Abstract
Rhizosphere isolate Pseudomonas sp. strain BW11M1, which belongs to the Pseudomonas putida cluster, secretes a heat- and protease-sensitive bacteriocin which kills P. putida GR12-2R3. The production of this bacteriocin is enhanced by DNA-damaging treatment of producer cells. We isolated a TnMod mutant of strain BW11M1 that had lost the capacity to inhibit the growth of strain GR12-2R3. A wild-type genomic fragment encompassing the transposon insertion site was shown to confer the bacteriocin phenotype when it was introduced into Escherichia coli cells. The bacteriocin structural gene was identified by defining the minimal region required for expression in E. coli. This gene was designated llpA (lectin-like putidacin) on the basis of significant homology of its 276-amino-acid product with mannose-binding lectins from monocotyledonous plants. LlpA is composed of two monocot mannose-binding lectin (MMBL) domains. Several uncharacterized bacterial genes encoding diverse proteins containing one or two MMBL domains were identified. A phylogenetic analysis of the MMBL domains present in eukaryotic and prokaryotic proteins assigned the putidacin domains to a new bacterial clade within the MMBL-containing protein family. Heterologous expression of the llpA gene also conveyed bacteriocin production to several Pseudomonas fluorescens strains. In addition, we demonstrated that strain BW11M1 and heterologous hosts secrete LlpA into the growth medium without requiring a cleavable signal sequence. Most likely, the mode of action of this lectin-like bacteriocin is different from the modes of action of previously described Pseudomonas bacteriocins.
Bacteria inhabit highly competitive environments, such as the plant root-soil interface (rhizosphere). In such niches, bacteria are constantly competing for nutrients and ecological space. As a consequence, bacteria have devised various offensive tools for intra- and interspecies competition, such as antibiotic substances, bacteriolytic enzymes, and bacteriocins. In all likelihood, the bacteriocins constitute the most abundant and diverse class of antimicrobial agents. Traditionally, these compounds have been defined as proteinaceous compounds that are produced by bacteria and have relatively narrow killing spectra. These toxins are able to kill bacterial competitors while causing little or no harm to the producing cells because of a specific immunity mechanism and/or posttranscriptional modifications (22).
The bacteriocinogenic properties of Pseudomonas aeruginosa are widely recognized. Analysis of large numbers of P. aeruginosa isolates from clinical and environmental sources has indicated that more than 70% of the isolates produce one or more bacteriocins (19, 21, 43). Bacteriocins of P. aeruginosa (termed pyocins) are classified into three groups on the basis of their structures. R- and F-type pyocins have complex structures similar to those of certain bacteriophage tails (41). S-type pyocins are single-subunit nucleases composed of three or four functional domains (45). This typical composite structure is also observed for the nuclease colicins, the counterparts of pyocins in Escherichia coli (25, 49). Because of their wide distribution, pyocins are useful tools for epidemiological studies (54), and because of their chimeric nature, colicins and S-type pyocins are of particular interest for investigating the evolutionary mechanisms involved in bacteriocin diversification (45, 49). For plant-associated pseudomonads, research has been focused mainly on plant-pathogenic Pseudomonas syringae strains (24, 29, 53). In this respect, bacteriocins have potential as biological control agents that can be used against bacterial pathogens (34). Several reports have also described the production of proteinaceous inhibitory substances by aquatic Pseudomonas spp. (23, 58). Still, pyocins remain the only Pseudomonas bacteriocins that have been characterized genetically and biochemically to date, except for the atypical bacteriocin small, an N-acylhomoserine lactone produced by Pseudomonas fluorescens F113 (33).
We have demonstrated previously that bacteriocin-producing P. fluorescens and Pseudomonas putida isolates are abundant in diverse plant rhizospheres of tropical soils, as well as temperate soils (44). One such isolate, Pseudomonas sp. strain BW11M1, which was originally isolated from banana roots in Sri Lanka, produces a potent antibacterial compound that is active against P. putida GR12-2R3 (44). This bacteriocin-like compound was designated putidacin. In this study, we cloned and characterized the gene encoding putidacin. Remarkably, the putidacin protein displays striking similarity, both in domain architecture and in conserved critical residues, to members of a superfamily of mannose-binding plant lectins. These lectins consist of one or more subunits with similar sequences and overall three-dimensional structures (namely, a β-prism arrangement of three bundles of β-sheets) (2). During synthesis on the endoplasmic reticulum, a signal peptide is cotranslationally removed, and the resulting proprotein is subsequently transported into the vacuoles, during which the proprotein usually undergoes final processing into two mature polypeptides. The homology of the BW11M1 bacteriocin with these plant proteins suggests a novel mode of bacteriocin action.
MATERIALS AND METHODS
Strains and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Most Pseudomonas cells were routinely grown in Trypticase soy medium (BD Bioscience) at 30°C; the exception was P. aeruginosa, which was cultured at 37°C. Luria-Bertani (LB) broth (39) was used to grow E. coli at 37°C. When required, antibiotics were added at the following concentrations: kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; and tetracycline, 10 μg/ml. Media were solidified with 1.5% agar. 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and isopropyl β-d-thiogalactoside (IPTG) (40 mg/liter; Duchefa Biochemie) were added to detect the presence of insert DNA cloned in pUC18/19-derived vectors in E. coli. All plasmids used for sequencing were propagated in E. coli TOP10F′ (Invitrogen). Frozen stocks were stored at −80°C by using a 1:2 mixture of 80% glycerol and a stationary-phase cell suspension.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli strains | ||
| TOP10F′ | F′[lacIq Tn10(Tetr)] mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacZ74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| GM2163 | F−ara-14 leuB6 fhuA31 lacY1 tsx78 glnV44 galK2 galT22 mcrA dcm-6 hisG4 rfbD1 rpsL136 dam-13::Tn9(Camr) xylA5 mtl-1 thi-1 mcrB1 hsdR2 | New England Biolabs |
| Pseudomonas sp. strains | ||
| BW11M1 | Strain isolated from banana rhizosphere | 63 |
| CMPG2065 | BW11M1 TnMod mutant; Kmr | This study |
| P. agarici LMG 2112 | Type strain | BCCMc |
| P. aeruginosa PAO1 | 56 | |
| P. aureofaciens LMG 1245a | Type strain | BCCM |
| P. cichorii LMG 2162 | Type strain | BCCM |
| P. chlororaphis LMG 5004 | Type strain | BCCM |
| P. fuscovaginae HPB 2646 | H. Maraitéd | |
| P. fluorescens strains | ||
| 2-79 | Strain isolated from wheat rhizosphere | 65 |
| F113 | Strain isolated from sugarbeet rhizosphere | 52 |
| OE 28.3 | Strain isolated from wheat rhizosphere | 13 |
| SBW25 | Strain isolated from sugarbeet leaves | 47 |
| WCS365 | Strain isolated from potato rhizosphere | 20 |
| P. marginalis LMG 2210b | Pathovar reference strain | BCCM |
| P. mendocina LMG 1223 | Type strain | BCCM |
| P. putida strains | ||
| GR12-2R3 | Rifampin-resistant derivative of strain GR12-2 isolated from grass rhizosphere | 36 |
| LMG 2257 | Type strain | BCCM |
| KT2440 | pWW0-cured, restriction-deficient derivative of P. putida mt-2 | 18 |
| P. resinovorans LMG 2274 | Type strain | BCCM |
| P. stutzeri LMG 11199 | Type strain | BCCM |
| P. syringae pv. glycinea LMG 5066 | Pathovar reference strain | BCCM |
| P. syringae pv. syringae LMG 1247 | Type strain | BCCM |
| P. syringae pv. tabaci LMG 5192 | BCCM | |
| P. syringae pv. tomato DC3000 | 66 | |
| P. tolaasii LMG 2342 | Type strain | BCCM |
| P. viridiflava LMG 2352 | Type strain | BCCM |
| Plasmids | ||
| pUC18/pUC19 | ColE1 replicon, lacZ, cloning vector; Apr | 69 |
| pCR2.1 | ColE1 replicon, f1 origin, lacZ; Apr Kmr | Invitrogen |
| pWTT2081 | E. coli-Pseudomonas shuttle vector based on pACYC184 and pVS1 replicons; Tcr | 62 |
| pPDM-1 | Plasmid deletion vector; Apr Kmr | Epicentre |
| pTnMod-OKm′ | Plasposon vector with pMB1 replicon between inverted repeats; Kmr | 14 |
| pCMPG6010 | pUC18 containing 10.9-kb genomic KpnI fragment with llpA from BW11M1 | This study |
| pCMPG6012 | pUC18 with 989-bp PCR-amplified fragment containing llpA gene cloned in KpnI/BamHI | This study |
| pCMPG6015 | pUC19 with 989-bp PCR-amplified fragment containing llpA gene cloned in KpnI/BamHI | This study |
| pCMPG6018 | pWTT2081 with insert from pCMPG6012, blunted and recloned in SmaI/BamHI | This study |
| pCMPG6020 | pPDM-1 with KpnI insert from pCMPG6010 | This study |
Now classified as P. chlororaphis.
P. marginalis pv. marginalis.
BCCM, Belgian Coordinated Collections of Microorganisms (http://www.belspo.be/bccm/index.htm).
H. Maraité, Université Catholique de Louvain, Louvain-la-Neuve, Belgium.
DNA manipulations.
