Abstract
So far, the extremely halophilic archaeon Haloferax volcanii has the best genetic tools among the archaea. However, the lack of an efficient gene knockout system for this organism has hampered further genetic studies. In this paper we describe the development of pyrE-based positive selection and counterselection systems to generate an efficient gene knockout system. The H. volacanii pyrE1 and pyrE2 genes were isolated, and the pyrE2 gene was shown to code for the physiological enzyme orotate phosphoribosyl transferase. A ΔpyrE2 strain was constructed and used to isolate deletion mutants by the following two steps: (i) integration of a nonreplicative plasmid carrying both the pyrE2 wild-type gene, as a selectable marker, and a cloned chromosomal DNA fragment containing a deletion in the desired gene; and (ii) excision of the integrated plasmid after selection with 5-fluoroorotic acid. Application of this gene knockout system is described.
The archaea represent one of the three fundamental divisions of life (24). Archaea have features that are present in both the eukaryotic and prokaryotic kingdoms, and this fact has been very important in understanding the evolution of cellular processes. Thus, archaeal transcription and translation can be envisaged as a mosaic of eukaryotic and bacterial elements (1). While the archaeal basal transcription machinery resembles that of RNA polymerase II of eukaryotes, the regulation of gene expression has many of the characteristics of bacterial systems (2). Particularly interesting is the fact that most of the known archaea are extremophiles, and studies of the structure-function relationships in archaeal proteins have provided valuable insights into the mechanisms that enable biochemical systems to adapt and function in extreme physiological conditions (9).
The extremely halophilic archaeon Haloferax volcanii is an obligate halophile that was first isolated from the Dead Sea (13). H. volcanii is a genetically stable prototroph that has become a model organism for molecular genetic studies of the archaea (6, 21, 25). The presence in H. volcanii of an efficient transformation system (5), several shuttle vectors (7, 10), and selectable markers (8, 15) has made a wide variety of molecular genetic studies possible. However, a key tool for genetic analysis, namely, the availability of an efficient gene knockout system, has been lacking. One important tool for the creation of gene knockouts is a counterselectable genetic marker. The counterselectable markers commonly used in bacteria include the Bacillus subtilis sacB gene, which encodes levan sucrase and confers sensitivity of many bacteria to sucrose (19), and the glkA gene, which encodes glucose kinase and confers sensitivity of Streptomyces to 2-deoxyglucose (23). In Saccharomyces cerevisiae, genes involved in uracil biosynthesis serve as effective counterselectable markers (3). The selection takes advantage of the fact that S. cerevisiae that can synthesize uracil de novo is sensitive to the toxic analogue 5-fluoroorotic acid (5-FOA), whereas mutations in the ura5 gene, which encodes orotate phosphoribosyl transferase (OPRTase), or the ura3 gene, which encodes orotidine-5′-phosphate decarboxylase, are resistant to 5-FOA. In bacteria the genes corresponding to ura5 and ura3 are pyrE and pyrF, respectively.
In this report we describe the isolation of two H. volcanii genes whose products exhibit homology to OPRTases and demonstrate that the pyrE2 gene codes for the physiological enzyme. Previously (16), other workers have employed the pyrF gene of Halobacterium salinarum as a counterselectable genetic marker for creating gene knockouts. Here we show that the H. volcanii pyrE2 gene can conveniently serve both as a selectable genetic marker and as a counterselectable genetic marker for efficiently creating gene knockouts in H. volcanii.
MATERIALS AND METHODS
Strains and culture conditions.
The properties of the various H. volcanii strains used in this work are given in Table 1. H. volcanii was routinely grown in rich (HY) medium containing (per liter) 206 g of NaCl, 36.9 g of MgSO4 · 7H2O, 5 ml of a 1 M KCl solution, 1.8 ml of a 75-mg/liter MnCl2 solution, and 50 mM Tris HCl (pH 7.2). After autoclaving and cooling, 5 ml of 10% (wt/vol) CaCl2 and 25 ml of filter-sterilized 20% (wt/vol) yeast extract (Difco) were added. Agar plates contained 18 g of Bacto Agar (Difco) per liter. For uracil-minus medium (CA medium), the yeast extract was replaced by Casamino Acids (Difco) at the same final concentration (0.5%, wt/vol). When needed, 5-FOA (US Biological) was added to HY medium to a final concentration of 150 μg/ml.
