Abstract
The DNA sequence coding for putative cellulosomal scaffolding protein ScaA from the rumen cellulolytic anaerobe Ruminococcus flavefaciens 17 was completed. The mature protein exhibits a calculated molecular mass of 90,198 Da and comprises three cohesin domains, a C-terminal dockerin, and a unique N-terminal X domain of unknown function. A novel feature of ScaA is the absence of an identifiable cellulose-binding module. Nevertheless, native ScaA was detected among proteins that attach to cellulose and appeared as a glycosylated band migrating at around 130 kDa. The ScaA dockerin was previously shown to interact with the cohesin-containing putative surface-anchoring protein ScaB. Here, six of the seven cohesins from ScaB were overexpressed as histidine-tagged products in E. coli; despite their considerable sequence differences, each ScaB cohesin specifically recognized the native 130-kDa ScaA protein. The binding specificities of dockerins found in R. flavefaciens plant cell wall-degrading enzymes were examined next. The dockerin sequences of the enzymes EndA, EndB, XynB, and XynD are all closely related but differ from those of XynE and CesA. A recombinant ScaA cohesin bound selectively to dockerin-containing fragments of EndB, but not to those of XynE or CesA. Furthermore, dockerin-containing EndB and XynB, but not XynE or CesA, constructs bound specifically to native ScaA. XynE- and CesA-derived probes did however bind a number of alternative R. flavefaciens bands, including an ∼110-kDa supernatant protein expressed selectively in cultures grown on xylan. Our findings indicate that in addition to the ScaA dockerin-ScaB cohesin interaction, at least two distinct dockerin-binding specificities are involved in the novel organization of plant cell wall-degrading enzymes in this species and suggest that different scaffoldins and perhaps multiple enzyme complexes may exist in R. flavefaciens.
The organization of microbial plant cell wall-degrading enzymes into multienzyme complexes or cellulosomes was first demonstrated at the molecular level in cellulolytic Clostridium species (2, 4, 5, 12, 14, 20, 23, 34, 40, 41). Early biochemical, immunochemical, and ultrastructural evidence suggested that a cellulosome-like organization might be found more generally in other gram-positive cellulolytic bacterial species (24). Recent molecular evidence has indeed verified the existence of cellulosome structures in Acetivibrio cellulolyticus and in Bacteroides cellulosolvens (9, 10). Molecular evidence has also emerged for cellulosome-like complexes in the ruminal anaerobes Ruminococcus flavefaciens (11, 21) and Ruminococcus albus (33) as well as anaerobic fungi (13, 28).
In Clostridium thermocellum, which has provided the paradigm for cellulosome organization, the complex is integrated via the binding of dockerin-containing enzyme subunits to nine cohesin domains present in a noncatalytic scaffolding protein (CipA). CipA also carries a cellulose-binding module (CBM) and a C-terminal dockerin that is involved in its attachment to cell wall anchoring proteins (16, 18, 26, 27).
As information on cellulosome structures from other species has increased, however, striking variations on the paradigm have emerged. The C-terminal dockerin is absent from many scaffoldins thus far examined, implying alternative anchoring mechanisms, while the scaffoldin protein of Acetivibrio includes a catalytic family 9 glycoside hydrolase domain. Recent work with R. flavefaciens has identified likely scaffolding and anchoring protein components that carry multiple cohesin domains that diverge very significantly in their sequences from those examined previously. The R. flavefaciens cohesins were considered to constitute a new group, designated type III (11). Furthermore the dockerins identified in R. flavefaciens enzymes also show among themselves a high degree of sequence divergence (1). In view of these findings, the present investigation explores further the organization of plant cell wall-degrading enzyme complexes in R. flavefaciens. In particular, we focused on the interactions between the dockerin of ScaA and different cohesins in ScaB. In addition, we examined the interaction between different enzyme-derived dockerin-containing probes and cohesins present in structural protein components. The investigations reported here firmly establish the role of the ScaA protein in R. flavefaciens as a scaffoldin to which a number of enzyme units can attach. Moreover, our results also demonstrate for the first time that the dockerins of the different enzyme subunits vary in their specificity of attachment, presumably to different types of cohesin-containing scaffoldins. Evidence is thus presented that indicates the participation of different enzyme subunits in the formation of different types of enzyme complexes.
MATERIALS AND METHODS
Strains and growth conditions.
R. flavefaciens 17 was grown anaerobically (7) either in modified M2GSC medium (30) or in Hungate-Stack medium (19) with 0.2% microcrystalline cellulose (Avicel PH101; Honeywill & Stein, London, United Kingdom) or 0.2% oat spelt xylan (Sigma, St. Louis, Mo.) as energy sources, as described previously (11). Escherichia coli XL10-gold, XL1-blue, or Solopack gold BL21(DE3)pLysS (supplied by Stratagene, La Jolla, Calif.) were used as hosts for transformation with constructs made in pET28a (Novagen, Madison Wis.). E. coli strains were routinely grown on Luria-Bertani medium with appropriate antibiotic selection.
Completion of the scaA gene sequence.
The ScaA coding sequence was completed by PCR walking from chromosomal DNA (Fig. 1), using the primers listed in Table 1. Size-fractionated R. flavefaciens 17 chromosomal DNA fragments of between 2 and 4 kb from a Sau3AI partial digestion were ligated with BamHI-cut pUC18. Amplification with the M13 forward primer (recognizing the vector sequence) and the reverse primer scacoh5r yielded three bands of 0.6, 0.8, and 1.1 kb. Sequencing revealed cohesin-encoding sequences at both ends of the 0.6- and 1.1-kb fragments, but the 0.8-kb fragment showed a cohesin sequence at one end only. The new primer ScaAcoh7r was designed within the unique region. A further amplification from ligated Sau3AI fragments with M13F and ScaAcoh7r gave a 0.9-kb product, which was sequenced and found to contain the start of the scaA gene. Further PCR amplifications and sequencing confirmed the presence of only three cohesins within the ScaA product, as detailed in Fig. 1.
FIG. 1.
ScaA sequencing strategy and domain architecture. The sequence of ScaA was completed by PCR walking, by sequencing extended products produced with ScaAcoh5r (∼0.8 kb) and then with 7r (∼0.9 kb) (Table 1; also see Materials and Methods). Additional primers and amplifications were used to obtain sequences for both strands and to confirm the relationship of the ScaA N terminus with unique ScaA dockerin, and ScaB sequences and their positions are also indicated. Domains within the ScaA protein are shown as follows: X, N terminal domain of unknown function; 1, 2, and 3, cohesin domains; Doc, dockerin. Solid boxes indicates threonine-rich linkers and striped boxes indicate N-terminal signal peptide.
TABLE 1.