Standard methods were used for DNA electrophoresis, transfer of DNA from agarose gels to nylon membranes (Amersham Biosciences), preparation of competent E. coli cells, and transformation of E. coli (51). Restriction endonucleases, the Klenow fragment, and alkaline phosphatase were used as specified by the supplier (Roche Diagnostics). DNA fragments were recovered from agarose gels with a Prep-a-Gene DNA purification kit (Bio-Rad). DNA ligation was performed by using a Rapid DNA ligation kit (Roche Diagnostics). Pseudomonas total DNA was isolated from 5 ml of early-stationary-phase cultures with a PureGene DNA purification kit (Gentra Systems). Plasmids were isolated by using a Qiaprep Spin Miniprep kit (Qiagen). Southern blot analyses were conducted with digoxigenin (DIG)-labeled probes according to the manufacturer's instructions (Roche Diagnostics). Probes were generated by PCR with Taq DNA polymerase by using a PCR DIG labeling kit (Roche Diagnostics) and a Mastercycler Personal (Eppendorf) according to the manufacturer's recommendations.
16S rRNA phylogeny of Pseudomonas sp. strain BW11M1.
The following three primer sets were used for PCR amplification of the 16S rRNA gene of strain BW11M1, corresponding to residues 10 to 1,536 of the E. coli 16S rRNA gene; forward1 (5′-AGTTTGATCATGGCTCAGATTG-3′) and reverse1 (5′-CAGAGTTAGCCGGTGCTTATTC-3′); forward2 (5′-CTACACACTGGAACTGAGACACGGTCC-3′) and reverse2 (5′-CTAAGCTGACGACAGCCATGCAGCACC-3′); and forward3 (5′-GGAGCATGTGGTTTAATTCG-3′) and reverse3 (5′-GGTGATCCAGCCGCAGGTTCC-3′). The PCR products were generated by using standard concentrations of Taq DNA polymerase, primers, and deoxynucleoside triphosphates (51) and were cloned into pCR2.1 by using a TA cloning kit (Invitrogen) for sequencing. A multiple alignment was constructed with the sequence obtained and the related 16S rRNA gene sequences of 22 P. putida and 11 P. fluorescens strains, which were retrieved from the small-subunit rRNA database (http://rrna.uia.ac.be/ssu/index.html) and truncated to 1,305 bp long (corresponding to E. coli residues 61 to 1,370). Phylogenetic analysis was performed by using the software package Treecon for Windows (61). An unrooted distance tree was constructed with the neighbor-joining program (50) from a similarity matrix of pairwise comparisons made by using the Jukes-Cantor algorithm (26) with Poisson correction.
Electrotransformation of Pseudomonas cells.
Competent Pseudomonas cells were prepared for electrotransformation in 15% glycerol-1 mM MOPS buffer by the method of Farinha and Kropinsky (16). Electroporation was performed with a Gene-Pulser (Bio-Rad) by using 40 μl of an electrocompetent Pseudomonas cell suspension and 10 to 200 ng of plasmid DNA at 2.5 kV, 25 μF, and 200 Ω. Two minutes after electroporation, the cell suspension was diluted in 1 ml of LB broth and incubated for 1.5 h at 30°C with shaking; this was followed by plating on selective medium.
Construction of a Pseudomonas sp. strain BW11M1 mutant library.
Random mutagenesis of Pseudomonas sp. strain BW11M1 was accomplished by introducing the transposon pTnMod-OKm′ by electroporation. Preliminary experiments had indicated that it was necessary to use nonmethylated plasmid DNA for efficient transformation of strain BW11M1. This was achieved by propagation of pTnMod-OKm′ in the methylation-deficient E. coli strain GM2163. Kanamycin-resistant transposon mutants were picked up with toothpicks and stored individually in 25% glycerol at −80°C in 96-well microtiter plates. Southern hybridization was carried out for 20 randomly chosen mutants by using a DIG-labeled PCR-generated kanamycin resistance gene probe (forward primer, 5′-GGCAATCAGGTGCGACAATCTA-3′; reverse primer, 5′-ATGAAGGAGAAAACTCACCGAGGC-3′), which confirmed that the mutants arose from single transposition of TnMod.
Deletion analysis of the LlpA biosynthesis region.
Mutant CMPG2065, which did not inhibit the growth of the indicator strain, was selected for plasmid rescue to clone the mutated region. Total DNA of this mutant was isolated and digested with several restriction enzymes that did not cut inside the inserted plasposon fragment (TnMod). The resulting fragments were self-ligated and transformed into E. coli TOP10F′ cells with selection on kanamycin. Initially, an E. coli clone carrying a circularized genomic PstI fragment was rescued, and the DNA sequences flanking TnMod were determined by using dye terminator chemistry (ALFexpress2 automated sequencer; Amersham Biosciences). The sequencing reactions were driven by two pTnMod-OKm′-specific primers (pseu-596 [5′-TCTGGCTGGATGATGGGGCG-3′] and pseu-597 [5′-CGGTTCCTGGCCTTTTGCTGGC-3′]) that were complementary to TnMod and oriented towards the genomic DNA flanking TnMod. A set of PCR primers (forward primer pseu-614 [5′-GGGTCGCCAATGAACAGCAAC-3′] and reverse primer pseu-615 [5′-CGTTGCTGGTGAAGGTGCTG-3′]) was designed to generate a DIG-labeled probe for isolation the wild-type genomic fragment from Pseudomonas sp. strain BW11M1. The PCR product (501 bp) was obtained by using the following reaction conditions: one cycle of 4 min at 94°C, followed by 30 cycles of 30 s at 94°C (denaturation), 30 s at 60°C (annealing), and 30 s at 72°C (extension), and one final cycle of 10 min at 72°C. The DIG-labeled fragment was used as a probe in Southern hybridization with BW11M1 total DNA digested with several combinations of restriction enzymes. A strong hybridization signal corresponding to a 10.9-kb KpnI DNA fragment was detected. A mixture of DNA fragments whose sizes coincided with the size of this positive signal was isolated from the agarose gel, purified, and ligated to dephosphorylated vector pUC18 digested with KpnI. The ligation mixture was transformed into E. coli TOP10F′. Approximately 900 transformants were picked and inoculated individually into wells of 96-well microtiter plates containing LB broth (0.2 ml) and ampicillin. All nine 96-well microtiter plates were then pooled by using a 96-prong replicator into a fresh microtiter plate containing LB medium supplemented with ampicillin. Following overnight growth of this pool plate, 0.1-μl aliquots from each well were transferred into the wells of a 96-well PCR plate (Molecular Bioproducts) for colony PCR with primers pseu-614 and pseu-615 by using a mastercycler gradient (Eppendorf). The KpnI insert recovered from a positive E. coli transformant was recloned into pPDM-1, giving pCMPG6020, which was used with an EZ::TN plasmid-based deletion machine (Epicentre). The orientation of the insert was checked by miniprep and restriction analysis. Using the supplier's protocol, we generated a population of random DNA sequence deletions and inversions of pCMPG6020 by combining 0.2 μg of plasmid DNA and 0.5 U of EZ::TN transposase in a 10-μl (total volume) mixture. E. coli TOP10F′ cells were then transformed with 1 μl of the deletion reaction mixture. Deletion and inversion clones (50% of the colonies obtained) were selected by replica plating by using LB medium plates supplemented with kanamycin (no growth) and ampicillin (growth), and they were analyzed further by restriction digestion. Deletion clones were assayed for bacteriocin production and sequenced. Sequence data were compiled by using the ALF Win Analyser software (Amersham Biosciences).
Analysis of DNA and protein sequences.
Potential open reading frames (ORFs) were predicted by using GeneMark (http://opal.biology.gatech.edu/GeneMark/heuristic_hmm2.cgi) (6). Homology searches were done with the BLAST programs (1) at the National Center for Biotechnology Information. Multiple-sequence alignments were constructed by using CLUSTALW (57). The GeneDoc program was used for editing the sequence alignment (http://www.psc.edu/biomed/genedoc/). Conserved protein domains were detected with the Pfam (protein family database) search tool (version 7.2) on the Sanger Center internet server (http://www.sanger.ac.uk/Software/Pfam/) and were confirmed with SMART (Simple Modular Architecture Research Tool, version 3.4) (http://smart.embl-heidelberg.de). Molecular weights and isoelectric points (pI) were estimated with the ProtParam tool on the ExPASy molecular biology server (http://us.expasy.org/tools/protparam.html). Potential signal peptide cleavage sites were predicted by using the SignalP World Wide Web prediction server (http://www.cbs.dtu.dk/services/SignalP/).
Phylogenetic analysis of LlpA domains.
Similar protein sequences were truncated to the length (104 to 124 amino acids) of known or predicted mannose-binding lectin domains (Pfam database accession no. PF01453 [agglutinin], equivalent to SMART database accession no. SM0108 [B_Lectin]). The alignment was further adapted manually according to secondary-structure predictions (Jpred2 [http://www.compbio.dundee.ac.uk/∼www-jpred/]). An unrooted distance tree was constructed as described above. The topology of the tree was evaluated by performing a bootstrap analysis of 1,000 resamples (17).
Heterologous expression of LlpA.
A 989-bp fragment containing llpA was amplified by PCR with Platinum Pfx DNA polymerase (Invitrogen) by using Pseudomonas sp. strain BW11M1 genomic DNA as the template. Primers were designed to anneal at positions −127 to −108 relative to the predicted llpA translation start site (primer pseu-632 [5′-TAGGTACCTCAAGTGCTCGCGTTGCGCG-3′; added KpnI site underlined]) and at positions 13 to 32 relative to the predicted stop codon (pseu-634 [5′-TAGGATCCAAGGCCGGGCCCGTTAAGGC-3′; added BamHI site underlined]). Thirty amplification cycles consisting of 30 s of denaturation at 94°C, 30 s of primer annealing at 55°C, and 45 s of primer extension at 68°C were performed, preceded by an initial 4 min of denaturation at 94°C and followed by a final 10 min of primer elongation at 68°C. The resulting 989-bp PCR product was purified by ethanol precipitation, digested with KpnI and BamHI, and ligated into the cognate sites of pUC18 (pCMPG6012) and pUC19 (pCMPG6015). Ligation mixtures were transformed into TOP10F′ cells for expression in E. coli. For heterologous expression in Pseudomonas, the KpnI/BamHI insert from pCMPG6012 was blunted with the Klenow enzyme and ligated in the SmaI and BamHI sites of pWTT2081. The resulting plasmid, pCMPG6018, was propagated in E. coli GM2163, and plasmid DNA was extracted for electroporation of P. fluorescens WCS365, P. fluorescens SBW25, and P. fluorescens OE 28.3 cells. The resulting Pseudomonas transformants were checked for the presence of pCMPG6018 by miniprep and restriction digestion. Bacteriocin production in heterologous hosts was investigated by using standard bacteriocin plate assays.