TABLE 1.
H. volcanii strains and plasmids used in this investigation
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| WR340 | His− | —a |
| WR341 | Cys− | — |
| WR445 | WR341/ΔhdrA ΔhdrB | 15 |
| WR472 | WR445 containing pGB53 (pop-in) | This study |
| WR473 | WR445/ΔpyrE1 | This study |
| WR479 | WR445/ΔpyrE1 ΔpyrE2 | This study |
| WR480 | WR340/ΔpyrE2 | This study |
| WR501 | WR480 containing pGB72 (pop-in) | This study |
| WR504 | WR480/Δcmi4 | This study |
| Plasmids | ||
| pGB53 | pBR-Nov containing the flanking sequences of pyrE1 | This study |
| pGB68 | pBR-Nov containing the flanking sequences of pyrE2 | This study |
| pGB70 | pUC19 containing the pyrE2 coding region under the ferredoxin promoter | This study |
| pGB72 | pGB70 containing the flanking sequences of cmi4 | This study |
| pBR-Nov | pBR containing the halobacterial novobiocin resistance gene gyrB | 15 |
Escherichia coli strains DH5α, XLI, and DH12S were grown in Luria-Bertani medium. When needed, ampicillin (Sigma) was added to the medium to a final concentration of 100 μg/ml.
Transformation procedures.
Transformation of halobacteria was carried out as previously described (5). E. coli was transformed by using the CaCl2 protocol (11) or a standard electroporation protocol.
Molecular genetic methods.
Restriction endonuclease digestion, agarose gel electrophoresis, and molecular cloning techniques were performed by using standard procedures (11). Isolation of total halobacterial DNA was performed as previously described (20). The oligodeoxynucleotide primers used in this study are shown in Table 2.
TABLE 2.
Oligodeoxynucleotide primers used in this study
| Primer | Sequence | Location |
|---|---|---|
| pyrE1-Rev | 5′-CGGGATCCGTTCTTCATACAGTACC | 5′ end of pyrE1; reverse primer |
| 1.4 us pyrE1 | 5′-CCATCGATGTCGACGACATCGAG | 1.4-kb upstream to pyrE1; forward primer |
| pyrE1-M | 5′-CGGGATCCCCCGCTTGCGACCTCCGTCTC | Middle of the pyrE1 ORF; forward primer |
| ds pyrE1 | 5′-GCGGTACCGTTGTCGCGCCAGTTGATTCCG | 1.1-kb downstream to pyrE1; reverse primer |
| 850 us pyrE2 | 5′-GGGGTACCGGGGCCTCTAATCGACGTAGGC | 850 bp upstream to pyrE2; forward primer |
| us pyrE2-Rev | 5′-CGGGATCCGCGTGGATTACCACGGCTCG | 5′ end of pyrE2; reverse primer |
| ds pyrE2 | 5′-CGGGATCCCGCCGACGGCTAATACACGC | 3′ end of pyrE2; forward primer |
| 850 ds pyrE2 Rev | 5′-GCCAAGCTTGTGCCTATTTCTACGTCACC | 850-bp downstream to pyrE2; reverse primer |
| N-pyrE2 (H. volcanii) | 5′-CGGCCATGGCGAACGCAGCACTCATCG | 5′ end of pyrE2 from H. volcanii; forward primer |
| C-pyrE2 (H. volcanii) | 5′-GCTCTAGATTAGCCGTCGGCGTCGGCCAGC | 3′ end of pyrE2 from H. volcanii; reverse primer |
| N-salpyrE2 | 5′-ATGAGTCCAACTGACGACCT | 5′ end of pyrE2 from H. salinarum; forward primer |
| C-salpyrE2 | 5′-TTACTCGGCGTCCAAGAGGTC | 3′ end of pyrE2 from H. salinarum; reverse primer |
| 300 us cmi4 | 5′-CGGAATTCATCGTCACCGGAATCGGTGAG | 300 bp upstream to cmi4; forward primer |
| us cmi4-Rev | 5′-CGCCCATGGCCGGAACGTGGCACGCCC | 5′ end of cmi4; reverse primer |
| ds cmi4 | 5′-CGCCCATGGACGACGACCTCTCGAACCGG | 3′ end of cmi4; forward primer |
| ds cmi4-Rev | 5′-CGCGGGGAGGTCGAACAGGC | Downstream to cmi4; reverse primer |
| 415 us cmi4 | 5′-AACACCGGCGCGCCGAGACC | 415-bp upstream to cmi4; forward primer |
(i) Southern blot analysis.