Oligonucleotide primers used in this studya
| Name | Sequence | Domain(s) amplifiedb |
|---|---|---|
| ScaAcoh1r2 | ATGTAGGAACGAGCTTTGTGC | ScaA dockerin (rev) |
| ScaAcoh5r | ATCAGCATTTGATGTCTGGC | ScaA cohesins (rev) |
| ScaAcoh7f | CAGTCGGCACTGTAACACC | ScaA X domain (fwd) |
| ScaAcoh7r | GCGAGGTTGTACTTGGAC | ScaA cohesins (rev) |
| ScaAcoh8f | GCTCCTTCAACTGTAGGC | ScaA X domain (fwd) |
| ScaAcoh8r | GCTGAGAAGTAAGGCTGGTC | ScaA X domain (rev) |
| ScaAcoh9f | GTCCAAGTACAACCTCGC | ScaA X domain (fwd) |
| AUnk-NcoI | TATACCATGGCTCCTTCAACTGTAGGCGCT | ScaA N terminal (fwd) |
| AUnk-XhoI | AAATCTCGAGTGTACCGGGTGTTGAACCGT | ScaA N terminal (rev) |
| Bcoh1-NcoI | ATATCCATGGCTGCCGGTGGTTTA | ScaB cohesin 1 (fwd) |
| Bcoh1-XhoI | CGGTCTCGAGATCCTTCTTAACAAT | ScaB cohesin 1 (rev) |
| Bcoh2-NcoI | AAAACCATGGTCAACAACACAGGTAAG | ScaB cohesin 2 (fwd) |
| Bcoh2-XhoI | CTCACTCGAGATCATTCTTGACTGTGAT | ScaB cohesin 2 (rev) |
| Bcoh3-NcoI | CAATCCATGGTACAGACGGTAGGTAAA | ScaB cohesin 3 (fwd) |
| Bcoh3-XhoI | AAAACTCGAGTACAGGCTTGCCGAC | ScaB cohesin 3 (rev) |
| Bcoh6-NcoI | AAAACCATGGCTCCTCAGGGCGGTATC | ScaB cohesin 6 (fwd) |
| Bcoh6-XhoI | ATCACTCGAGAATAACGGGCTTCTTTAC | ScaB cohesin 6 (rev) |
| Bcoh7-NcoI | AATTCCATGGTAGTTCCCAAGGATTCT | ScaB cohesin 7 (fwd) |
| Bcoh7-XhoI | ATATCTCGAGAGGAGCGGGCTTTTCAAA | ScaB cohesin 7 (rev) |
| EndB-NcoI | AATTCCATGGCGCCCGTCAACGGTCTG | EndB full length (fwd) |
| EndB-XhoI | CACGCTCGAGTTCGGGAAGCTTGTCTAT | EndB full length (rev) |
| XynE-NcoI | CTGACCATGGTTACAAATCCTACTATCAAT | XynE dockerin + family 1 carbohydrate esterase (fwd) |
| XynE-XhoI | AGTGCTCGAGCTTGAAAGCATATCTGAAGA | XynE dockerin + family 1 carbohydrate esterase (rev) |
| XynB-EcoRI | AAGGAATTCACCACACAGCCTTCGAGC | XynB family 3 carbohydrate esterase + dockerin (fwd) |
| XynB-XhoI | AACCGCTCGAGCTTGCAACTTTACCGATGAGG | XynB family 3 carbohydrate esterase + dockerin (rev) |
| CesA-EcoRI | AAGGAATTCAAGCACTTTGTTGTCGG | CesA family 3 carbohydrate esterase + dockerin (fwd) |
| CesA-XhoI | AACCGCTCGAGCAGGGAAGTTGTAGC | CesA family 3 carbohydrate esterase + dockerin (rev) |
Residues shown in italics were added to the 5′ ends to generate restriction enzyme cleavage sites (underlined).
Abreviations: fwd, forward; rev, reverse.
Overexpression and purification of recombinant proteins.
His-tagged proteins were expressed from pET28a constructs following transformation into E. coli Solopack Gold BL21(DE3)pLysS. Cells were recovered and lysed following growth and IPTG (isopropyl-β-d-thiogalactopyranoside) induction at 16 or 37°C, and the His-tagged product was purified from cell lysates with Ni-nitrilotriacetic acid resin, all as described previously (11).
Antibodies against ScaA cohesin 2.
A rabbit antibody against the cohesin2 from the protein ScaA was raised following standard procedures. Immune serum was collected and subjected to purification using an Affi-Gel protein A column (Bio-Rad Laboratories Ltd., Hemel Hempstead, United Kingdom). The column was equilibrated with 10 bed volumes of binding buffer (10 mM sodium phosphate buffer, 0.15 M NaCl, pH 8.2). After the sample was applied, the column was washed with 10 bed volumes of binding buffer, and elution of the immunoglobulin fraction was performed using 5 bed volumes of 0.1 M sodium citrate, pH 3.0. The eluant was then concentrated using a protein concentrator tube (Vivaspin 4 [molecular mass cutoff, 5,000 Da]; VivaScience, Lincoln, United Kingdom) and stored at 4°C after the addition of 0.1% sodium azide to avoid microbial growth.
Biotinylation of recombinant proteins.
Biotinylation was carried out by incubating purified protein with a 20-fold-greater concentration of N-hydroxysuccinimido biotin (Sigma Chemical Co.) in 100 mM HEPES buffer, pH 8.0, for 1 h at room temperature. The excess of unincorporated biotin was washed out using a concentrator (Vivaspin 4 [molecular mass cutoff, 10,000 Da]; VivaScience). The sample was stored at 4°C with 0.05% (wt/vol) sodium azide. Detection was performed using peroxidase-conjugated streptavidin and chemiluminescence.
Western blotting and detection of protein-protein interactions.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene difluoride (PVDF) transfer membrane (Immobilon-P; Millipore, Bedford, United Kingdom) in a semidry MultiPhor II (Pharmacia Biotech, Little Chalfont, United Kingdom) or Biometra Dry Blot system as described previously (11). Membranes were blocked for 1 h at ambient temperature in blocking buffer composed of T-TBS buffer (50 mM Tris, 150 mM NaCl, 10 mM CaCl2, 2 mM dithiothreitol, 0.1% Tween [pH 7.5]) containing 2% bovine serum albumin. They were then incubated with the recombinant His-tagged protein or with the biotin-labeled protein (approximately 5 μg of protein · ml−1 in blocking buffer for 1 h at room temperature). The membrane was again incubated for 1 h at room temperature with a peroxidase-conjugated antibody [Anti-His(C-term)-HRP; Invitrogen, San Diego, Calif.] at 0.2 μg · ml−1 in the case of His-tagged proteins, or peroxidase-conjugated streptavidin (0.1 μg ml−1; Sigma) in the case of biotin-labeled proteins, in blocking buffer, after which the membrane was washed three times with T-TBS buffer and twice with TBS buffer (the same buffer, but lacking Tween). The bands were visualized using a chemiluminescence approach (Western blotting with SuperSignal Substrate [Pierce, Rockford, Ill.]) following the manufacturer's instructions.