Protein sample preparation for gel electrophoresis.
Bacterial cultures (250 ml) were grown overnight to an optical density at 600 nm of 1.6 and then centrifuged for 10 min at 9,000 × g. The growth medium was not supplemented with antibiotics, as this would have interfered with bacteriocin detection of protein fractions. The supernatant was filtered through 0.45-μm-pore-size filters (Millipore) and lyophilized for 48 to 72 h. The lyophilized supernatant was then dissolved in 5 ml of double-distilled water and stored at −20°C until it was used. Protein samples for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) were prepared by purification of lyophilisate with phenol extraction, followed by ammonium acetate precipitation by a protocol described by De Mot and Vanderleyden (12), with the following minor modifications. In the first step, 1 ml of dissolved lyophilisate was diluted with an equal volume of extraction buffer. After protein precipitation, the resulting pellet was dissolved in 200 μl of solubilization buffer containing 9.8 M urea, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 0.5% IPG buffer (pH 3 to 10; Amersham Biosciences), and 65 mM dithiothreitol and stored at 4°C. Protein fractions were resolved by SDS-PAGE to estimate relative protein concentrations.
Protein electrophoresis and N-terminal amino acid sequencing.
SDS-PAGE was performed under reducing conditions as described by Laemmli (32) by using a 5% polyacrylamide stacking gel and a 12.5% polyacrylamide separating gel calibrated with a broad-range SDS-PAGE standard (Bio-Rad). Electrophoresis was performed with a Mini-Protean unit (Bio-Rad) at 200 V for 50 min. For two-dimensional PAGE, proteins were resolved by isoelectric focusing by using 7-cm Immobiline dry strip gels (pH 6 to 11) (Amersham Biosciences). One hundred microliters of phenol-extracted lyophilisate was applied to the anodic side of each strip gel, and the isoelectric focusing running conditions used were those described in the manufacturer's protocol. The second-dimension SDS-PAGE was performed with a 1-mm-thick SDS-12.5% polyacrylamide gel by using the Mini-Protean system (Bio-Rad). SDS-polyacrylamide gels were stained with Coomassie blue R-250 to visualize protein. For N-terminal microsequencing, two-dimensional PAGE gels were electroblotted onto polyvinylidene difluoride membranes by using a Multiphor II electrophoresis system (Amersham Biosciences). The N-terminal amino acid sequences of proteins were determined from the Coomassie blue-stained polyvinylidene difluoride membranes by automated Edman degradation by using a Procise 491 cLC protein sequencer (Applied Biosystems, Foster City, Calif.).
Bacteriocin assays.
The ability to produce bacteriocin was detected by examining deferred antagonism (19). Two-microliter portions of a stationary-phase culture were spotted onto agar plates and incubated for 8 h at the appropriate temperature. For screening of 96-well microtiter plates, samples were stamped onto suitable agar growth medium in square petri dishes. To prevent further cell growth, the plates were then exposed to chloroform vapor (20 min) and subsequently overlaid with 3 ml of soft agar (0.5%) seeded with 100 μl of a cell culture of the indicator strain (108 CFU/ml). Bacteriocin production was assessed after overnight incubation at 30°C by determining the formation of clear zones of growth inhibition in the indicator lawns around the test colonies.
To test the effect of UV irradiation on bacteriocin production by strain BW11M1, a plate onto which a BW11M1 cell culture was spotted was exposed to UV light (312 nm) for 30 s after an initial incubation for 6.5 h. Heat inactivation of LlpA was determined by placing preincubated and chloroform-treated producer plates in an oven at 75°C for 15 min. After cooling at room temperature for 30 min, the plates were overlaid with an indicator lawn. Sensitivity to proteolytic enzymes was tested by spotting 10 μl of pronase E (20 mg/ml; Sigma) or proteinase K (20 mg/ml, Sigma) near (presumed) bacteriocin-producing colonies which had been previously incubated and killed with chloroform. After the drops dried, producer plates were incubated for 1 h at 37°C, which allowed optimal proteolytic activity. Subsequently, an indicator lawn was applied as usual. In all cases, treated plates were examined after overnight incubation, and inhibition zones were compared to zones on control plates.
For in-gel detection of proteins with bacteriocin activity, SDS-PAGE gels were rinsed in distilled water for 30 min with gentle shaking and incubated overnight at 4°C in two changes of 50 mM MES (2-morpholinoethanesulfonic acid)-NaOH (pH 6) containing 0.1% (wt/vol) Triton X-100 (35). The following day, each gel was washed with distilled water, excess fluid was removed with Kimwipes, and an overlay consisting of soft agar seeded with the indicator strain was applied.
Mannose binding assays.
Agglutination assays were carried out in small glass tubes by combining 30 μl of a fivefold-diluted suspension of rabbit blood with 10 μl of BW11M1 culture lyophilisate in the presence or absence of 1 M ammonium sulfate. The lyophilisates were washed first with 10 volumes of MilliQ water by ultrafiltration by using Vivaspin 10,000 molecular-weight-cutoff centrifugal concentrators (Vivascience). Agglutination of erythrocytes was assessed visually. In addition, the possible mannose-binding capacity of LlpA was investigated by affinity chromatography. One hundred microliters of BW11M1 culture lyophilisate was buffered with two washes consisting of 5 volumes of 50 mM Tris-HCl (pH 8.5) by ultrafiltration, and the final volume was adjusted to 1.5 ml with 50 mM Tris-HCl (pH 8.5). This sample was then combined with 1 ml of a mannose-Sepharose 4B (Sigma) slurry equilibrated with 1 M ammonium sulfate in 50 mM Tris-HCl (pH 8.5) (equilibration buffer) in a 15-ml polypropylene centrifuge tube. Proteins were allowed to adsorb to the resin for 2 h on a rocking platform. After the resin was washed twice with 10 ml of equilibration buffer, bound proteins were desorbed with 1 ml of 0.1 M d-mannose in equilibration buffer. LlpA bacteriocin activity was ascertained by applying 5-μl aliquots of 10-fold-concentrated flowthrough (wash) and eluted fraction onto an indicator lawn.
Nucleotide sequence accession numbers.
The GenBank accession number for the nucleotide sequence of the 16S ribosomal DNA of Pseudomonas sp. strain BW11M1 is AY118111. The nucleotide sequence of llpA determined in this study has been deposited in the GenBank database under accession number AY118112.
RESULTS
Selection of a fluorescent Pseudomonas rhizosphere isolate exhibiting bacteriocin activity against P. putida GR12-2R3.
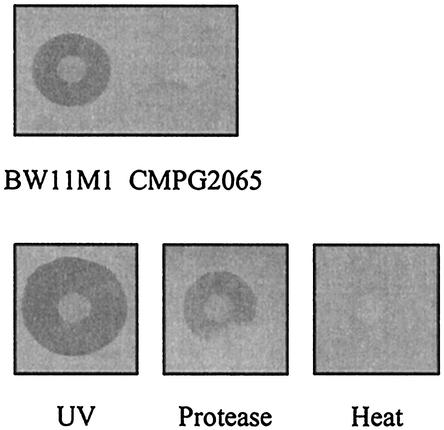
From an initial screening to examine bacteriocin production by root-colonizing fluorescent Pseudomonas, we selected a fluorescent Pseudomonas strain, strain BW11M1 isolated from a banana rhizosphere in Sri Lanka (63). This strain displayed strong antibacterial activity against the plant-growth-promoting strain P. putida GR12-2R3 (Fig. 1). This antibacterial activity was eliminated by heat and protease treatment. Conversely, exposure of strain BW11M1 to UV light resulted in an enlarged inhibition zone (Fig. 1).
FIG. 1.
Growth inhibition of P. putida GR12-2R3 by strain BW11M1. Mutant CMPG2065 did not exhibit antibacterial activity against the indicator strain. UV-irradiated BW11M1 exhibited enlarged inhibition halos (UV). A droplet of pronase E spotted near the producing colony eliminated the inhibition effect (Protease). Bacteriocin activity was not observed on producer plates exposed to 75°C for 15 min (Heat).
The taxonomic position of strain BW11M1 within the fluorescent pseudomonads was evaluated by analysis of its 16S rRNA gene sequence. BLASTN analysis of the sequence obtained and phylogenetic distance analysis based on a multiple alignment of Pseudomonas 16S rRNA gene sequences placed strain BW11M1 within the P. putida cluster (44). According to conventional rules of bacteriocin naming, we named the bacteriocin produced by P. putida-related strain BW11M1 a putidacin.
Cloning and characterization of the llpA gene of Pseudomonas sp. strain BW11M1 encoding putidacin, a novel lectin-like bacteriocin.