The nucleotide sequence of Halobacterium sp. strain NRC-1 pyrE2 was obtained from the complete genome sequence (14) (accession no. NC 002607). The H. salinarum S9 pyrE2 gene was obtained by PCR amplification of the chromosomal DNA of this archaeon (obtained from Felicitas Pfeifer, Technical University, Darmstadt, Germany) by using the N-salpyrE2 and C-salpyrE2 primers (Table 2). Hybridization probes were labeled with a digoxigenin (DIG) DNA labeling kit (Roche Diagnostics GmbH, Mannheim, Germany). The standard hybridization buffer contained 0.6 M NaCl, 0.06 M sodium citrate, 0.1% N-laurylsarcosine, 0.02% sodium dodecyl sulfate, 2% blocking reagent (Roche Diagnostics GmbH), and 50% (vol/vol) formamide. Hybridization was performed at 42°C. Chemiluminescent detection of the labeled fragments was performed as described in the instructions to a DIG luminescence detection kit (Roche Diagnostics GmbH).
(ii) DNA sequence analysis.
Nucleotide sequences of cloned fragments were determined by using an ABI373 automated sequencer (Perkin-Elmer ABI) as described by the supplier. DNA database searches were performed by using the National Center for Biotechnology Information Blast web site (http://www.ncbi.nlm.nih.gov/BLAST/). Multiple-sequence alignments were constructed by using the ClustalW program (22) on a web server (http://www.ch.embnet.org/software/ClustalW.html).
Plasmid construction.
The plasmids used in this work are shown in Table 1, and the oligodeoxynucleotide PCR primers used are shown in Table 2.
(i) pGB53, used for creating a deletion in the pyrE1 gene.
A 1.4-kb DNA fragment containing the upstream flanking sequence of the H. volcanii pyrE1 gene was amplified by PCR by using primers pyrE1-Rev and 1.4 us pyrE1, which contain BamHI and ClaI restriction sites, respectively. A 1.4-kb DNA fragment containing 300 bp of the 3′ coding region of H. volcanii pyrE1 and 1.1 kb of the downstream sequence was amplified by PCR by using primers pyrE1-M and ds pyrE1, which contain BamHI and Asp718 restriction sites, respectively. The two PCR products were cloned by triple ligation into pBR-Nov digested with ClaI and Asp718.
(ii) pGB68, used for pyrE2 gene deletion.
An 850-bp fragment upstream from the first codon of the H. volcanii pyrE2 gene was amplified by PCR with primers 850 us pyrE2 and us pyrE2-Rev, which contain Asp718 and BamHI restriction sites, respectively. Similarly, an 850-bp fragment containing the downstream flanking sequence of this gene was amplified by PCR with primers 850 ds pyrE2-Rev and ds pyrE2, which contain HindIII and BamHI restriction sites. The two fragments were cloned by triple ligation into pBR-Nov digested with HindIII and Asp718.
(iii) pGB70.
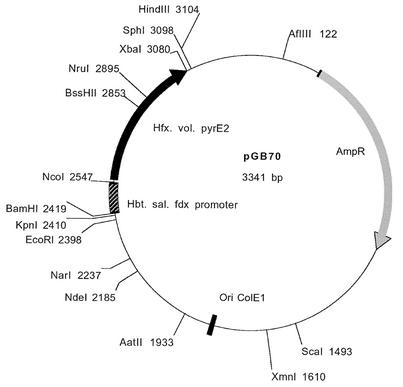
The coding region of pyrE2 was amplified by PCR by using primers N-pyrE2 and C-pyrE2, which contain NcoI and XbaI restriction sites, respectively. The amplified coding region was fused to the promoter region of the Halobacterium halobium ferredoxin gene (17) and then cloned into pUC19 digested with BamHI and XbaI. A schematic diagram of pGB70 is shown in Fig. 1.
FIG. 1.
Restriction map of H. volcanii gene knockout plasmid pGB70. Hbt. sal., Halobacterium salinarum.
(iv) pGB72, used for creating a deletion in the cmi4 gene.