Glycoprotein staining.
Proteins were transferred to a PVDF membrane following SDS-PAGE. The membrane was then stained for glycoprotein using a digoxigenin glycan detection kit (Boehringer, Mannheim, Germany) in an enzyme immunoassay following the manufacturer's guidelines. The proteins were labeled on the membrane with digoxigenin to avoid a shift in the molecular weight caused by the attachment of the digoxigenin-spacer to the glycoprotein.
Assay of cellulose and xylan binding.
Substrate binding was studied by incubating the protein (approximately 10 μg in 10 μl) with 5 mg of prewetted cellulose (Avicel) or water-insoluble oat spelt xylan and 10 μl of 50 mM sodium phosphate buffer (pH 6.8)-1 mM dithiothreitol either for 1 h at 37°C or for 16 h at 4°C, with shaking. After incubation the substrate was pelleted at 10,000 × g and washed three times with 50 mM sodium phosphate buffer. Any residual bound protein was then eluted from the pellet by heating at 100°C for 5 min with SDS-PAGE loading buffer, and the released material was analyzed by SDS-PAGE (22).
Transmission immunogold labeling EM.
R. flavefaciens was grown anaerobically in M2CGS medium for 48 h and then spun down at 2,500 × g for 10 min and resuspend in sterilized phosphate-buffered saline (PBS) three times for washing. The cells were resuspended in PBS containing rabbit anti-ScaA/Coh2 antibody (5 μg · ml−1) and incubated for 1 h at 37°C in a shaker incubator (200 rpm). Cells were centrifuged again and resuspended in PBS three times to wash out the excess of unbound proteins. Gold-labeled goat anti-rabbit antibody (British BioCell International, Cardiff, United Kingdom) was used as a second antibody at 5 μg · ml−1 in PBS for 1 h at 37°C in a shaker incubator. After the second incubation cells were washed with PBS three times to remove the excess of unbound material and then embedded in the fixative solution (4% paraformaldehyde and 1% glutaraldehyde) overnight and further processed to be visualized by transmission electron microscopy (transmission EM) (Tillydrone Electron Microscopy Unit, Department of Zoology, University of Aberdeen, Aberdeen, United Kingdom). A negative control was carried out using cells with the second incubation reagent only.
Phylogenetic analysis.
Phylogenetic trees were generated using the ClustalW program (http://www2.ebi.ac.uk/clustalw/). Dockerin sequences were obtained from the GenBank website (http://www.ncbi.nlm.nih.gov/) or via the carbohydrate-active enzymes server (CAZy website [http://afmb.cnrs-mrs.fr/∼pedro/CAZY/db.html]), designed by Coutinho and Henrissat (6, 8). The following enzyme-borne dockerin sequences were used in this work (accession numbers in parentheses): XynB (Z35226), XynD (S61204), XynE (AJ272430), EndA (Z83304), EndB (AJ298117), and CesA (AJ238716) from R. flavefaciens; EgV (AB028320), EgVI (AB028321), and EgVII (AB028321) from R. albus; Xyn1 (Z49970) from Ruminococcus sp.; CbhA (X80993), CelS (L06942), CelD (X04584), CelF (X60545), CelH (M31903), CelP (AJ275974), and XynA (AF047761) from C. thermocellum; CelF (M87018), CelC (M87018), CelG (M87018), CelE (M87018), CelH (AF316823), and CelM (AF316823) from Clostridium cellulolyticum; PelA (AF105330) and ExgS (U34793) from Clostridium cellulovorans; and Clostridium josui CelD (AB004845). Sources for the scaffoldin-borne dockerin sequences in this work were R. flavefaciens ScaA (AJ278969), C. thermocellum CipA (L08665), B. cellulosolvens CipBc (AF224509), and A. cellulolyticus CipV (AF155197).
Updated nucleotide sequence accession number.
The sequence under accession number AJ278969 has been updated to include the complete sequence of ScaA.
RESULTS
Complete sequence of the ScaA scaffoldin and detection of ScaA in R. flavefaciens 17.
We reported previously the identification and complete sequencing of a gene, scaB, which is postulated to be involved in cellulosome anchoring in R. flavefaciens 17 (11). The ScaB gene encodes a 1,852-amino-acid polypeptide containing seven cohesin domains. Immediately upstream of scaB, we detected a second open reading frame, scaA, whose product was predicted to contain several cohesin domains plus a C-terminal dockerin. ScaA was postulated to function as a cellulosomal scaffolding protein. The N-terminal sequences of ScaA were, however, lacking. In the present work, we first completed the sequence of ScaA by PCR walking from genomic DNA (Fig. 1). The complete product of ScaA has 880 amino acids, including a total of three very similar, repeated cohesin domains and the C-terminal dockerin. The N terminus consists of a signal peptide sequence and a unique N-terminal domain of 231 amino acids (residues 29 to 259) that is unrelated to any other sequence in the database. The start codon of ScaA is preceded by a ribosome binding site (ΔG, −11.8 kCal [42] when matched with the Bacillus subtilis consensus).
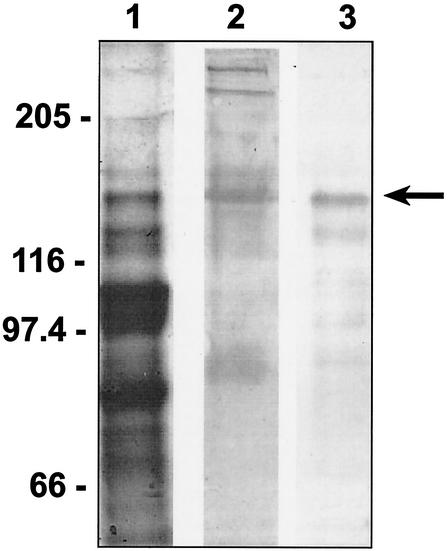
A recombinant form of the second cohesin domain of the ScaA protein (ScaA-Coh2) had previously been purified after cloning into pET28a and overexpression in E. coli (11). In order to detect expression of the ScaA product in R. flavefaciens 17, polyclonal antibodies were raised against the purified cloned ScaA-Coh2 domain, as described in Materials and Methods. This antibody preparation was found to bind specifically to a polypeptide of 130 kDa present among extracellular proteins from R. flavefaciens 17, which was also present among supernatant proteins eluted with SDS after binding to microcrystalline cellulose (Fig. 2). This protein corresponds to an isolated band of 130 kDa in Coomassie-stained gels that showed evidence of glycosylation. EM studies using immunogold labeling revealed that the ScaA protein is distributed both on the cell surface and presumably detached cell surface material (Fig. 3). The extensive labeling of cell surfaces observed in the figure is indicative of most of the cells observed in the sample. In contrast, some of the apparently detached material was heavily labeled whereas some was unlabeled. At least a portion of the former fraction would represent tangentially sectioned cell surfaces.