To obtain mutants with impaired putidacin production, random transposon mutagenesis of Pseudomonas sp. strain BW11M1 was carried out (14). When some 10,500 kanamycin-resistant TnMod mutants were screened, CMPG2065, which did not produce a growth inhibition halo on bacteriocin assay plates seeded with P. putida GR12-2R3, was isolated. The presence of a single TnMod insertion in CMPG2065 was confirmed by hybridization. Using a plasposon rescue procedure, we cloned the DNA region flanking TnMod. A 10.9-kb genomic KpnI fragment encompassing the plasposon insertion site was isolated from a size-fractionated KpnI sublibrary of strain BW11M1 by colony PCR screening. Expression of this KpnI fragment in E. coli by using the pUC18-derived plasmid pCMPG6010 resulted in acquisition of the putidacin production phenotype. The KpnI fragment was recloned in the pPDM-1 vector in order to use the EZ::TN plasmid-based deletion method. With this procedure a collection of deletion and inversion clones carrying fragments of the original KpnI fragment were generated in E. coli TOP10F′. Deletion clones were selected by restriction analysis and arranged according to insert size. Standard bacteriocin plate assays allowed us to narrow down the region in the 10.9-kb KpnI fragment required for putidacin production. Sequence analysis of a 1.3 kb-region (G+C content, 66%) revealed the presence of a single ORF comprised of 831 bp preceded by a plausible ribosome binding site (AGGAGA) at positions −13 to −8 relative to the proposed ATG start codon. Further deletions affecting this ORF resulted in loss of putidacin production in E. coli. This ORF coded for a 276-amino-acid protein with a theoretical molecular mass of 29,967 Da and a predicted isoelectric point of 9.12. The deduced amino acid sequence showed similarities with the sequences of members of a superfamily of monocot lectins with mannose specificity. The gene was therefore designated llpA (lectin-like putidacin). Sequence analysis of the upstream region of llpA did not reveal obvious transcription factor binding sites or a putative P box. Such a conserved regulatory element has been found in the promoter region of S pyocin genes 60 to 100 bp upstream of the ribosome binding site and was shown to be involved in the induction of S pyocin gene expression following DNA-damaging treatments (15, 38). The site of insertion of the TnMod element in mutant CMPG2065 was found to be located between bp 515 and 516 of the coding region of llpA.
Homology of LlpA with monocot mannose-binding lectins.
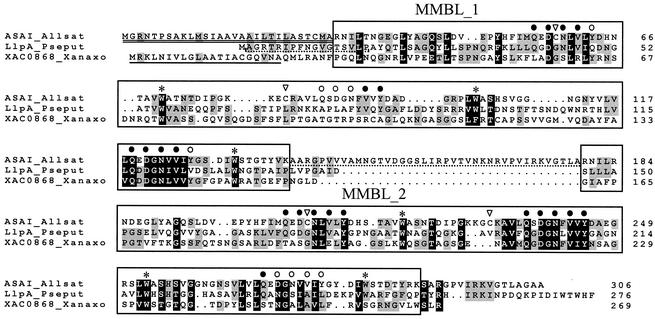
A BLASTP search for the LlpA amino acid sequence revealed no close matches. The highest overall homology (22% identity and 35% similarity) was found with a lectin type I precursor protein isolated from garlic (Allium sativum) bulbs (ASAI; SwissProt accession no. Q38788) belonging to the superfamily of monocot mannose-binding lectins (60). ASAI is a mannose-binding lectin derived from a single precursor (preprolectin) consisting of two very similar, tandemly arranged lectin domains (8). Each mature ASAI protein contains three mannose-binding sites made up of identical amino acid residues (namely, Gln, Asp, Asn, and Tyr) that bind a mannose molecule through a network of four hydrogen bonds. In addition, a hydrophobic residue, Val, interacts with mannose through a hydrophobic interaction. These residues are arranged in a QXDXNXVXY motif. A Pfam domain search identified two monocot mannose-binding lectin domains in LlpA that were separated by seven amino acids and were referred to as MMBL domains (corresponding to Pfam accession no. PF01453 [agglutinin]); these domains were MMBL_1, ranging from amino acid 14 to amino acid 138 (Pfam E value, 0.14), and MMBL_2, ranging from amino acid 146 to amino acid 254 (Pfam E value, 9.8 × 10−6). Sequence analysis further indicated that MMBL_1 and MMBL_2 are quite dissimilar (23% identity and 33% similarity). A pairwise alignment with ASAI revealed the presence of two perfect or close matches with the characteristic QXDXNXVXY motif in each MMBL domain of LlpA (Fig. 2). The equivalent of the third motif (in MMBL_2) and, in particular, the counterpart of the second motif (in MMBL_1) are much less well conserved in LlpA MMBL_2 and LlpA MMBL_1, respectively. However, three highly conserved Trp (W) residues were detected in each MMBL domain. These hydrophobic residues are situated at the center of each of the lectin subunits, close to the threefold axis, and form a stabilizing hydrophobic core (48).
FIG. 2.
Multiple-sequence alignment of LlpA (LlpA_Pseput), the precursor protein of ASAI, a mannose-specific lectin found in bulbs of garlic (A. sativum) (ASAI_Allsat), and hypothetical protein XAC0868 from X. axonopodis pv. citri (XAC0868_Xanaxo). Gaps are indicated by dots. Predicted MMBL domains are enclosed in boxes. Amino acids involved in mannose binding in ASAI are indicated by solid circles when they are present in LlpA or by open circles when they are not present in LlpA. The labeled tryptophan residues (asterisks) are part of a stabilizing hydrophobic core structure and are conserved in nearly all homologous proteins. Cysteine residues typical of plant MMBL domains are also identified (inverted open triangles). The signal peptide of ASAI is double underlined. A SignalP search revealed the presence of a putative 21-amino-acid signal sequence (underlined) for XAC0868. The linker peptide of ASAI which is cleaved off during final processing is underlined with a dashed line. The N-terminal amino acid sequence of LlpA determined by sequencing is also underlined.
Remarkably, we were unable to detect significant homology of LlpA with previously described bacteriocins, with the possible exception of a protein from Ruminococcus albus strain 7. The latter protein, encoded by the inh1 gene, is described as an inhibitor of the growth of Ruminococcus flavescens strains. The inh1 gene product, which has an estimated length of 339 amino acids, contains a single N-terminal MMBL domain showing significant homology to MMBL_2 of LlpA (30% identity and 47% similarity). No significant homology matches were found for the C-terminal region of this protein. Interestingly, we predicted from a SignalP analysis that unlike LlpA, this second bacterial lectin-like bacteriocin contains a possible 49-amino-acid signal peptide (Fig. 3).
FIG. 3.
Phylogenetic analysis of MMBL domains and domain structures of bacterial and selected eukaryotic proteins containing one or two MMBL domains. An unrooted neighbor-joining distance tree was constructed from a multiple alignment of amino acid sequences of known and inferred MMBL domains found in eukaryotic and bacterial proteins. The suffixes _1 and _2 indicate tandem MMBL domains present in certain polypeptides. Bootstrap values based on 1,000 replicate trees are shown at the appropriate nodes when they were more than 50%. Scale bar = 0.1 substitution per site. Monocot lectins with a two-domain precursor are exemplified by the A. sativum lectin precursor (clone 2; SwissProt accession no. Q38788), while GNA (G. nivalis agglutinin precursor; SwissProt accession no. Q39903) represents lectins containing only one MMBL domain. Other selected proteins containing MMBL domains are B. oleracea S-locus receptor-like kinase (SwissProt accession no. P93068), C. latifolia curculin precursor (SwissProt accession no. P19667), D. discoideum comitin precursor (SwissProt accession no. Q03380), R. albus strain 7 bacterial inhibitor (SwissProt accession no. Q8VPZ8), S. coelicolor strain A3(2) putative secreted esterase SCO3053 (SwissProt accession no. Q93J50), Synechocystis sp. strain PCC6803 hypothetical protein (SwissProt accession no. P73139), and X. axonopodis pv. citri hypothetical protein XAC0868 (GenBank accession no. NP_641220). Additional proteins with MMBL domains homologous to the LlpA MMBL domains were found in the unfinished genomes of M. smegmatis strain mc2155 (Mycsme:contig 3310, 1763047-1763703; retrieved from The Institute for Genome Research) and R. metallidurans strain CH34 (Reut_p_1061∗; retrieved from the DOE Genome Institute; a truncated version of this protein, without the first 244 residues, is found in GenBank [accession no. ZP_00022141]). The complex domain structure of a large Synechocystis protein with one MMBL domain (SwissProt accession no. P73139) is not shown. The domains of the MMBL domain-containing proteins are drawn to scale, and all Pfam matches are indicated. The known cleavage sites of the signal peptides are indicated by inverted solid triangles. The cleavage sites predicted by a SignalP search are indicated by inverted open triangles. aa, amino acids.
Phylogenetic analysis of the MMBL domains of LlpA.
The apparent similarity of LlpA to the superfamily consisting of monocot mannose-binding lectins was further explored by performing a molecular phylogenetic analysis of its MMBL domains. A multiple-sequence alignment of amino acids was constructed for the LlpA MMBL domains and the corresponding domains of the garlic lectin ASAI and the snowdrop (Galanthus nivalis) lectin GNA, as well as comitin, a chimeric mannose-specific lectin with actin-binding properties found in the slime mold Dictyostelium discoideum (27). Also included in this analysis was curculin, a sweet-tasting protein from lumbah (Curculigo latifolia), since it is evolutionarily related to the mannose-binding lectins yet is devoid of carbohydrate-binding activity (3). Additional lectin-like proteins containing one or more MMBL domains were retrieved from BLAST searches, which also included unfinished microbial genomes at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/cgi-bin/Entrez/genom_table_cgi).