A 300-bp fragment upstream from the first codon of the H. volcanii cmi4 gene was amplified by PCR with primers 300 us cmi4 and us cmi4-Rev, which contain EcoRI and NcoI restriction sites, respectively. Similarly, a fragment containing the last 92 bp of cmi4 and 176 bp of the downstream flanking sequence of this gene was amplified by PCR with the ds cmi4 (containing an NcoI restriction site) and pUC reverse primers. The template for the second PCR product was a subclone of a 1.4-kb BsiWI-ClaI genomic fragment that contained the cmi4 gene cloned in pUC19 digested with AccI and Asp718. The two PCR fragments were cloned by triple ligation into pGB70 digested with EcoRI and Asp718.
Nucleotide sequence accession numbers.
The nucleotide sequences of the H. volcanii pyrE1 and pyrE2 genes have been deposited in the EMBL nucleotide sequence database under accession numbers AJ492197 and AJ492198, respectively.
RESULTS
Cloning of pyrE1 and its flanking sequences.
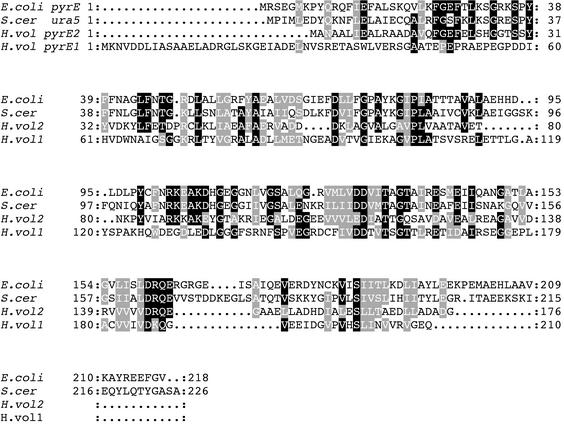
When a collection of random H. volcanii genomic clones was sequenced, a sequence was identified in a BLAST search which exhibited homology to part of the Halobacterium sp. strain NRC-1 pyrE1 gene. To clone the entire gene and the flanking sequences, H. volcanii chromosomal DNA was digested with the PstI and BsiWI restriction enzymes and analyzed by Southern blotting by using the incomplete pyrE-containing clone as a probe. A DNA fragment of about 3 kb was found to hybridize to the probe. A genomic DNA minilibrary of PstI-BsiWI 3-kb fragments was constructed and screened by using the incomplete pyrE1 probe, and this resulted in isolation of a clone containing the entire coding region of the H. volcanii pyrE1 homologue and its flanking sequences. As shown in Fig. 2, the deduced amino acid sequence of the protein encoded by the pyrE1 gene of H. volcanii exhibits low but significant homology with the sequences of the E. coli and S. cerevisiae OPRTases. The H. volcanii pyrE1 structural gene contains 630 bp and encodes a putative protein, PyrE1,consisting of 210 amino acids. Like other halophilic proteins, PyrE1 is acidic and contains 20% negatively charged amino acid residues and 8% positively charged residues. BLAST analysis of the sequence immediately upstream of the pyrE1 gene revealed an open reading frame (ORF) homologous to the gcd gene coding for glucose dehydrogenase (18). The TGA termination codon of gcd overlaps the translation initiation ATG codon of the pyrE1 gene. A similar arrangement occurs in the corresponding Halobacterium sp. strain NRC-1 (H. salinarum) (14) and Haloferax mediterranei DNA regions (unpublished results).
FIG. 2.
Multiple alignment of the E. coli PyrE, S. cerevisiae Ura5 (S.cer), H. volcanii PyrE1 (H.vol1), and H. volcanii PyrE2 (H.vol2) proteins.
Construction of a deletion in pyrE1 and phenotypic analysis.