FIG. 2.
Detection of native ScaA with antibodies raised against purified, recombinant ScaA-Coh2. Proteins from supernatants of cellobiose-grown R. flavefaciens 17 cultures were separated by SDS-PAGE and analyzed by Coomassie blue staining (lane 1) or glycostaining (lane 2). A duplicate sample was transferred to PVDF membranes and probed with anti-ScaA-Coh2 antibodies (lane 3). Numbers at left are molecular masses in kilodaltons.
FIG. 3.
Electron micrograph showing the distribution of ScaA, detected by immunogold labeling, in cellulose-grown R. flavefaciens 17 at late stationary phase.
The unique N-terminal domain of ScaA was overexpressed in E. coli BL21 after cloning into pET28a, and the histidine-tagged product was purified. No evidence was found that it could mediate binding to cellulose (Avicel) or a water-insoluble fraction of birch wood xylan (results not shown), and it is therefore currently best described as an X domain.
Interactions of different ScaB cohesins with ScaA.
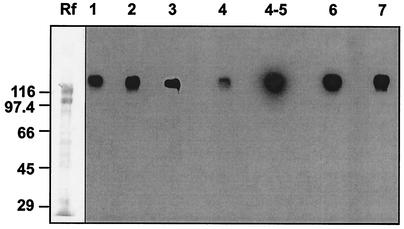
We established previously that two adjacent cohesin domains (4 and 5) of the putative anchoring protein ScaB recognize the dockerin of ScaA, and interact specifically with a polypeptide of 130 kDa (now confirmed as ScaA) among native R. flavefaciens 17 proteins (11). The cohesin domains of ScaB show considerable sequence divergence, however, apart from Coh5 and Coh6, which resemble each other closely (11). In order to examine the interactions of the ScaB cohesins in more detail, His-tagged constructs were prepared for six of the seven individual cohesins of ScaB, and the purified products were probed against native R. flavefaciens extracellular proteins. As shown in Fig. 4, all six of the ScaB cohesins, when tested individually, interacted with a band of approximately 130 kDa. Although we were unable to recover stable clones for ScaB-Coh5, the close resemblance of its sequence to that of ScaB-Coh6 (11) and the behavior of the combined ScaB-Coh4-Coh5 construct suggest that it fails to recognize additional proteins. Therefore, it appears that all seven ScaB cohesins recognize predominantly a single protein present in cellulose-grown R. flavefaciens 17. Additional bands smaller than 130 kDa were detectable with longer exposures, suggesting that some binding occurs to additional proteins, but this could be the result of lower affinity binding to enzyme dockerins. Alternatively, multiple-modular proteins like ScaA are known to undergo fragmentation (25, 31, 32, 35), which could account for the observed interaction of dockerin-containing probes with smaller bands.
FIG. 4.
Recognition of a common 130-kDa protein band by different cohesin domains from ScaB. Total R. flavefaciens 17 supernatant proteins (approximately 7 μg) from a cellulose-grown culture were loaded onto successive lanes of an SDS-PAGE gel, separated, and subjected to Western blotting. Membrane strips containing the electrophoretically transferred material were then incubated with each of the designated cohesin probes (standardized by His tag detection, corresponding to approximately 10 to 50 μg of protein). Binding was detected by chemiluminescence using peroxidase-conjugated anti-His tag antibody. Lanes are marked 1 through 7 according to the cohesin probe used. Lane Rf, profile of Coomassie-stained R. flavefaciens supernatant proteins. Numbers at left are molecular masses in kilodaltons.
In order to confirm the identity of the common 130-kDa band, it was recovered from the SDS-PAGE gel and subjected to limited peptide sequencing. It was shown to contain the amino acid sequence ADTAKGFAV that is present in the unique N-terminal domain of the ScaA product, as predicted from the gene sequence. This further confirms that the 130-kDa protein band corresponds to ScaA, and we can conclude that the difference noted above between the observed molecular size and that predicted for the mature protein from the amino acid sequence (90,198 Da) would presumably be accounted for by glycosylation.
Interaction of ScaA-Coh2 with dockerin-containing enzymes from R. flavefaciens.
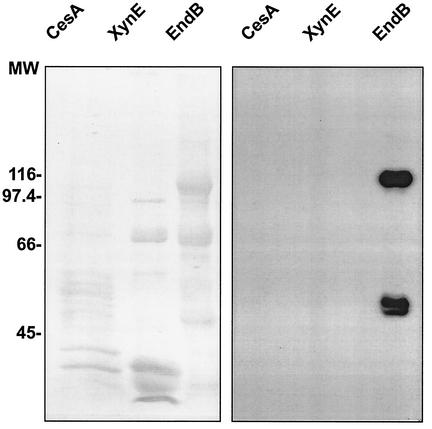
ScaA-Coh2 was shown previously to interact with a dockerin-containing fragment of the enzyme XynD (11). Owing to the distinctive variation in dockerin sequences between different enzymes from R. flavefaciens 17 (1), it was of interest to assess the ability of other dockerin-containing enzyme subunits from R. flavefaciens to bind to ScaA. Appropriate catalytically active fragments from the enzymes EndB, XynB, XynE, and CesA (1, 15, 38) were all overexpressed and purified as His-tagged products after cloning in pET28a vectors. The interaction of the resultant dockerin-containing fragments to the purified ScaA-Coh2 domain was examined in a series of Western blotting experiments, using biotinylated ScaA-Coh2 as the probe in each case. ScaA-Coh2 was able to recognize the dockerin of EndB, but no binding was detected to XynE or to CesA (Fig. 5). This observation is of interest since, as noted previously (1, 38), the dockerin sequences of XynE and CesA show significant divergence from those of XynB, XynD and EndB, and this divergence includes residues implicated in binding specificity in cellulosomal enzymes from Clostridium spp. (35).
FIG. 5.