Figure 3 shows that the MMBL domain is also found in combination with a variety of other domains. The 269-amino-acid hypothetical protein XAC0868 from Xanthomonas axonopodis pv. citri strain 306 apparently has a domain structure very similar to that of LlpA (11). A multiple-sequence alignment of this protein with LlpA and ASAI showed that this protein is also comprised of two dissimilar MMBL domains (for amino acids 29 to 156, no Pfam hits above the threshold levels; for amino acids 161 to 266, Pfam E value of 2.4 × 10−5) separated by an intervening 4-amino-acid sequence (Fig. 2). These domains exhibit only 16% amino acid identity, compared to levels of identity of 18 and 33% with the corresponding domains of LlpA. In addition, two of the three Trp residues conserved in the MMBL domains were also observed in the MMBL domains of the Xanthomonas LlpA-like protein.
Ralstonia metallidurans strain CH34 encodes an 852-amino-acid hypothetical protein with two apparent MMBL domains located in the C-terminal half of the protein. No homology to the N-terminal sequence of this protein was found. We also detected an ORF in Mycobacterium smegmatis strain mc2155 encoding a putative 219-amino-acid protein comprised of an obvious MMBL domain connected to a LysM domain by a proline-rich linker sequence. The LysM domain is one of the most common modules in bacterial cell surface proteins. It occurs most often in cell wall-degrading enzymes, in which it probably anchors the catalytic domains to their substrates (4). MMBL domains are also present in S-locus glycoproteins and S-locus receptor kinases of higher plants. S-locus glycoproteins and S-locus receptor kinase glycoproteins reside in the plant stigma as part of a self-incompatibility mechanism to prevent self-fertilization (67). SFR2 from cauliflower (Brassica oleracea), a representative S-locus receptor kinase, contains an obvious MMBL domain in its N-terminal half (28) (Fig. 3). However, to our knowledge, carbohydrate binding by the dicot proteins has not been reported so far. Additional bacterial MMBL domains were detected in a 413-kDa putative protein from the cyanobacterium Synechocystis sp. strain PCC 6803 (30) and in a putative secreted trypsin-like hydrolase from Streptomyces coelicolor 3A(2) (5). The large Synechocystis protein contains eight FG-GAP repeats typically found in the N-terminal part of alpha subunits of integrins (55). The MMBL domain is located between the fifth and sixth FG-GAP repeat.
Due to the occurrence of lectin domains in such functionally and structurally diverse proteins from unrelated organisms (Fig. 3), we were interested in performing a comparative analysis of the individual MMBL domains of LlpA. From a multiple-sequence alignment of MMBL_1 and MMBL_2 of LlpA with known or derived homologous MMBL domains, an unrooted phylogenetic tree was inferred by a neighbor-joining distance analysis (Fig. 3). The organization of the monocot mannose-binding lectin cluster was comparable to previously described tree topologies (60). Interestingly, the bacterial MMBL domains clearly cluster in a bacterial clade. To our surprise, this bacterial clade included the MMBL domain of a eukaryotic protein, namely, SFR2 of B. oleracea. It is noteworthy that the bacterial MMBL domains are distinguished by a lack of two highly conserved cysteine residues, one which is located within the first mannose-binding motif (QXDCNXVXY) and one which is near the second motif (Fig. 2). In GNA lectin from snowdrop, these cysteine residues are linked through a disulfide bridge (60). Interestingly, comitin and SFR2 also lack these conserved cysteine residues.
Expression of llpA confers bacteriocin activity in E. coli and P. fluorescens.
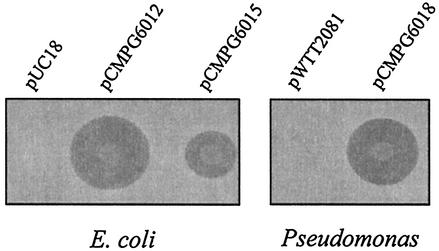
A 989-bp fragment containing llpA was amplified by high-fidelity PCR and expressed in E. coli under control of the lacZ promoter (pCMPG6012) and not under control of the lacZ promoter (pCMPG6015). The resulting E. coli transformants inhibited the growth of P. putida GR12-2R3 (Fig. 4). Inhibition zones were not observed on bacteriocin assay plates containing E. coli cells carrying pUC18, thus confirming that the inhibitory effect was due to expression of llpA. Comparison of the diameters of the inhibition zones surrounding E. coli cells expressing LlpA revealed enhanced LlpA production when llpA was properly orientated with respect to lacZ promoter pCMPG6012. To explore possible expression in other Pseudomonas species, the insert from pCMPG6012 was recloned into the E. coli-Pseudomonas shuttle vector pWTT2081. In this way, llpA was successfully expressed in P. fluorescens after electroporation of pCMPG6018 into P. fluorescens strains WCS365, SBW25, and OE 28.3. Bacteriocin plate assays showed that the inhibition zone around the putidacin-producing P. fluorescens cells carrying pCMPG6018 was comparable to or even slightly larger than the inhibition zone for strain BW11M1 (Fig. 4; data not shown for strains SBW25 and OE 28.3). Construct pCMPG6018 could not be used for complementation of CMPG2065, possibly due to the presence of an endogenous plasmid belonging to the same incompatibility group as pWTT2081, which is derived from the pVS1 replicon.
FIG. 4.
Bacteriocin plate assays of spotted Pseudomonas and E. coli cell suspensions with P. putida GR12-2R3 as the indicator strain. The bacterial strains tested for putidacin production were P. fluorescens WCS365(pWTT2081), P. fluorescens WCS365(pCMPG6018), E. coli TOP10F′(pUC18), E. coli TOP10F′(pCMPG6012), and E. coli TOP10F′(pCMPG6015). The diameters of the growth inhibition zones around bacteriocinogenic colonies were considered to be proportional to the amounts of LlpA produced by the strains tested.
Putidacin activity of renatured LlpA after SDS-PAGE.
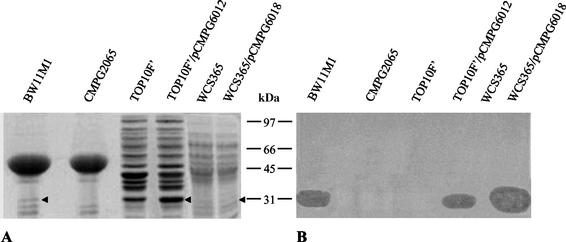
Culture supernatants of E. coli TOP10F′(pCMPG6012) and P. fluorescens WCS365(pCMPG6018) were collected and concentrated 50-fold by lyophilization. A protein with a molecular mass of ca. 30 kDa, the predicted size of LlpA, was visible after SDS-PAGE of supernatant proteins of Pseudomonas sp. strain BW11M1. This band was clearly absent in CMPG2065, due to the TnMod insertion in llpA (Fig. 5). In the case of heterologous expression of llpA in E. coli, a band with increased thickness was observed around 30 kDa, probably due to comigration of LlpA with an E. coli protein. The expected extra band in the SDS-PAGE profiles of protein samples from P. fluorescens WCS365 carrying pCMPG6018 was difficult to detect due to the presence of several protein bands of similar sizes in the supernatant of the wild type. The unusual stability of LlpA allowed us to detect putidacin inhibitory activity after an unstained SDS-PAGE gel was washed by applying a lawn of the indicator strain on top of it. This procedure revealed a clearing zone corresponding to the 30-kDa band previously observed in concentrated culture filtrate of strain BW11M1 and E. coli(pCMPG6012) (Fig. 5). This clearing zone was also visible in the case of the protein sample from P. fluorescens WCS365 containing pCMPG6018.
FIG. 5.
Separation of proteins extracted from 50-fold-concentrated culture filtrate resolved on an SDS-12.5% PAGE gel. The organisms used were Pseudomonas sp. strain BW11M1, an llpA mutant of strain BW11M1 (CMPG2065), E. coli TOP10F′, E. coli TOP10F′(pCMPG6012), P. fluorescens WCS365, and P. fluorescens WCS365(pCMPG6018). The arrowheads indicate the inferred position of LlpA. (A) Coomassie blue-stained gel. (B) Buffer-equilibrated gel overlaid with soft agar containing P. putida GR12-2R3. The positions of molecular mass markers (in kilodaltons) are indicated between the panels.
Secretion of LlpA does not require a cleavable signal sequence.
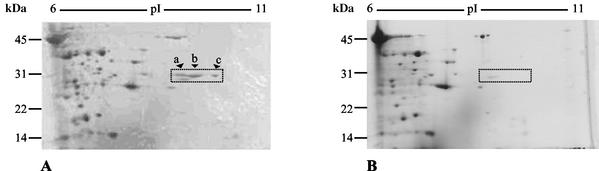
To confirm the predicted start site of llpA, we wanted to determine the N-terminal sequence of LlpA purified by two-dimensional PAGE. Unexpectedly, two-dimensional PAGE of a concentrated culture filtrate of Pseudomonas sp. strain BW1M11 revealed the presence of three closely spaced protein spots which were not present in a concentrated culture filtrate of mutant CMPG2065 (Fig. 6). All three of these spots had relative molecular masses corresponding to the molecular mass anticipated for LlpA, but they had slightly different pI values. The same three extra protein spots were visible on two-dimensional PAGE gels containing proteins from a concentrated culture filtrate of E. coli TOP10F′ cells carrying pCMPG6012 and were absent on two-dimensional PAGE gels containing proteins from TOP10F′ carrying pUC18 (data not shown). The N-terminal sequences of the three proteins were as follows: AG(R)T(R)IPFXXVXTX(V)(L) (spot a), AGRTRIPFNGVGTSVLP (spot b), and AGRTRI (spot c), where X is an unidentified residue and uncertain amino acids are in parentheses. The sequences obtained agree with translation of the llpA nucleotide sequence from the predicted ATG start site, with the N-terminal methionine removed. Since the initial yields obtained with the sequencer for all three protein spots were below the detection limit for the Coomassie blue-stained membranes, the major part of the protein is in all probability blocked by a formylated or acetylated methionine. Our findings indicate that the three proteins are isoforms encoded by the llpA gene. The nature of the apparent modifications that result in small changes in the pI is unknown. Similar observations have been reported for the E. coli proteome; for this proteome 18% of the spots identified on two-dimensional PAGE gels represented isoforms in which protein products of the same gene had different observed pI values and/or molecular masses (37).
