) | 2.980 (52) | 2.449 (58) | 2.497 (45) |
| tmax (h)c | 2.00 (0.55, 4.00) | 2.00 (0.75, 4.03) | 4.00 (1.98, 6.02) | 4.00 (2.00, 4.05) | 4.00 (0.75, 12.00) | 4.00 (2.02, 6.00) |
| Ctau (ng/ml or μg/ml)* | 57 (25) | 68 (30) | 0.620 (104) | 0.755 (75) | 0.406 (77) | 0.467 (61) |
| AUCtau (ng·h/ml or μg·h/ml)* | 2,722 (20) | 3,114 (24) | 17.404 (78) | 19.724 (56) | 12.160 (47) | 12.870 (37) |
| t1/2 (h)c | 14.68 (7.22, 22.45) | 16.40 (12.23, 23.22) | 3.20 (2.24, 6.95) | 4.25 (2.55, 12.40) | 2.96 (2.19, 8.03) | 4.15 (2.38, 14.60) |
AUCtau, area under the concentration versus time curve over the dosing interval; BID, twice a day; Cmax, maximum observed concentration of drug; Ctau, observed drug concentration at the end of the dosing interval; QD, once a day; RTV, ritonavir; SQV, saquinavir; SQV/r, ritonavir-boosted saquinavir; TDF, tenofovir disoproxil fumarate; TFV, tenofovir; t1/2, estimate of the terminal elimination half-life of the drug; Tmax, time (observed time point) of Cmax. Values in parentheses are percent coefficients of variation (CV%).
*, Values for TFV PK are in nanograms, and values for SQV PK and RTV PK are in micrograms.
Median values are given (with minimum and maximum values in parentheses).
TABLE 2.
Statistical comparisons of steady-state PK parameters of tenofovir, saquinavir, and ritonavir after administration of tenofovir DF and ritonavir-boosted saquinavir alone and in combinationa
| Parameterb | TFV PK
|
SQV PK
|
RTV PK
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geometric least-squares means
|
GMR (%) | 90% CI | Geometric least-squares means
|
GMR (%) | 90% CI | Geometric least-squares means
|
GMR (%) | 90% CI | ||||
| TDF at 300 mg QD + SQV/r at 1,000/100 mg BID (n = 35) | TDF at 300 mg QD (n = 35) | SQV/r at 1,000/100 mg BID + TDF at 300 mg QD (n = 32) | SQV/r at 1,000/100 mg BID (n = 32) | SQV/r at 1,000/100 mg BID + TDF 300 mg QD (n = 32) | SQV/r at 1,000/100 mg BID (n = 32) | |||||||
| Cmax (ng/ml or μg/ml)* | 327 | 285 | 114.5 | 107.4-122.1 | 2.664 | 2.184 | 122.0 | 105.5-141.0 | 2.278 | 2.068 | 110.1 | 95.0-127.6 |
| Ctau (ng/ml or μg/ml)*c | 68 | 55 | 122.5 | 115.8-129.6 | 0.591 | 0.402 | 147.1 | 122.6-176.4 | 0.394 | 0.321 | 122.6 | 103.3-145.6 |
| AUCtau (ng·h/ml or μg·h/ml)* | 3,031 | 2,669 | 113.5 | 108.6-118.7 | 17.306 | 13.467 | 128.5 | 111.8-147.7 | 12.008 | 10.841 | 110.8 | 100.4-122.2 |
AUCtau, area under the concentration versus time curve over the dosing interval; BID, twice a day; CI, confidence interval; Cmax, maximum observed concentration of drug; Ctau, observed drug concentration at the end of the dosing interval; GMR, geometric least-squares means ratio; QD, once a day; RTV, ritonavir; SQV, saquinavir; SQV/r, ritonavir-boosted saquinavir; TDF, tenofovir disoproxil fumarate; TFV, tenofovir.
See Table 1, footnote b.
n = 34.
Figure 2B shows the mean (plus the SD) steady-state saquinavir plasma concentration versus time profiles after administration of multiple doses of ritonavir-boosted saquinavir alone (day 39) or with multiple doses of tenofovir DF (day 24). Steady-state saquinavir trough values (Ctau) were somewhat less variable when saquinavir mesylate was coadministered with tenofovir DF, and all subjects achieved saquinavir Ctau values of >0.100 μg/ml (Table 1). Steady-state saquinavir Cmax was 22% higher but not significantly different (i.e., the ratio of geometric least-squares means was within a confidence interval range of 70 to 143%) when ritonavir-boosted saquinavir mesylate was coadministered with tenofovir DF versus ritonavir-boosted saquinavir mesylate alone, whereas Ctau and AUCtau values for ritonavir-boosted saquinavir significantly increased by 47 and 29%, respectively, with the upper boundary of the respective 90% confidence intervals falling outside the 70 to 143% confidence interval range (Table 2).