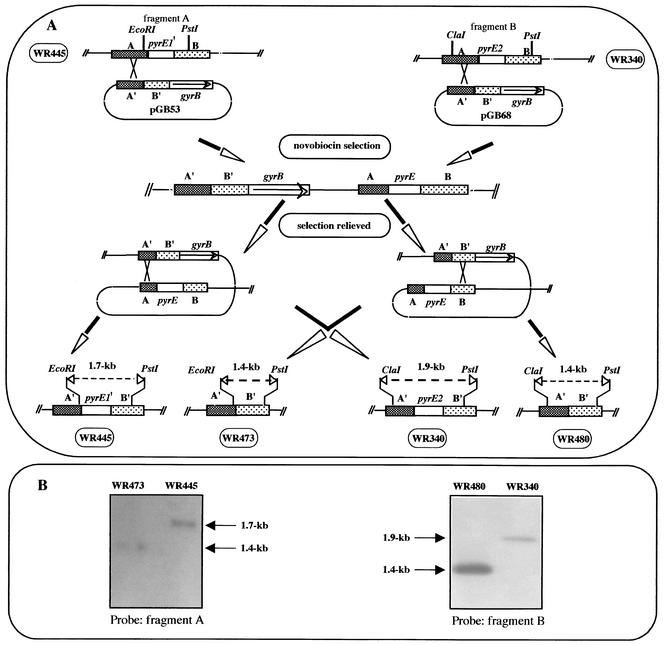
To determine whether the pyrE1 gene encodes a functional OPRTase, deletion of the first 330 bp of the gene was performed by using the pop-in-pop-out method shown in Fig. 3. A 2.8-kb DNA fragment containing a deletion of the first 330 bp of the pyrE1 gene was cloned into an E. coli plasmid that carries a halobacterial novobiocin resistance gene (gyrB) (8) to create plasmid pGB53. pGB53 was transformed into H. volcanii WR445 (pop in). Following transformation, novobiocin-resistant colonies were obtained, and the chromosomal DNAs of several colonies were screened by using Southern blot hybridization. One clone, in which pGB53 had integrated into the pyrE1 flanking region, was designated WR472. Excision of pGB53 in WR472 was performed by propagating WR472 for approximately 30 generations in rich liquid medium containing uracil and lacking novobiocin, and the cultures were spread on agar plates containing the same medium. Colonies were screened by replica plating on media with and without novobiocin; novobiocin-sensitive colonies were picked, and their DNAs were analyzed by Southern blot hybridization. Excision of pGB53 by a homologous recombination event may result in either reconstitution of the wild-type allele or deletion of 330 bp from the chromosomal pyrE1 gene (Fig. 3A). A strain in which excision of pGB53 resulted in deletion of 330 bp from the pyrE1 gene was designated WR473 (Fig. 3B). This excision did not affect the adjacent coding region of the gcd gene. H. volcanii WR473 was found to be partially resistant to 5-FOA (it grew in HY medium containing 150 μg 5-FOA per ml but did not grow in HY medium containing 450 μg of 5-FOA per ml), and surprisingly, it did not require uracil for growth.
FIG. 3.
Schematic diagram of disruption of the pyrE1 gene in strain WR445 and disruption of the pyrE2 gene in strain WR340 and Southern blot analyses of the mutant strains. (A) Plasmids pGB53 and pGB68 were constructed as described in Materials and Methods. The plasmids were integrated into the chromosome by homologous recombination between the chromosomal sequence A and the plasmid sequence A′, and novobiocin-resistant recombinants were selected. Following relief of selection, recombination events could result in either reconstitution of the wild-type allele or deletion of the chromosomal pyrE gene. pyrE1′ is the first 330 bp of the pyrE1 gene. (B) Analysis of pyrE1 mutant. Total DNA was prepared from WR445 (parental strain) and WR473 (ΔpyrE1), digested with the EcoRI and PstI restriction enzymes, and analyzed by Southern blotting by using fragment A as a probe. For analysis of the ΔpyrE2 mutant, total DNA was prepared from WR430 (parental strain) and WR480 (ΔpyrE2), digested with the ClaI and PstI restriction enzymes, and analyzed by Southern blotting by using fragment B as a probe.
Cloning of pyrE2.
Because WR473 is prototrophic for uracil and Halobacterium sp. strain NRC-1 possesses two pyrE genes (14), we supposed that H. volcanii might also have two pyrE genes. H. volcanii genomic DNA was digested with various combinations of restriction enzymes, and the DNA was resolved by electrophoresis and blotted onto nylon filters. The filters were hybridized to a PCR product of H. salinarum pyrE2 labeled with DIG. A 5.7-kb MluI-Asp718 fragment was found to hybridize to H. salinarum pyrE2 DNA and was subsequently cloned. The sequence of this fragment revealed that it contains the H. volcanii pyrE2 homologue and its flanking sequence. The H. volcanii pyrE2 structural gene is 531 bp long and encodes protein PyrE2, which has 176 amino acid residues (Fig. 2). Similar to PyrE1, PyrE2 contains 20% acidic amino acid residues and 9% basic residues. A comparison of the deduced amino acid sequences of PyrE2 and PyrE1 showed that these proteins have limited sequence similarity (Fig. 2).