FIG. 6.
Two-dimensional PAGE of proteins extracted from concentrated culture filtrates of Pseudomonas sp. strain BW11M1 (A) and an llpA mutant of strain BW11M1 (CMPG2065) (B). The arrowheads indicate the positions of three protein spots (spots a, b, and c) that were not detected in the culture supernatant of llpA mutant CMPG2065. Proteins a, b, and c were subjected to N-terminal amino acid sequencing. The positions of molecular mass markers (in kilodaltons) are indicated on the left.
Lack of mannose binding of LlpA.
We examined the possible mannose binding of LlpA by standard procedures used for studying mannose-binding lectins (10, 59). Rabbit erythrocytes are used in agglutination assays to assess the mannose binding of a novel lectin. However, when we combined a concentrated cell-free culture supernatant of strain BW11M1 with rabbit blood cells, no agglutination was observed. Another method to ascertain mannose binding relies on affinity chromatography. Concentrated protein samples from a supernatant of strain BW11M1 were mixed with a resin consisting of d-mannose attached to cross-linked agarose beads in the presence of ammonium sulfate. LlpA activity was detected in the wash fraction, indicating that LlpA was not capable of binding d-mannose.
Antibacterial spectrum of LlpA.
We investigated the bacteriocin activity of LlpA against several Pseudomonas strains belonging to all major groups of pseudomonads according to Moore et al. (40). Table 2 summarizes the results of a bacteriocin screening analysis in which we used 23 Pseudomonas strains other than P. putida GR12-2R3 as indicator strains. Growth inhibition was obvious for specific strains of Pseudomonas marginalis, Pseudomonas mendocina, Pseudomonas syringae, and Pseudomonas viridiflava. To verify that the observed inhibitory effects were due to the production of LlpA, we examined the sensitivities of the Pseudomonas indicator strains to the llpA mutant CMPG2065, as well as to LlpA-producing heterologous hosts (namely, E. coli and P. fluorescens strains WCS365, OE 28.3, and SBW25). In all cases, CMPG2065 did not inhibit the indicator strain or produced markedly reduced growth inhibition halos (P. marginalis), indicating that each of the indicator strains that is sensitive to Pseudomonas sp. strain BW11M1 is also sensitive to LlpA. However, strain BW11M1 is likely to produce at least one other bacteriocin which is active against P. putida KT2440 and LMG 2257. This was inferred from the inability of the heterologous hosts expressing llpA to inhibit the growth of these indicator strains (Table 2), in contrast to strain BW11M1, which was able kill these indicator organisms.
TABLE 2.
Antibacterial spectra of Pseudomonas sp. strain BW11M1, llpA mutant CMPG2065, and E. coli TOP10F′ expressing llpA against representative Pseudomonas indicator strains grouped as described by Moore et al.a
| Indicator strain | Growth inhibitionc by:
|
||
|---|---|---|---|
| BW11M1 | CMPG2065 | TOP10F′ (pCMPG6012)b | |
| P. aeruginosa lineage | |||
| P. aeruginosa PAO1 | − | − | − |
| P. stutzeri LMG 11199T | − | − | − |
| P. resinovorans lineage | |||
| P. resinovorans LMG 2274T | − | − | − |
| P. mendocina lineage | |||
| P. mendocina LMG 1223T | + | − | + |
| P. fluorescens lineage | |||
| P. aureofaciens LMG 1245T | − | − | − |
| P. chlororaphis LMG 5004T | − | − | − |
| P. fluorescens LMG 1794T | − | − | − |
| P. fluorescens F113 | − | − | − |
| P. fluorescens 2-79 | − | − | − |
| P. fluorescens OE 28.3 | − | − | − |
| P. fluorescens SBW25 | − | − | − |
| P. fluorescens WCS365 | − | − | − |
| P. marginalis LMG 2210 | + | +d | + |
| P. tolaasii LMG 2342T | − | − | − |
| P. viridiflava LMG 2352T | + | − | + |
| P. syringae lineage | |||
| P. syringae pv. glycinea LMG 5066 | + | − | + |
| P. syringae pv. syringae LMG 1247T | + | − | + |
| P. syringae pv. tabaci LMG 5192 | + | − | + |
| P. syringae pv. tomato DC3000 | − | − | − |
| P. cichorii lineage | |||
| P. cichorii LMG 2162T | − | − | − |
| P. putida lineage | |||
| P. putida GR12-2R3 | + | − | + |
| P. putida LMG 2257T | + | + | − |
| P. putida KT2440 | + | + | − |
| P. agarici lineage | |||
| P. agarici LMG 2112T | − | − | − |
| Other | |||
| P. fuscovaginae HPB 2646 | − | − | − |
See reference 40.
Pseudomonas hosts carrying pCMPG6018 display an identical antibacterial spectrum.
−, no growth inhibition; +, growth inhibition.
The diameter of the inhibition halo was reduced.
DISCUSSION
Several strains of fluorescent Pseudomonas spp. isolated from roots of tropical (banana, rice) and temperate (wheat, maize) crops have been reported to produce one or more types of bacteriocins with relatively narrow activity spectra (44). One of these strains, strain BW11M1 isolated from a banana rhizosphere in Sri Lanka, is characterized by strong antibacterial activity against P. putida GR12-2R3. This organism was preliminarily classified as P. fluorescens (63), but we reassigned it to the P. putida lineage on the basis of 16S rRNA phylogeny (40). Initial experiments suggested that the antibacterial compound that is produced by Pseudomonas sp. strain BW11M1 and is active against strain GR12-2R3 may represent a novel type of Pseudomonas bacteriocin, which is considerably smaller than the S-type pyocins of P. aeruginosa (65 to 80 kDa). So far, pyocins are the only Pseudomonas bacteriocins that have been characterized genetically. In this study, we identified and characterized the structural gene llpA, encoding a novel type of bacteriocin. LlpA is a 30-kDa protein consisting of two MMBL-like domains connected by a short linker sequence. It exhibits significant homology to mannose-binding lectins found in monocotyledonous plants, and ASAI lectin from garlic is the most significant match. Previously, these monocot mannose-binding lectins have been found in at least 10 different plant families. Because of the abundance of lectins in storage organs, such as bulbs, tubers, rhizomes, and roots, it is believed that plants accumulate lectins as possible nitrogen reserves (46). A number of lectins, including GNA from G. nivalis, have been shown to exhibit insecticidal effects on sucking insects (60). Antifungal activity has been reported for a mannose-binding lectin isolated from corm of the orchid Gastrodia elata (64). In general, it appears that mannose-binding lectins isolated from leaves are likely to fulfill a specific role as plant protectants, whereas the abundant bulb-specific lectins are regarded as vegetative storage proteins. Despite its significant homology to mannose-binding lectins, LlpA does not appear to bind mannose, which is one of the properties of curculin, a lectin-related protein from fruits of C. latifolia (3). However, at this stage we cannot exclude the possibility that there is carbohydrate-binding activity with mannose oligomers or other complex derivatives.
Precursor proteins of monocot mannose-binding lectins have one or two MMBL domains. Precursors with two MMBL domains are usually processed into two subunits with molecular masses of 11 to 14 kDa. However, in some lectins, like SCAfet from bluebell (Scilla campanulata), the two MMBL domains are not proteolytically separated (68). Tandem MMBL domains can be very similar (ASAI from garlic) or dissimilar. Two dissimilar MMBL domains were detected in LlpA.
To our surprise, genome analysis revealed the presence of MMBL domains in several other, mostly uncharacterized, bacterial proteins. Most interesting is the presence of a structural llpA homologue in the genome of X. axonopodis, the causative agent of citrus canker. A functional llpA homologue (inh1) may be present in R. albus strain 7. Apparently, inh1 encodes a heat-labile bacteriocin with a single MMBL domain. This bacteriocin suppresses the growth of another ruminal species, R. flavescens (J. Chen, D. M. Stevenson, and P. J. Weimar, unpublished data). The production of a bacteriocin-like inhibitor by a ruminal cellulolytic bacterium was first reported for R. albus strain 8 (42). This bacteriocin-like inhibitory substance has not been characterized at the molecular level, impeding confirmation that it is a true bacteriocin. However, we detected two homologues of inh1 in unfinished genomic sequences of R. albus strain 8. inh1 and its two homologues are predicted to encode very similar proteins exhibiting more than 70% sequence identity and comprising a single N-terminal MMBL domain fused to a C-terminal region having an unknown function. Bacteriocins produced by ruminococcal bacteria are said to be part of the survival strategy of ruminal bacteria conferring competitive fitness in the ruminal environment (9). Inh1 and most other MMBL domain proteins of bacterial origin carry a potential N-terminal signal sequence that may direct these proteins to the extracellular environment. However, such a signal sequence appears to be lacking in LlpA and also was not detected in the MMBL-like protein from M. smegmatis. Despite the fact that bacterial proteins carrying MMBL domains have diverse domain structures and mostly unknown functions, a phylogenetic analysis indicated that bacterial MMBL domains clearly cluster. The MMBL domains in LlpA from strain BW11M1 exhibit the greatest similarity to the corresponding MMBL domains detected in an unknown ORF of the phylogenetically related organism X. axonopodis pv. citri. This similarity suggests that the predicted protein, XAC0868, may possess bacteriocin activity as well. This ORF is not present in the Xanthomonas campestris pv. campestris genome (11).