Figure 2C shows the mean (plus the SD) steady-state ritonavir plasma concentration versus time profiles after administration of multiple doses of ritonavir-boosted saquinavir mesylate alone (day 39) or with multiple doses of tenofovir DF (day 24). Steady-state pharmacokinetic parameters for ritonavir after administration of ritonavir-boosted saquinavir mesylate alone or with tenofovir DF are summarized in Table 1. Steady-state ritonavir Cmax and AUCtau were not significantly different between treatments, whereas the trough ritonavir value (Ctau) significantly increased 23% when ritonavir-boosted saquinavir mesylate was coadministered with tenofovir DF compared to the administration of ritonavir-boosted saquinavir mesylate alone (Table 2).
In the exploratory analyses, tenofovir steady-state pharmacokinetics were similar after administration of tenofovir DF alone (day 8) or with a single dose of either unboosted (day 9) or ritonavir-boosted (day 10) saquinavir mesylate. The geometric least-squares mean ratios and associated 90% confidence intervals for tenofovir Cmax, Ctau, and AUCtau were each contained within the confidence interval range of 70 to 143% after administration of tenofovir DF with a single dose of either unboosted or ritonavir-boosted saquinavir mesylate compared to tenofovir DF alone. In contrast, higher saquinavir Cmax and AUCinf values were observed when saquinavir mesylate was coadministered with either a single (day 2) or multiple (day 9) doses of tenofovir DF than when saquinavir mesylate was administered alone (day 1). The saquinavir Cmax value significantly increased by 31 and 29%, and the AUCinf increased by 66 and 49%, upon coadministration with a single or multiple doses of tenofovir DF, respectively, compared to administration of a single dose of saquinavir mesylate alone.
Safety.
Thirty-two subjects completed the entire 39-day treatment phase, and all scheduled pharmacokinetic samplings. Three subjects discontinued the study prematurely because of adverse events: a male subject experienced grade 2 erectile dysfunction; a female subject experienced grade 2 menorrhagia that was associated with low hemoglobin (onset at grade 1, reaching maximum grade 3 severity 9 days after the study drugs were discontinued, and improving to grade 1 by the time she was lost to follow-up); and grade 2 dyspnea occurred in a second female subject. Of these adverse events, the erectile dysfunction and menorrhagia were considered by the investigator to be related to the study drugs; the low hemoglobin was interpreted as a result of the subject's menorrhagia but was not directly related to the study drugs, and the dyspnea was assessed as unrelated to the study drugs. The other five subjects discontinued the study for reasons unrelated to the study treatment (three subjects withdrew consent for personal reasons, and two subjects had problems with inadequate venous access for the serial venipunctures required for pharmacokinetic sampling).
Overall, one or more treatment-emergent adverse events were reported in 37 of 40 (93%) subjects; none was assessed as serious. The majority (73%) of the reported adverse events were considered by the investigator to be related to study treatment. The most frequently reported (in ≥10% of subjects overall) adverse events related to the study treatment were headache (45%), nausea (35%), dizziness (20%), fatigue (20%), loose stools (17.5%), vomiting (17.5%), upper abdominal pain (15%), and dyspepsia (10%). Almost all events were assessed as grade 1 (87%) or grade 2 (12%) severity; only one subject experienced an adverse event of grade 3 severity (decreased hemoglobin), and no subject had an adverse event of grade 4 severity.
Laboratory abnormalities of grade 3 or grade 4 toxicity were reported in eight subjects, including single incidences of grade 3 (3+ on dipstick) hematuria in six female subjects coincident with normal menses. As described previously, one subject had a grade 3 low hemoglobin (reported as adverse event) coincident with ongoing menorrhagia, and another subject had an asymptomatic high creatine phosphokinase (grade 4) that was attributed to strenuous exercise.
DISCUSSION
The purpose of this study was to determine the pharmacokinetics of tenofovir, saquinavir mesylate, and ritonavir under steady-state conditions when tenofovir DF (300 mg once daily [QD]) and ritonavir-boosted saquinavir mesylate (1,000 mg/100 mg twice daily [BID]) were coadministered to establish recommendations regarding the concurrent use of these drugs. A secondary objective was to assess the effects of single and multiple (steady-state) doses of tenofovir DF on exposure to saquinavir mesylate, and the effects of a single dose of ritonavir-boosted saquinavir mesylate on exposure to tenofovir. These exploratory investigations were conducted to elucidate possible mechanisms for observed drug-drug interactions between tenofovir DF and the HIV protease inhibitors atazanavir and lopinavir/ritonavir (5, 9; Reyataz [atazanavir] product summary [Bristol Meyer Squibb Company, Princeton, NJ]).