Affinity blotting of dockerin-containing constructs from CesA, XynE, and EndB using recombinant ScaA-Coh2. Dockerin-containing fragments of the three enzymes and the ScaA-Coh2 cohesin domain, were constructed as six-His-tagged fusions (Table 1), and the products were purified. The enzyme constructs were separated by SDS PAGE, transferred to a PVDF membrane, and probed with biotinylated ScaA-Coh2 (right-hand panel). Coomassie-stained lanes from the same gel are shown in the left-hand panel. Significant fragmentation of the recombinant proteins was observed in all preparations. The estimated molecular masses for the CesA, XynE and EndB constructs are 45,427, 41,625, and 87,040 Da, respectively. EndB fragmented into two major fractions, of which the full-length construct and the smaller (∼50-kDa) fragment were labeled by the cohesin-containing probe. No labeling of XynE or CesA was observed. Numbers at left are molecular weights (MW) in thousands.
Interactions of dockerin-containing probes, derived from XynE and CesA.
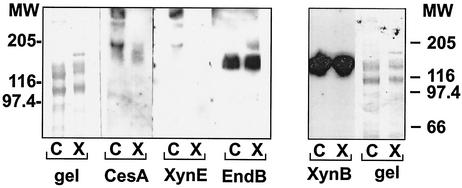
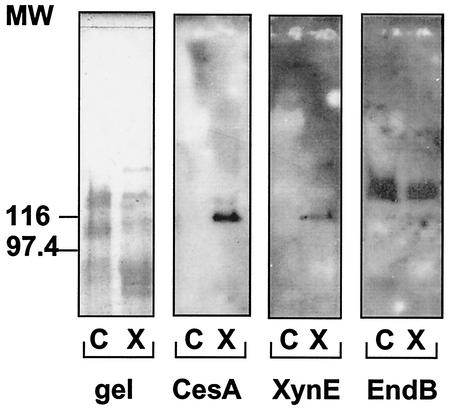
Dockerin-containing fragments of the enzymes EndB, XynE, CesA, and XynB were also used as probes of cell-derived material. The EndB- and XynB-derived probes recognized a native 130-kDa protein band corresponding in size to ScaA in both cellulose- and xylan-grown cells of R. flavefaciens 17 (Fig. 6 and 7). The experiment shown in Fig. 6 compares the binding of biotin-labeled, dockerin-containing fragments of EndB, XynE, or CesA to native R. flavefaciens cell-associated proteins. A band at around 45 kDa (not shown in the figures) consistently appeared in the presence or absence of biotinylated probe and reflected a native biotinylated R. flavefaciens protein that could be used as an internal standard. In contrast to the labeling pattern of EndB- and XynB-derived probes, there was no evidence of binding of XynE or CesA to the 130-kDa ScaA polypeptide. Some binding was however observed with CesA, and to a lesser extent with XynE, to components larger than 180 kDa in cell-associated material from cellulose-grown cultures, suggesting that XynE and CesA can bind to a native protein(s) other than ScaA. The biotinylated XynE and CesA fragments were also used to probe supernatant proteins (Fig. 7). Again, no binding of XynE or CesA to ScaA was detected, but binding was detected to a protein of approximately 110 kDa present in xylan-grown cultures. In conclusion, it is clear from Fig. 5, 6, and 7 that XynE and CesA differ from EndB in their ability to bind extracellular proteins from R. flavefaciens 17. Thus, the dockerins of XynE and CesA do not appear to recognize any ScaA cohesin but may recognize cohesins in other proteins yet to be identified.
FIG. 6.
Affinity blotting of cell-associated proteins from R. flavefaciens 17 by dockerin-containing constructs from CesA, XynE, and EndB (a) and XynB (b). Cultures were grown on either microcrystalline cellulose (C) or birch wood xylan (X) as the energy source. Proteins were separated in successive lanes of the same SDS-PAGE gel before transfer to a PVDF membrane. The molecular size of the XynB product used as probe was 44,782 Da. The blots were probed with the indicated biotinylated protein construct, and labeled bands were detected by chemiluminescence using peroxidase-conjugated streptavidin. A band of around 45 kDa (not shown in the figure), representing a native biotinylated protein present in R. flavefaciens cells, was detected in all samples and was used as an internal standard. The EndB-induced labeling of the 130-kDa band derived from both cellulose- and xylan-grown cultures was confirmed by immunochemical staining using a nonbiotinylated construct and anti-His-Tag antibodies (not shown). Numbers at left and right are molecular weights (MW) in thousands.
FIG. 7.
Binding of biotinylated CesA, XynE, and EndB probes to extracellular material of R. flavefaciens 17 cultures grown on either cellulose (C) or birchwood xylan (X). Concentrated culture supernatants were subjected to SDS-PAGE (gel). Duplicate samples were transferred to membranes and probed using the designated biotinylated construct. Numbers at left are molecular weights (MW) in thousands.
DISCUSSION
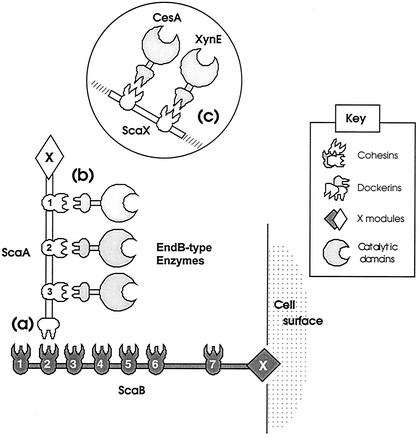
In a previously proposed model for the organization of plant cell wall-degrading enzymes in R. flavefaciens 17 (11), a scaffoldin-type protein (ScaA) was postulated to carry repeated cohesin domains that interact with enzyme dockerins, thereby incorporating them into the cellulosome. The ScaA scaffoldin contains a C-terminal dockerin that was further proposed to interact with the cohesins of a second scaffoldin-type protein (ScaB), putatively considered to serve as a cell-surface anchoring protein. The present work provides further support for the main elements of this model by revealing the complete primary structure of ScaA and by confirming that ScaA corresponds to a native 130k-Da protein that interacts with the enzymes XynD, XynB, and EndB (Fig. 8). The present study also confirms and clarifies the interactions postulated previously to occur between ScaA and ScaB (11). It appears that despite the considerable sequence diversity noted among six of the seven ScaB cohesins, all of the ScaB cohesins bound specifically to ScaA when tested against proteins expressed by cellulose-grown R. flavefaciens cells. Since the 130-kDa ScaA band was the dominant partner for each of the six divergent ScaA cohesins, when tested individually, we found little evidence for the possibility that some ScaB cohesins might play a role in binding enzyme dockerins. In view of the two distinctive types of labeling patterns by the dockerin-containing probes used in this work, we do not discount the further possibility that scaffolding proteins other than ScaA might exist that bind to ScaB, perhaps induced under different growth conditions, and this will be examined in future work.
FIG. 8.