Construction of a ΔpyrE2 strain and phenotypic analysis.
Deletion of the H. volcanii pyrE2 gene was performed by using the strategy that was used to create a deletion in the pyrE1 gene (Fig. 3). Plasmid pGB68 contains the 850-bp upstream and 850-bp downstream flanking sequences of pyrE2 and the gene coding for novobiocin resistance as a selectable marker. The deletion was created in H. volcanii WR340, and the strain with pyrE2 deleted, designated WR480, was found to be 5-FOA resistant and to require uracil for growth.
Construction of a ΔpyrE1 ΔpyrE2 strain and phenotypic analysis.
By using the pop-in-pop-out approach, an H. volcanii strain containing deletions in both the pyrE1 and pyrE2 genes was constructed. Strain WR473 (ΔpyrE1) was transformed with pGB68, and an isolate containing the ΔpyrE2 deletion was obtained. As expected, the phenotype of the ΔpyrE1 ΔpyrE2 strain (WR479) was similar to that of WR480 (ΔpyrE2) (namely, 5-FOA resistance and uracil auxotrophy).
Construction of a Δcmi4 mutation by using the ΔpyrE2 strain for positive and negative selection.
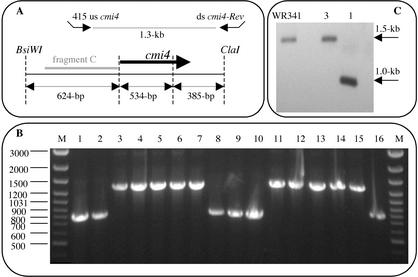
We used WR480 (ΔpyrE2), which is unable to grow without uracil and is 5-FOA resistant, and the pyrE2 gene to develop an effective tool for creating gene knockouts in H. volcanii. The coding region of pyrE2 was fused to the constitutive ferredoxin promoter, and the construct was inserted into pUC19 to obtain plasmid pGB70 (Fig. 1). pGB70 served as the basic knockout vector into which the flanking sequences of the desired gene to be deleted were cloned. To determine the efficiency of this system, we performed a knockout of the cmi4 gene, an H. volcanii gene whose expression we have shown to be conditioned medium induced. Two 300-bp fragments containing the 5′ and 3′ flanking sequences of the cmi4 gene were fused and cloned into pGB70 to create plasmid pGB72 (see Materials and Methods). Plasmid pGB72 was transformed into H. volcanii WR480 with selection for transformants that grew without added uracil (pop in). A single colony of one of the pop-in isolates (WR501) was inoculated into liquid medium lacking uracil and grown for 48 h. The culture was then spread onto HY medium plates containing 5-FOA. Since only ΔpyrE2 cells can grow on 5-FOA-containing media, it was expected that only cells in which pGB72 had been excised from the chromosome could grow. Excision of pGB72 was expected to leave in the chromosome either the wild-type cmi4 gene or a deletion of the cmi4 gene. PCR analysis was used to analyze the genotypes of 16 isolates. As shown in Fig. 4B, in six isolates excision of pGB72 resulted in deletion of the cmi4 gene, and in the other 10 isolates excision of pGB72 resulted in reversion to the parental cmi4 gene. These results were confirmed by a Southern blot analysis of one of the clones (Fig. 4C). Δcmi4 mutants did not show any phenotype when they were grown in rich or minimal medium.
FIG. 4.
Partial restriction map of the cmi4 gene and its flanking sequences (A) and PCR analysis (B) and Southern blot analysis (C) of cells that underwent excision of the integrated plasmid and could grow on media containing 5-FOA. (A) The long solid arrow represents the cmi4 gene. The locations of PCR primers 415 us cmi4 and ds cmi4-Rev are indicated by short arrows, and fragment C was used as a probe for Southern blot analysis. (B) PCR were performed with DNA isolated from 16 colonies that grew on media containing 5-FOA by using primers 415 us cmi4 and ds cmi4-Rev. Lanes M contained molecular weight markers. (C) Total DNA was prepared from WR341 (parental strain), one cmi4 deletion strain (as determined by PCR) (lane 1), and a cmi4 reconstituted strain (as determined by PCR) (lane 3). The DNA was digested with the BsiWI and ClaI restriction enzymes and analyzed by Southern blotting by using fragment C as a probe.