Although LlpA is an entirely novel type of Pseudomonas bacteriocin, it shares several traits with previously characterized S pyocins of P. aeruginosa, including (i) a relatively narrow activity spectrum within the genus Pseudomonas, (ii) sensitivity to heat and nonspecific proteases, (iii) enhanced production following DNA-damaging treatment, (iv) secretion without a cleavable N-terminal sequence, (v) genetic determinants located on the chromosome (in contrast to many other bacteriocins of plasmid origin), and (vi) heterologous expression and secretion in E. coli. However, S pyocins, as well as their counterparts in E. coli (colicins), are each produced as a complex of two proteins, a large protein (65 to 80 kDa) responsible for the killing activity and a small, highly specific immunity protein (about 10 kDa) which is cosynthesized with the cognate bacteriocin. The immunity protein binds very tightly to the cytotoxic domain of the bacteriocin, ensuring that the toxin is inactive inside the producer cell (31). The pyocin phenotype is encoded by a two-gene operon consisting of the kill and imm genes. Such a highly specific self-protection mechanism seems to be absent in strain BW11M1. No immunity gene has been found in the immediate vicinity of the llpA structural gene. Furthermore, llpA can be expressed in E. coli and other Pseudomonas species, suggesting that putidacin exerts no toxic effect within the producer cells. We were unable to express llpA in the sensitive organism P. putida GR12-2R3. This does not necessarily imply that an immunity protein is necessary. It may well be that LlpA's toxic action is dependent on interaction with the cell envelope surface of the sensitive target cell. Thus, GR12-2R3 cells expressing putidacin would be killed not by intracellular LlpA but by the secreted protein, resulting in suicide.
Plant-growth-promoting pseudomonads can suppress plant diseases by direct antagonism between the bacteria and soilborne pathogens, by iron depletion through the production of siderophores, or by induction of systemic resistance (7). The use of fluorescent pseudomonads to improve crop yields has enormous potential, but to date attempts to do this have had only limited success. This is due in part to a lack of understanding of the population dynamics in the environment. If plant-growth-promoting pseudomonads are to work successfully as inoculants, it is crucial to understand how these inoculants compete with the indigenous rhizosphere population. We have previously explored the potential of plant-associated P. fluorescens and P. putida to produce a large variety of pyocin S-like bacteriocins with nuclease activity (45). The present study revealed yet another type of Pseudomonas bacteriocin, which most likely has a distinct mode of action. It is tempting to speculate that LlpA interacts with sensitive bacterial cells by binding to a specific, possibly mannosylated outer membrane receptor, resulting in cell death through an unknown mechanism. The high incidence and anticipated diverse range of activities of P. putida and P. fluorescens bacteriocins imply that these compounds play a significant role in rhizosphere population dynamics.
Acknowledgments
A.H.A.P. is indebted to the Instituut voor de Aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen (IWT) for a predoctoral fellowship. P.P. received a postdoctoral fellowship from the Fund for Scientific Research (FWO-Vlaanderen).
We thank Els Van Damme for helpful discussions and for assistance with the agglutination tests. We are also grateful to Annabelle Zgoda for providing a protocol for the in-gel detection procedure. Preliminary sequence data were obtained from the DOE Joint Genome Institute for R. metallidurans strain CH34 (http://www.jgi.doe.gov/JGI_microbial/html/ralstonia/ralston_homepage.html database version 19/06/2002), and from the Institute for Genomic Research web site for R. albus strain 8 and M. smegmatis strain mc2155 (http://www.tigr.org/; database version 07/05/2002). We thank B. Lugtenberg and P. Rainey for providing cultures of P. fluorescens WCS365 and SBW25, respectively.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barre, A., Y. Bourne, E. J. M. Van Damme, W. J. Peumans, and P. Rougé. 2001. Mannose-binding plant lectins: different structural scaffolds for a common sugar-recognition process. Biochimie 83:645-651. [DOI] [PubMed] [Google Scholar]
- 3.Barre, A., E. J. M. Van Damme, W. J. Peumans, and P. Rougé. 1997. Curculin, a sweet-tasting and taste-modifying protein, is a non-functional mannose-binding lectin. Plant. Mol. Biol. 33:691-698. [DOI] [PubMed] [Google Scholar]
- 4.Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113-1119. [DOI] [PubMed] [Google Scholar]
- 5.Bentley, S. D., K. F. Chater, A. M. Cerdeño-Tárraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C.-H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M.-A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 6.Besemer, J., and M. Borodovsky. 1999. Heuristic approach to deriving models for gene finding. Nucleic Acids Res. 27:3911-3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloemberg, G., and B. Lugtenberg. 2001. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 4:343-350. [DOI] [PubMed] [Google Scholar]
- 8.Chandra, N. R., G. Ramachandraiah, K. Bachhawat, T. K. Dam, A. Surolia, and M. Vijayan. 1999. Crystal structure of a dimeric mannose-specific agglutinin from garlic: quaternary association and carbohydrate specificity. J. Mol. Biol. 285:1157-1168. [DOI] [PubMed] [Google Scholar]
- 9.Chen, J., and P. J. Weimar. 2001. Competition among three predominant ruminal cellulolytic bacteria in the absence or presence of non-cellulolytic bacteria. Microbiology 147:21-30. [DOI] [PubMed] [Google Scholar]
- 10.Dam, T. K., K. Bachhawat, P. G. Rani, and A. Surolia. 1998. Garlic (Allium sativum) lectins bind to high mannose oligosaccharide chains. J. Biol. Chem. 273:5528-5535. [DOI] [PubMed] [Google Scholar]
- 11.da Silva, A. C. R., J. A. Ferro, F. C. Reinach, C. S. Farah, L. R. Furlan, R. B. Quaggio, C. B. Monteiro-Vitorello, M. A. Van Sluys, N. F. Almeida, L. M. C. Alves, A. M. do Amaral, M. C. Bertolini, L. E. A. Camargo, G. Camarotte, F. Cannavan, J. Cardozo, F. Chambergo, L. P. Ciapina, R. M. B. Cicarelli, L. L. Coutinho, J. R. Cursino-Santos, H. El-Dorry, J. B. Faria, A. J. S. Ferreira, R. C. C. Ferreira, M. I. T. Ferro, E. F. Formighieri, M. C. Franco, C. C. Greggio, A. Gruber, A. M. Katsuyama, L. T. Kishi, R. P. Leite, E. G. M. Lemos, M. V. F. Lemos, E. C. Locali, M. A. Machado, A. M. B. N. Madeira, N. M. Martinez-Rossi, E. C. Martins, J. Meidanis, C. F. M. Menck, C. Y. Miyaki, D. H. Moon, L. M. Moreira, M. T. M. Novo, V. K. Okura, M. C. Oliveira, V. R. Oliveira, H. A. Pereira, A. Rossi, J. A. D. Sena, C. Silva, R. F. de Souza, L. A. F. Spinola, M. A. Takita, R. E. Tamura, E. C. Teixeira, R. I. D. Tezza, M. Trindade dos Santos, D. Truffi, S. M. Tsai, F. F. White, J. C. Setubal, and J. P. Kitajima. 2002. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459-463. [DOI] [PubMed] [Google Scholar]
- 12.De Mot, R., and J. Vanderleyden. 1989. Application of two-dimensional protein analysis for strain fingerprinting and mutant analysis of Azospirillum species. Can. J. Microbiol. 35:960-967. [Google Scholar]
- 13.De Mot, R., and J. Vanderleyden. 1991. Purification of root-adhesive outer membrane protein of root-colonizing Pseudomonas fluorescens. FEMS Microbiol. Lett. 81:323-328. [Google Scholar]
- 14.Dennis, J. J., and G. J. Zylstra. 1998. Plasposons: modular self-cloning minitransposon derivatives for rapid genetic analysis of gram-negative bacterial genomes. Appl. Environ. Microbiol. 64:2710-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duport, C., C. Baysse, and Y. Michel-Briand. 1995. Molecular characterization of pyocin S3, a novel S-type pyocin from Pseudomonas aeruginosa. J. Biol. Chem. 270:8920-8927. [DOI] [PubMed] [Google Scholar]
- 16.Farinha, M. A., and A. M. Kropinsky. 1990. High efficiency electroporation of Pseudomonas aeruginosa using frozen cell suspensions. FEMS Microbiol. Lett. 70:221-226. [DOI] [PubMed] [Google Scholar]
- 17.Felsenstein, J. 1995. Confidence limits on phylogenies: an approach using bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 18.Franklin, F. C., M. Bagdasarian, M. M. Bagdasarian, and K. N. Timmis. 1981. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc. Natl. Acad. Sci. USA 78:7458-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fyfe, J. A. M., G. Harris, and J. R. W. Govan. 1984. Revised pyocin typing method for Pseudomonas aeruginosa. J. Clin. Microbiol. 20:47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geels, F. P., and B. Schippers. 1983. Selection of antagonistic fluorescent Pseudomonas spp. and their root colonization and persistence following treatment of seed potatoes. Phytopathol. Z. 108:193-206. [Google Scholar]
- 21.Hamon, Y. 1956. Contribution à l'étude des pyocins. Ann. Inst. Pasteur 91:81-90. [PubMed] [Google Scholar]
- 22.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubert, E., O. Lobos, P. Brevis, and C. Padilla. 1998. Purification and characterization of the bacteriocin PsVP-10 produced by Pseudomonas sp. J. Appl. Microbiol. 84:910-913. [DOI] [PubMed] [Google Scholar]
- 24.Iacobellis, N. S., A. M. Contesini, and G. Surico. 1995. Bacteriocin production by Pseudomonas syringae subsp. savastanoi. Phytopathol. Mediterr. 34:15-22. [Google Scholar]
- 25.James, R., C. Kleanthous, and G. R. Moore. 1996. The biology of E colicins: paradigms and paradoxes. Microbiology 142:1569-1580. [DOI] [PubMed] [Google Scholar]
- 26.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism, vol. 3. Academic Press, New York, N.Y.