Schematic representation of dockerin-cohesin interactions involved in cellulosome organization in R. flavefaciens 17. Three different specificities of cohesin-dockerin interaction are shown: between the ScaA dockerin and ScaB cohesins (a), between the ScaA cohesins and enzymes with EndB-type dockerins (b), and between the cohesins of a putative 110-kDa scaffoldin (ScaX) and the CesA and XynE dockerins (c). Although ScaB is a cell-associated protein, the proposed interaction of the C-terminal X domain with the cell surface awaits experimental verification.
Two novel features of the R. flavefaciens system revealed by this work are of particular interest—evidence of functional diversity among enzyme dockerins and the lack of a CBM in the ScaA polypeptide. While our present results confirm that a group of enzymes including XynD, EndB, and XynB bind to ScaA, as discussed above, the enzymes XynE and CesA clearly exhibit an alternative specificity, failing to bind either to the native ScaA protein or to the recombinant ScaA-Coh2. A difference in cohesin-binding specificity between the dockerins of EndB, XynB, and XynD on the one hand and XynE and CesA on the other, was previously suspected because of the divergence in their sequences (1). This observation may have profound implications, since it represents the first case in which distinct dockerin-binding specificities have been demonstrated among microbial plant cell wall-degrading enzyme complexes within a single microbial species. In Clostridium spp. different dockerin-containing enzyme subunits appear to bind in an essentially random manner to the different cohesins present in the scaffolding protein (3, 17, 35, 39). Nevertheless, Park et al. (36) have suggested recently that different cohesins of the C. cellulovorans scaffoldin may exhibit different efficiencies and/or specificities. Our evidence in R. flavefaciens indicates further that the spectrum of cellulosomal enzymes in this rumen bacterium may be determined by the type of dockerin present in a given enzyme subunit.
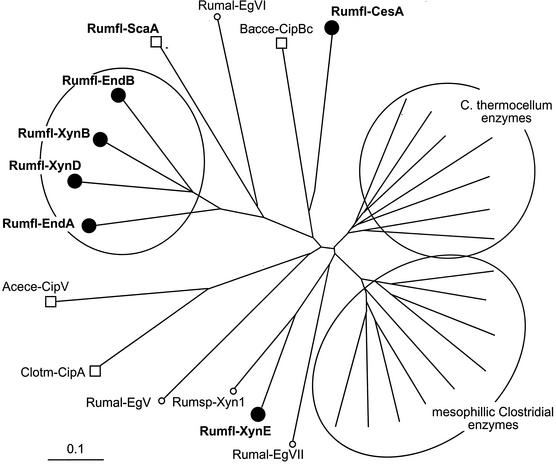
Phylogenetic analysis of known dockerin sequences derived from enzymes and scaffoldins of various bacteria (Fig. 9) revealed that those of R. flavefaciens EndA, EndB, XynB, and XynD form a relatively tight group which is significantly different from the dockerins of XynE and CesA. All of the R. flavefaciens dockerins are, in fact, markedly distinct from the known enzyme-borne dockerins of the clostridia. The divergent specificities of the R. flavefaciens dockerins observed in this work are further supported by their predicted specificity determinants (Table 2). Earlier work on the interspecies specificities of the clostridial cohesin-dockerin interaction has suggested that four amino acid residues (positions 10 and 11 in the characteristic duplicated segment) of the ∼70-residue dockerin domain may serve as recognition codes for binding to the cohesin domain (29, 35). In this context, the relevant positions of the EndA-type dockerins are characterized by LA(S)-XD, whereas those of the XynE-type dockerins—SF(L)-VA—are entirely different. The latter specificity residues are also consistent with those of Ruminococcus sp. strain Xyn1, which is indeed phylogenetically related to XynE (Fig. 9), and it is thus likely that the former enzyme would also show a similar binding specificity. It is interesting that the predicted specificity residues of the R. flavefaciens dockerins are all distinct from those of other species—both for enzyme- and scaffoldin-bearing dockerins. The present results thus appear to support further the role of these residues as specificity determinants.
FIG. 9.
Phylogenetic relationship of R. flavefaciens dockerin domains. Dockerins from the indicated R. flavefaciens enzymes (designated Rumfl-EndA, etc.) are shown as filled circles. Scaffoldin-borne dockerins from R. flavefaciens (Rumfl-ScaA), C. thermocellum (Clotm-CipA), A. cellulolyticus (Acece-CipV), and B. cellulosolvens (Bacce-CipBc) are shown as squares. Other dockerin-borne enzymes are from R. albus (Rumal-EgV, etc.), Ruminococcus sp. (Rumsp-Xyn1), and a selection of enzymes from C. thermocellum and mesophilic clostridia (C. cellulolyticum, C. cellulovorans, and C. josui). For a precise list of the proteins and their accession numbers, consult Materials and Methods. The scale bar indicates the percentage (0.1) of amino acid substitutions.
TABLE 2.
Predicted specificity residues of dockerins derived from the indicated proteins
| Enzyme or dockerin | Protein | Residues in duplicated segmenta
|
|
|---|---|---|---|
| 1st | 2nd | ||
| Ruminococcal enzyme from: | |||
| R. flavefaciens | EndA | LA | GD |
| EndB | LA | ND | |
| XynB | LA | AD | |
| XynD | LS | GD | |
| CesA | SF | VA | |
| XynE | SL | VA | |
| Ruminococcus sp. strain | Xyn1 | SF | VA |
| R. albus | EgV | VT | VS |
| EgVI | MA | ND | |
| EgVII | DT | MA | |
| C. thermocellum enzymes | Consensusb | ST | ST |
| Mesophilic clostridial enzymes | Consensusb | A(L/I) | A(I/L) |
| Scaffoldin dockerins from: | |||
| R. flavefaciens | ScaA | VA | AV |
| C. thermocellum | CipA | LL | MQ |
| A. cellulolyticus | CipV | LE | LE |
| B. cellulosolvens | CipBc | SD | SD |
The four residues indicated represent positions 10 and 11 of the calcium-binding motifs in the first and second duplicated segments, respectively (35).
The consensus residues represent the dominant amino acid(s) that appears in the designated position from the indicated group of cellulosomal enzymes.
The identities of the proteins that bind XynE and CesA are as yet unknown. The observed level of binding of the latter was less than that detected between EndB and ScaA, perhaps suggesting lower abundance or affinity of these as yet undescribed binding proteins. The possibility is raised, however, that an alternative cohesin-containing scaffoldin or cell surface attachment proteins may exist for XynE and CesA and that these might show different regulatory responses to the growth substrate (e.g., xylan versus cellulose). Interestingly, while the activities are known for both catalytic domains (xylanase and esterase) of XynE and for the N-terminal domain (esterase) of CesA, that of the unique C-terminal domain of CesA is still unclear (1). Xylanase and esterase activities are also represented in the ScaA-based complex, which includes enzyme-borne domains with cellulase and mixed-link β-glucanase activities. Therefore, we cannot yet determine whether this difference in organization of XynE and CesA relative to the other enzymes reflects a distinctive role in plant cell wall breakdown.