This study was conducted in healthy subjects to eliminate the potentially confounding effects of background antiretroviral and other therapies and to avoid the need for multiple, short-term changes in existing treatment regimens of HIV-infected patients for the purpose of examining pharmacokinetics. Previous analyses have demonstrated that the pharmacokinetics of tenofovir are comparable between healthy subjects and HIV-infected patients and, while minor differences may be observed in the pharmacokinetics of saquinavir, differences observed in healthy subjects are insufficient to warrant exploration of drug-drug interactions in patients (5).
Tenofovir DF is a prodrug, converted by plasma and tissue esterases to tenofovir, which is then phosphorylated intracellularly to the active form, tenofovir diphosphate. Unlike protease inhibitors, tenofovir is not metabolized by CYP450 isoenzymes, nor is it an inducer or inhibitor of these enzymes. Tenofovir is renally eliminated as unchanged drug by a combination of glomerular filtration and active tubular secretion (5). In contrast, saquinavir mesylate is metabolized by CYP450, with >90% mediated by CYP3A4 (Invirase product summary [Roche]). Therefore, the potential for an interaction between such pharmacokinetically distinct compounds is low. Furthermore, no interactions between tenofovir and a variety of drugs have been observed, including other medications that are cleared predominantly by CYP450 isoenzymes, such as the protease inhibitors nelfinavir mesylate (G. Kruse, S. Esser, H. Stocker, A. Breske, C. Mockling-Hoff, A. Hill, and M. Kurowski, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-446, 2004) (2) and indinavir (B. Kearney, J. Flaherty, J. Wolf, J. Sayre, S. Gill, and D. Coakley, Abstr. 8th European Conf. Clin. Aspects Treatment of HIV-Infection, abstr. 171, 2001). Atazanavir remains the only protease inhibitor to have a clinically meaningful interaction with tenofovir (an ∼24% increase in AUC0-24 of tenofovir and an ∼25% decrease in AUC0-24 of atazanavir), and pharmacokinetic boosting of atazanavir with 100 mg of ritonavir is recommended when these two drugs are used in combination (9; Reyataz [atazanavir] product summary [Bristol Meyer Squibb]). The mechanism of this interaction remains unclear.
In the present study, similar steady-state tenofovir plasma concentration-time profiles were observed when tenofovir DF was coadministered with single or multiple doses of saquinavir mesylate alone or with ritonavir compared to tenofovir DF alone. These data indicate that neither saquinavir nor ritonavir has a clinically meaningful effect on the pharmacokinetics of tenofovir after its oral administration.
Coadministration with tenofovir DF under steady-state conditions increased saquinavir Cmax (22%), Ctau (47%), and AUCtau (29%), and all subjects achieved saquinavir Ctau values of >0.100 μg/ml, which exceeds the minimum target levels recommended in the current U.S. Department of Health and Human Services HIV Treatment Guidelines for Adults and Adolescents (http://aidsinfo.nih.gov/guidelines). Tenofovir-dependent increases in saquinavir exposure are not clinically meaningful because they are small relative to the >10-fold increase in exposure that results from the pharmacokinetic boosting by ritonavir routinely used in clinical practice (Invirase product summary [Roche]). Steady-state saquinavir trough values (Ctau) were somewhat less variable upon coadministration with tenofovir DF. In the exploratory assessments, a single dose of tenofovir DF increased the saquinavir Cmax by 31% and the AUCinf by 66%, whereas multiple doses of tenofovir DF increased Cmax by 29% and AUCinf by 49%. The reason why saquinavir exposures after single dose administration should be higher upon its coadministration with tenofovir DF is unclear but could be due to both saquinavir and the prodrug (disoproxil) form of tenofovir being putative substrates for the drug transporter p-glycoprotein, which is known to actively efflux drugs at their site of absorption (6-8, 10; A. Ray, L. Tong, K. Robinson, B. Kearney, T. Cihlar, and G. Rhodes, unpublished data). A direct effect on absorption would explain similar saquinavir pharmacokinetic parameters after coadministration of saquinavir mesylate with either single or multiple doses of tenofovir DF, indicating no time-dependent effect on the absorption and disposition processes.