DISCUSSION
Genome analysis depends largely on the ability to assign functions to the various putative ORFs identified by DNA sequencing. For a given gene, the most straightforward approach is to create a knockout in that gene and to characterize the phenotype of the mutant. In the pop-in-pop-out approach, the first step results in integration of the vector into the chromosome, creating a tandem arrangement of the wild-type and mutant gene copies; in the second step, the two alleles are resolved, resulting either in the desired knockout strain or in the parental strain. Two advantages of this method are (i) that isolation of the initial integrant provides a means to assess successful transformation and insertion of the vector into the chromosome and (ii) that the selectable marker used for construction is removed at the end of the process and thus can be used again.
We previously employed the pop-in-pop-out method to create Δhdr mutations in H. volcanii (15), and in this work we created ΔpyrE1 and ΔpyrE2 strains by using a plasmid vector carrying the novobiocin resistance gene for selection. A disadvantage of the novobiocin marker is that it is not possible to positively select for excision of the plasmid when this marker is used. The low frequency of spontaneous excision necessitates tedious screening of cells for mutants in which the selectable marker has been lost. In contrast, the uracil biosynthetic pathway for selection and counterselection is widely used in S. cerevisiae (3), and use of this pathway for counterselection was recently introduced for H. salinarum by employing the pyrF gene (16).
In this paper we describe construction of a selection-counterselection system based on the H. volcanii pyrE gene. pyrE encodes the enzyme OPRTase, which participates in uracil biosynthesis. We identified in the H. volcanii genome two genes that encode proteins that exhibit low but significant (∼30%) homology with E. coli PyrE and S. cerevisiae Ura5 (Fig. 2). Previously, genome sequence analysis showed that the phylogenetically distinct halophilic archaeon Halobacterium sp. strain NRC-1 (14) also contains two ORFs (designated pyrE1 and pyrE2) that exhibit homology with pyrE genes of many organisms. Interestingly, in a search of the databases of complete microbial genomes we noticed that some archaea have two ORFs that are homologous to pyrE1 and pyrE2, while other archaea have only one pyrE ORF that is more similar to pyrE2.
In order to demonstrate the functionality of the two pyrE genes, deletion mutations of both genes were created. It was found that the ΔpyrE1 mutant has partial resistance to 5-FOA and is a uracil prototroph, whereas the ΔpyrE2 mutant shows the expected phenotype, 5-FOA resistance and uracil auxotrophy. Plausibly, pyrE2 encodes the physiological OPRTase of the cell.
It is not clear what the cellular function of pyrE1 is. On the one hand, the ΔpyrE1 strain is prototrophic, and the deletion could not be complemented by an intact copy of pyrE1 (a ΔpyrE2 mutant is auxotrophic to uracil). On the other hand, the partial resistance of the ΔpyrE1 strain to 5-FOA indicates that pyrE1 is involved in uracil biosynthesis. It seems likely that the level of OPRTase activity encoded by pyrE1 is, by itself, insufficient to enable growth without uracil but is sufficient to increase sensitivity to 5-FOA. In the three halophilic archaea known to contain pyrE1 (H. salinarum, H. volcanii, and H. mediterranei) (unpublished data), pyrE1 occurs in an operon with the gene encoding for glucose dehydrogenase (18). This is not the case in other archaea containing two pyrE genes.
In this study we developed for H. volcanii a new genetic tool that enables efficient creation of gene knockouts. The pyrE2 gene provides a valuable genetic marker that enables both positive and negative selection. We demonstrated the efficiency of this system for creating deletions by employing the cmi4 gene as a target for gene knockouts. Our previous attempts to delete cmi4 failed mainly due to the very low level of spontaneous plasmid excision even after long exposure to nonselective conditions (unpublished data). When the pyrE2-based system was used, the desired Δcmi4 mutants were obtained at a high frequency (6 of 16 isolates) (Fig. 4B).
Acknowledgments
We thank Anat Krauskopf and Gerald Cohen for critical reading of the manuscript.