ScaA represents the first case of a cellulosomal scaffolding protein that does not possess an identifiable cellulose-binding module. Nevertheless ScaA corresponds to a 130-kDa native protein that was previously detected in supernatant material that binds to cellulose with a His-tagged EndB probe (38). This suggests that substrate binding, considered to be one of the major functions of the cellulosome, must be mediated through a somewhat-different mechanism in this species. One possibility is that a subunit(s) that binds the scaffolding protein, rather than the scaffolding protein itself, would contain a substrate-binding module(s). Indeed, the EndB cellulase, shown here to bind to ScaA Coh2, was recently found to contain a novel CBM that might play a role in helping the complex attach to cellulose (38). Other possibilities are that additional, as-yet-unidentified, enzymatic or nonenzymatic subunits of the cellulosome may play a role in binding. Alternatively, it has been suggested for R. albus (37) that cellulose-binding pilus-like structures might be involved in delivering the bacterial cell and its enzyme complexes into contact with the substrate.
This work has provided further insights into the complexity and diversity of cellulosome organization within cellulose-digesting microorganisms. Ruminal strains of R. flavefaciens have evolved in an ecosystem that provides a well-regulated environment, but one that is also highly competitive and subject to rapid turnover. The organization of the enzymes that provide this bacterium with its main source of energy presumably reflects its adaptation to survival in this ecosystem.
Acknowledgments
M.R. acknowledges support of a Conicit PhD studentship and funding from the Technion. R.R.I. is supported by the Scottish Executive Rural Affairs Department. This work was supported in part by grants from the Israel Science Foundation (administered by the Israel Academy of Sciences and Humanities, Jerusalem) and by research grant US-2783-96 from BARD, the U.S.-Israel Binational Agricultural Research and Development Fund. Parts of this research were performed in the Otto Meyerhof Center for Biotechnology, established by the Minerva Foundation (Munich, Germany), with funding from the Technion-Niedersachsen Cooperation (Hannover, Germany).
Footnotes
For a commentary on this article, see page 701 in this issue.
REFERENCES
- 1.Aurilia, V., J. C. Martin, S. I. McCrae, K. P. Scott, M. T. Rincon, and H. J. Flint. 2000. Three multidomain esterases from the cellulolytic rumen anaerobe Ruminococcus flavefaciens 17 that carry divergent dockerin sequences. Microbiology 146:1391-1397. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, E. A., E. Morag, and R. Lamed. 1994. The cellulosome-a treasure-trove for biotechnology. Trends Biotechnol. 12:379-386. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, E. A., L. J. Shimon, Y. Shoham, and R. Lamed. 1998. Cellulosomes—structure and ultrastructure. J. Struct. Biol. 124:221-234. [DOI] [PubMed] [Google Scholar]
- 4.Béguin, P., and M. Lemaire. 1996. The cellulosome: an exocellular, multiprotein complex specialised in cellulose degradation. Crit. Rev. Biochem. Mol. 31:201-236. [DOI] [PubMed] [Google Scholar]
- 5.Belaich, J.-P., C. Tardif, A. Belaich, and C. Gaudin. 1997. The cellulolytic system of Clostridium cellulolyticum. J. Biotechnol. 57:3-14. [DOI] [PubMed] [Google Scholar]
- 6.Bourne, Y., and B. Henrissat. 2001. Glycoside hydrolases and glycosyltransferases: families and functional modules. Curr. Opin. Struct. Biol. 11:593-600. [DOI] [PubMed] [Google Scholar]
- 7.Bryant, M. P. 1972. Commentary on the Hungate technique for culture of anaerobic bacteria. Am. J. Clin. Nutr. 25:1324-1328. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho, P. M., and B. Henrissat. 1999. Carbohydrate-active enzymes: an integrated database approach, p. 3-12. In H. J. Gilbert, G. J. Davies, B. Henrissat, and B. Svensson (ed.), Recent advances in carbohydrate bioengineering. The Royal Society of Chemistry, Cambridge, United Kingdom.
- 9.Ding, S.-Y., E. A. Bayer, D. Steiner, Y. Shoham, and R. Lamed. 1999. A novel cellulosomal scaffoldin from Acetivibrio cellulolyticus that contains a family 9 glycoside hydrolase. J. Bacteriol. 181:6720-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding, S.-Y., E. A. Bayer, D. Steiner, Y. Shoham, and R. Lamed. 2000. A typical scaffoldin of the Bacteroides cellulosolvens cellulosome that contains eleven type II cohesins. J. Bacteriol. 182:4915-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding, S.-Y., M. T. Rincon, R. Lamed, J. C. Martin, S. I. McCrae, V. Aurilia, Y. Shoham, E. A. Bayer, and H. J. Flint. 2001. Cellulosomal scaffoldin-like proteins from Ruminococcus flavefaciens. J. Bacteriol. 183:1945-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doi, R. H., M. Goldstein, S. Hashida, J. S. Park, and M. Takagi. 1994. The Clostridium cellulovorans cellulosome. Crit. Rev. Microbiol. 20:87-93. [DOI] [PubMed] [Google Scholar]
- 13.Fanutti, C., T. Ponyi, G. W. Black, G. P. Hazlewood, and H. J. Gilbert. 1995. The conserved noncatalytic 40-residue sequence in cellulases and hemicellulases from anaerobic fungi functions as a protein docking domain. J. Biol. Chem. 270:29314-29322. [DOI] [PubMed] [Google Scholar]
- 14.Felix, C. R., and L. G. Ljungdahl. 1993. The cellulosome—the exocellular organelle of Clostridium. Annu. Rev. Microbiol. 47:791-819. [DOI] [PubMed] [Google Scholar]
- 15.Flint, H. J., J. Martin, C. A. McPherson, A. S. Daniel, and J.-X. Zhang. 1993. A bifunctional enzyme, with separate xylanase and β(1,3-1,4)-glucanase domains, encoded by the xynD gene of Ruminococcus flavefaciens. J. Bacteriol. 175:2943-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujino, T., P. Béguin, P., and J. P. Aubert. 1993. Organization of a Clostridium thermocellum gene cluster encoding the cellulosomal scaffolding protein CipA and a protein possibly involved in attachment of the cellulosome to the cell surface. J. Bacteriol. 175:1891-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gal, L., S. Pagès, C. Gaudin, A. Belaich, C. Reverbel-Leroy, C. Tardif, and J.-P. Belaich. 1997. Characterization of the cellulolytic complex (cellulosome) produced by Clostridium cellulolyticum. Appl. Environ. Microbiol. 63:903-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerngross, U. T., M. P. M. Romaniec, T. Kobayashi, N. S. Huskisson, and A. L. Demain. 1993. Sequencing of a Clostridium thermocellum gene (cipA) encoding the cellulosomal SL-protein reveals an unusual degree of internal homology. Mol. Microbiol. 8:325-334. [DOI] [PubMed] [Google Scholar]