The results of the present study in healthy subjects are consistent with the results of studies reported by Boffito et al. in HIV-infected patients, in which tenofovir DF (300 mg QD) was added to existing antiretroviral regimen that included ritonavir-boosted saquinavir mesylate (1,000/100 mg BID). In the first study, coadministration with ritonavir-boosted saquinavir did not significantly affect tenofovir pharmacokinetic parameters (Cmax, Ctau, AUCtau, t1/2, or Tmax) at steady state (day 13) relative to values measured on day 1 (1). Furthermore, the tenofovir steady-state pharmacokinetic parameters when tenofovir DF was coadministered with ritonavir-boosted saquinavir mesylate (AUC0-24 = 3,005 ng · h/ml; Cmax = 287 ng/ml; Ctau = 64 ng/ml; t1/2 = 13.0 h; Tmax = 1.7 h) are comparable to values measured in the present study (see Table 1). In the second study, neither saquinavir or ritonavir pharmacokinetics were determined to have been affected by the addition of tenofovir DF to their background antiretroviral regimen (M. Boffito, A. D'Avolio, G. Di Perri, M. Sciandra, S. Bonora, D. Back, A. Hill, G. Moyle, M. Nelson, C. Higgs, J. Tomkins, B. Gazzard, and A. Pozniak, Abstr. 5th Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 4.19, 2004). Such complementary data indicate that the results of the present study in healthy subjects are applicable to HIV-infected patients.
In summary, the results of the present study confirm the lack of a clinically meaningful drug-drug interaction between tenofovir DF and ritonavir-boosted saquinavir mesylate under steady-state conditions simulating actual clinical practice. Coadministration of tenofovir DF and ritonavir-boosted saquinavir mesylate was generally well tolerated in this study. No dose adjustments are recommended when these agents are to be coadministered as part of an antiretroviral regimen.
Acknowledgments
This study was supported by Gilead Sciences, Inc.
REFERENCES
- 1.Boffito, M., D. Back, M. Stainsby-Tron, A. Hill, G. DiPerri, G. Moyle, M. Nelson, J. Tomkins, B. Gazzard, and A. Pozniak. 2005. Pharmacokinetics of saquinavir hard gel/ritonavir (1000/100 mg twice daily) when administered with tenofovir disoproxil fumarate in HIV-1-infected subjects. Br. J. Clin. Pharmacol. 59:38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boffito, M., A. Pozniak, B. Kearney, C. Higgs, A. Mathias, L. Zhong, and J. Shah. 2005. Lack of a pharmacokinetic drug interaction between tenofovir disoproxil fumarate and nelfinavir mesylate. Antimicrob. Agents Chemother. 49:4386-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, C. L., R. P. G. van Heeswijk, K. Gallicano, and D. W. Cameron. 2003. A review of low-dose ritonavir in protease inhibitor combination therapy. Clin. Infect. Dis. 36:1585-1592. [DOI] [PubMed] [Google Scholar]
- 5.Kearney, B. P., J. F. Flaherty, and J. Shah. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 43:595-612. [DOI] [PubMed] [Google Scholar]
- 6.Lee, C. G., M. M. Gottesman, C. O. Cardarelli, M. Ramachandra, K. T. Jeang, S. V. Ambudkar, I. Pastan, and S. Dey. 1998. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry 37:3594-3601. [DOI] [PubMed] [Google Scholar]
- 7.Sankatsing, S. U., J. H. Beijnen, A. H. Schinkel, J. M. Lange, and J. M. Prins. 2004. P glycoprotein in human immunodeficiency virus type 1 infection and therapy. Antimicrob. Agents Chemother. 48:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivas, R. V., D. Middlemas, P. Flynn, and A. Fridland. 1998. Human immunodeficiency virus protease inhibitors serve as substrates for multidrug transporter proteins MDR1 and MRP1 but retain antiviral efficacy in cell lines expressing these transporters. Antimicrob. Agents Chemother. 42:3157-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taburet, A.-M., C. Piketty, C. Chazallon, I. Vincent, L. Gérard, V. Calvez, F. Clavel, J.-P. Aboulker, J.-P. Girard, et al. 2004. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents and Chemother. 48:2091-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Washington, C. B., G. E. Duran, M. C. Man, B. I. Sikic, and T. F. Blaschke. 1998. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 19:203-209. [DOI] [PubMed] [Google Scholar]
- 11.Yeni, P. G., S. M. Hammer, M. S. Hirsch, M. S. Saag, M. Schechter, C. C. J. Carpenter, M. A. Fischl, J. M. Gatell, B. G. Gazzard, D. M. Jacobsen, D. A. Katzenstein, J. S. Montaner, D. D. Richman, R. T. Schooley, M. A. Thompson, S. Vella, and P. A. Volberding. 2004. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 292:251-265. [DOI] [PubMed] [Google Scholar]