- 27.Jung, E., P. Fucini, M. Stewart, A. A. Noegel, and M. Schleicher. 1996. Linking microfilaments to intracellular membranes: the actin-binding and vesicle-associated protein comitin exhibits a mannose-specific lectin activity. EMBO J. 15:1238-1246. [PMC free article] [PubMed] [Google Scholar]
- 28.Kai, N., G. Suzuki, M. Watanabe, A. Isogai, and K. Hinata. 2001. Sequence comparisons among dispersed members of the Brassica S multigene family in an S9 genome. Mol. Gen. Genet. 265:526-534. [DOI] [PubMed] [Google Scholar]
- 29.Kairu, G. M. 1997. Biochemical and pathogenic differences between Kenyan and Brazilian isolates of Pseudomonas syringae pv. garcae. Plant Pathol. 46:239-246. [Google Scholar]
- 30.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109-136. [DOI] [PubMed] [Google Scholar]
- 31.Kleanthous, C., and D. Walker. 2001. Immunity proteins: enzyme inhibitors that avoid the active site. Trends Biochem. Sci. 26:624-631. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Laue, B. E., Y. Jiang, S. R. Chhabra, S. Jacob, G. S. Stewart, A. Hardman, J. A. Downie, F. O'Gara, and P. Williams. 2000. The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N-(3-hydroxy-7-cis-tetradecenoyl)homoserine lactone, via HdtS, a putative novel N-acylhomoserine lactone synthase. Microbiology 146:2469-2480. [DOI] [PubMed] [Google Scholar]
- 34.Lavermicocca, P., S. L. Lonigro, F. Valerio, A. Evidente, and A. Visconti. 2002. Reduction of olive knot disease by a bacteriocin from Pseudomonas syringae pv. ciccaronei. Appl. Environ. Microbiol. 68:1403-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lepeuple, A.-S., E. Van Gemert, and M.-P. Chapot-Chartier. 1998. Analysis of the bacteriolytic enzymes of the autolytic Lactococcus lactis subsp. cremoris strain AM2 by renaturing polyacrylamide gel electrophoresis: identitification of a prophage-encoded enzyme. Appl. Environ. Microbiol. 64:4142-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lifshitz, R., J. W. Kloepper, M. Kozlowski, C. Simonson, J. Carlson, E. M. Tipping, and I. Zaleska. 1987. Growth promotion of canola (rapeseed) seedlings by a strain of Pseudomonas putida under gnotobiotic conditions. Can. J. Microbiol. 33:390-395. [Google Scholar]
- 37.Link, A. J., K. Robison, and G. M. Church. 1997. Comparing the predicted and observed properties of proteins encoded in the genome of Escherichia coli K12. Electrophoresis 18:1259-1313. [DOI] [PubMed] [Google Scholar]
- 38.Matsui, H., Y. Sano, H. Ishihara, and T. Shinomiya. 1993. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. J. Bacteriol. 175:1257-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Moore, E. R. B., M. Mau, A. Arnscheidt, E. C. Böttger, R. A. Hutson, M. D. Collins, Y. Van de Peer, R. De Wachter, and K. N. Timmis. 1996. The determination and comparison of the 16S rRNA gene sequences of species of the genus Pseudomonas (sensu stricto) and estimation of the natural intrageneric relationships. Syst. Appl. Microbiol. 19:478-492. [Google Scholar]
- 41.Nakayama, K. 2000. The R-type pyocin of Pseudomonas aeruginosa is related to P2 phage, and the F-type is related to lambda phage. Mol. Microbiol. 38:213-231. [DOI] [PubMed] [Google Scholar]
- 42.Odenyo, A. A., R. I. Mackie, D. A. Stahl, and B. A. White. 1994. The use of 16S rRNA-targeted oligonucleotide probes to study competition between ruminal fibrolytic bacteria: development of oligonucleotide probes for Ruminococcus species and evidence for bacteriocin production. Appl. Environ. Microbiol. 60:3688-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padilla, C., M. Salazar, and O. Faúndez. 1992. Range of action and genetic bacteriocin codification of Pseudomonas aeruginosa isolated from three different ecological niches. J. Appl. Bacteriol. 73:497-500. [DOI] [PubMed] [Google Scholar]
- 44.Parret, A. H. A., and R. De Mot. 17 October 2000, release date. Bacteriocin production by rhizosphere-colonizing fluorescent Pseudomonas. In Proceedings of the 5th International PGPR Workshop. Auburn University Web Site [Online.] http://www.ag.auburn.edu/argentina/pdfmanuscripts/parret.pdf.
- 45.Parret, A. H. A., and R. De Mot. 2002. Bacteria killing their own kind: novel bacteriocins of Pseudomonas and other γ-proteobacteria. Trends Microbiol. 10:107-112. [DOI] [PubMed] [Google Scholar]
- 46.Peumans, W. J., and E. J. M. Van Damme. 1995. Lectins as plant defense proteins. Plant Physiol. 109:347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rainey, P. B., and M. J. Bailey. 1996. Physical and genetic map of the Pseudomonas fluorescens SBW25 chromosome. Mol. Microbiol. 19:521-533. [DOI] [PubMed] [Google Scholar]
- 48.Ramachandraiah, G., N. R. Chandra, A. Surolia, and M. Vijayan. 2002. Re-refinement using processed data to improve the quality of the structure: a case study involving garlic lectin. Acta Crystallogr. D Biol. Crystallogr. 58:414-420. [DOI] [PubMed] [Google Scholar]
- 49.Riley, M. A. 1998. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 32:255-278. [DOI] [PubMed] [Google Scholar]
- 50.Saitou, N., and M. Nei. 1987. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 51.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 52.Shanahan, P., D. J. O'Sullivan, P. Simpson, J. D. Glennon, and F. O'Gara. 1992. Isolation and characterization of an antibiotic-like compound from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl. Environ. Microbiol. 58:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smidt, M. L., and A. K. Vidaver. 1982. Bacteriocin production by Pseudomonas syringae PsW-1 in plant tissue. Can. J. Microbiol. 28:600-604. [DOI] [PubMed] [Google Scholar]
- 54.Spencker, F. B., S. Haupt, M. C. Claros, S. Walter, T. Lietz, R. Schille, and A. C. Rodloff. 2000. Epidemiologic characterization of Pseudomonas aeruginosa in patients with cystic fibrosis. Clin. Microbiol. Infect. 6:600-607. [DOI] [PubMed] [Google Scholar]
- 55.Springer, T. 1997. Folding of the N-terminal, ligand-binding region of integrin α-subunits into a β-propeller domain. Proc. Natl. Acad. USA 94:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, S. K. Folger, A. Kas, K. Larbigß, R. Lim, K. Smith, D. Spencer, G. K.-S. Wong, Z. Wu, I. T. Paulsenk, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 57.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vachée, A., D. A. A. Mossel, and H. Leclerc. 1997. Antimicrobial activity among Pseudomonas and related strains of mineral water origin. J. Appl. Microbiol. 83:652-658. [DOI] [PubMed] [Google Scholar]
- 59.Van Damme, E. J. M., C. H. Astoul, A. Barre, P. Rougé, and W. J. Peumans. 2000. Cloning and characterization of a monocot mannose-binding lectin from Crocus vernus (family Iridaceae). Eur. J. Biochem. 267:5067-5077. [DOI] [PubMed] [Google Scholar]
- 60.Van Damme, E. J. M., W. J. Peumans, A. Barre, and P. Rougé. 1998. Plant lectins: a composite of several distinct families of structurally and evolutionarily related proteins with diverse biological roles. Crit. Rev. Plant Sci. 17:575-692. [Google Scholar]
- 61.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Appl. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 62.Van der Bij, A. J., L. A. de Weger, W. T. Tucker, and B. J. J. Lugtenberg. 1996. Plasmid stability in Pseudomonas fluorescens in the rhizosphere. Appl. Environ. Microbiol. 62:1076-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlassak, K., L. Van Holm, L. Duchateau, J. Vanderleyden, and R. De Mot. 1992. Isolation and characterization of fluorescent Pseudomonas associated with the roots of rice and banana grown in Sri Lanka. Plant Soil 145:51-63. [Google Scholar]
- 64.Wang, X., G. Bauw, E. J. M. Van Damme, W. J. Peumans, Z.-L. Chen, M. Van Montagu, G. Angenon, and W. Dillen. 2001. Gastrodianin-like mannose-binding proteins: a novel class of plant proteins with antifungal properties. Plant J. 25:651-661. [DOI] [PubMed] [Google Scholar]
- 65.Weller, D. M., and R. J. Cook. 1983. Suppression of Take-all of wheat by seed treatments with fluorescent pseudomonads. Phytopathology 73:463-469. [Google Scholar]
- 66.Whalen, M. C., R. W. Innes, A. F. Bent, and B. J. Staskawicz. 1991. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 3:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wheeler, M. J. 2001. The molecular and genetic basis of pollen-pistil interactions. New Phytol. 151:565-584. [DOI] [PubMed] [Google Scholar]
- 68.Wright, L. M., E. J. M. Van Damme, A. Barre, A. K. Allen, F. Van Leuven, C. D. Reynolds, P. Rougé, and W. J. Peumans. 1999. Isolation, characterization, molecular cloning and molecular modelling of two lectins of different specificities from bluebell (Scilla campanulata) bulbs. Biochem. J. 340:299-308. [PMC free article] [PubMed] [Google Scholar]
- 69.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]