- 19.Hungate, R. E., and R. J. Stack. 1982. Phenylpropanoic acid: growth factor for Ruminococcus albus. Appl. Environ. Microbiol. 44:79-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakiuchi, M., A. Isui, K. Suzuki, Fujino, E. Fujino, T. Kimura, S. Karita, K. Sakka, and K. Ohmiya. 1998. Cloning and DNA sequencing of the genes encoding Clostridium josui scaffolding protein CipA and cellulase CelD and identification of their gene products as major components of the cellulosome. J. Bacteriol. 180:4303-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby, J., J. C. Martin, A. S. Daniel, and H. J. Flint. 1997. Dockerin-like sequences in cellulases and xylanases from the rumen cellulolytic bacterium Ruminococcus flavefaciens. FEMS Microbiol. Lett. 149:213-219. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 23.Lamed, R., E. Setter, and E. A. Bayer. 1983. Characterization of a cellulose binding, cellulase-containing complex in Clostridium thermocellum. J. Bacteriol. 156:828-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamed, R., J. Naimark, E. Morgenstern, and E. A. Bayer. 1987. Specialized cell surface structures in cellulolytic bacteria. J. Bacteriol. 169:3792-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamed, R., R. Kenig, E. Morag, S. Yaron, Y. Shoham, and E. A. Bayer. 2001. Nonproteolytic cleavage of aspartyl proline bonds in the cellulosomal scaffoldin subunit from Clostridium thermocellum. Appl. Biochem. Biotechnol. 90:67-74. [DOI] [PubMed] [Google Scholar]
- 26.Leibovitz, E., and P. Béguin. 1996. A new type of cohesin domain that specifically binds the dockerin domain of the Clostridium thermocellum cellulosome integrating protein CipA. J. Bacteriol. 178:3077-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemaire, M., H. Ohayon, P. Gounon, T. Fujino, P. Béguin. 1995. OlpB, a new outer layer protein of Clostridium thermocellum, and binding of its S-layer-like domains to components of the cell envelope. J. Bacteriol. 177:2451-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, X., H. Chen, and L. Ljungdahl. 1997. Two cellulases, CelA and CelC, from the polycentric anaerobic fungus Orpinomyces strain PC-2 contain N-terminal docking domains for a cellulase-hemicellulase complex. Appl. Environ. Microbiol. 63:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mechaly, A., S. Yaron, R. Lamed, H.-P. Fierobe, A. Belaich, J.-P. Belaich, Y. Shoham, and E. A. Bayer. 2000. Cohesin-dockerin recognition in cellulosome assembly: experiment versus hypothesis. Proteins 39:170-177. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki, K., J. C. Martin, R. Marinsek-Logar, and H. J. Flint. 1997. Degradation of xylans by the rumen anaerobe Prevotella bryantii (formerly Prevotella ruminicola subsp. brevis) B14. Anaerobe 3:373-381. [DOI] [PubMed] [Google Scholar]
- 31.Morag, E., E. A. Bayer, and R. Lamed. 1991. Anomalous dissociative behavior of the major glycosylated component of the cellulosome of Clostridium thermocellum. Appl. Biochem. Biotechnol. 30:129-136. [DOI] [PubMed] [Google Scholar]
- 32.Morag, E., E. A. Bayer, and R. Lamed. 1992. Unorthodox intra-subunit interactions in the cellulosome of Clostridium thermocellum. Appl. Biochem. Biotechnol. 33:205-217. [Google Scholar]
- 33.Ohara, H., S. Karita, T. Kimura, K. Sakka, and K. Ohmiya. 2000. Characterisation of the cellulolytic complex (cellulosome) from Ruminococcus albus. Biosci. Biotechnol. Biochem. 64:254-260. [DOI] [PubMed] [Google Scholar]
- 34.Pages, S., A. Belaich, C. Tardif, C. Reverbel-Leroy, C. Gaudin, and J.-P. Belaich. 1996. Interaction between the endoglucanase CelA and the scaffolding protein CipC of the Clostridium cellulolyticum cellulosome. J. Bacteriol. 178:2279-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pages, S., A. Belaich, J. P. Belaich, E. Morag, R. Lamed, Y. Shoham, and E. A. Bayer. 1997. Species-specificity of the cohesin-dockerin interaction between Clostridium thermocellum and Clostridium cellulolyticum: prediction of specificity determinants of the dockerin domain. Proteins 29:517-527. [PubMed] [Google Scholar]
- 36.Park, J.-S., Y. Matano, and R. H. Doi. 2001. Cohesin-dockerin interactions of cellulosomal subunits of Clostridium cellulovorans. J. Bacteriol. 183:5431-5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pegden, R. S., M. A. Larson, R. J. Grant, and M. Morrison. 1998. Adherence of the gram-positive bacterium Ruminococcus albus to cellulose and identification of a novel form of cellulose-binding protein which belongs to the Pil family of proteins. J. Bacteriol. 180:5921-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rincon, M. T., S. I. McCrae, J. Kirby, K. P. Scott, and H. J. Flint. 2001. EndB, a multidomain family 44 cellulase from Ruminococcus flavefaciens 17, binds to cellulose via a novel cellulose binding module and to another R. flavefaciens protein via a dockerin domain. Appl. Environ. Microbiol. 67:4426-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salamitou, S., O. Raynaud, M. Lemaire, M. Coughlan, P. Béguin, and J. P. Aubert. 1994. Recognition specificity of the duplicated segments present in the Clostridium thermocellum endoglucanase gene CelD and in the cellulosome integrating protein CipA. J. Bacteriol. 176:2822-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz, W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56:634-649. [DOI] [PubMed] [Google Scholar]
- 41.Shoseyov, O., M. Takagi, M. A. Goldstein, and R. H. Doi. 1992. Primary sequence analysis of Clostridium cellulovorans cellulase binding protein A. Proc. Natl. Acad. Sci. USA 89:3483-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tinoco, I., P. N. Borer, B. Dengler, M. D. Levine, O. C. Uhlenbeck, D. M. Crothers, and J. Gralla. 1973. Improved estimation of secondary structure in ribonucleic acids. Nat. New Biol. 246:40-41. [DOI] [PubMed] [Google Scholar]