REFERENCES
- 1.Bell, S. D., and S. P. Jackson. 1998. Transcription and translation in Archaea: a mosaic of eukaryal and bacterial features. Trends Microbiol. 6:222-228. [DOI] [PubMed] [Google Scholar]
- 2.Bell, S. D., C. P. Magill, and S. P. Jackson. 2001. Basal and regulated transcription in Archaea. Biochem. Soc. Trans. 29:392-395. [DOI] [PubMed] [Google Scholar]
- 3.Boeke, J. D., F. LaCroute, and G. R. Fink. 1984. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol. Gen. Genet. 197:345-346. [DOI] [PubMed] [Google Scholar]
- 4.Charlebois, R. L., W. L. Lam, S. W. Cline, and W. F. Doolittle. 1987. Characterization of pHV2 from Halobacterium volcanii and its use in demonstrating transformation of an archaebacterium. Proc. Natl. Acad. Sci. USA 84:8530-8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cline, S., F. Pfeifer, and W. Doolittle. 1995. Transformation of halophilic archaea, p. 197-204. In F. Robb (ed.), Protocols for archaeal research. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 6.Danner, S., and J. Soppa. 1996. Characterization of the distal promoter element of halobacteria in vivo using saturation mutagenesis and selection. Mol. Microbiol. 19:1265-1276. [DOI] [PubMed] [Google Scholar]
- 7.Holmes, M., F. Pfeifer, and M. Dyall-Smith. 1994. Improved shuttle vectors for Haloferax volcanii including a dual-resistance plasmid. Gene 146:117-121. [DOI] [PubMed] [Google Scholar]
- 8.Holmes, M. L., and M. L. Dyall-Smith. 1990. A plasmid vector with a selectable marker for halophilic archaebacteria. J. Bacteriol. 172:756-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hough, D. W., and M. J. Danson. 1999. Extremozymes. Curr. Opin. Chem. Biol. 3:39-46. [DOI] [PubMed] [Google Scholar]
- 10.Lam, W. L., and W. F. Doolittle. 1989. Shuttle vectors for the archaebacterium Halobacterium volcanii. Proc. Natl. Acad. Sci. USA 86:5478-5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 12.Mevarech, M., and R. Werczberger. 1985. Genetic transfer in Halobacterium volcanii. J. Bacteriol. 162:461-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullakhanbhai, M. F., and H. Larsen. 1975. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch. Microbiol. 104:207-214. [DOI] [PubMed] [Google Scholar]
- 14.Ng, W. V., S. P. Kennedy, G. G. Mahairas, B. Berquist, M. Pan, H. D. Shukla, S. R. Lasky, N. S. Baliga, V. Thorsson, J. Sbrogna, S. Swartzell, D. Weir, J. Hall, T. A. Dahl, R. Welti, Y. A. Goo, B. Leithauser, K. Keller, R. Cruz, M. J. Danson, D. W. Hough, D. G. Maddocks, P. E. Jablonski, M. P. Krebs, C. M. Angevine, H. Dale, T. A. Isenbarger, R. F. Peck, M. Pohlschroder, J. L. Spudich, K. W. Jung, M. Alam, T. Freitas, S. Hou, C. J. Daniels, P. P. Dennis, A. D. Omer, H. Ebhardt, T. M. Lowe, P. Liang, M. Riley, L. Hood, and S. DasSarma. 2000. Genome sequence of Halobacterium species NRC-1. Proc. Natl. Acad. Sci. USA 97:12176-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortenberg, R., O. Rozenblatt-Rosen, and M. Mevarech. 2000. The extremely halophilic archaeon Haloferax volcanii has two very different dihydrofolate reductases. Mol. Microbiol. 35:1493-1505. [DOI] [PubMed] [Google Scholar]
- 16.Peck, R. F., S. Dassarma, and M. P. Krebs. 2000. Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker. Mol. Microbiol. 35:667-676. [DOI] [PubMed] [Google Scholar]
- 17.Pfeifer, F., J. Griffig, and D. Oesterhelt. 1993. The fdx gene encoding the [2Fe-2S] ferredoxin of Halobacterium salinarium (H. halobium). Mol. Gen. Genet. 239:66-71. [DOI] [PubMed] [Google Scholar]
- 18.Pire, C., J. Esclapez, J. Ferrer, and M. J. Bonete. 2001. Heterologous overexpression of glucose dehydrogenase from the halophilic archaeon Haloferax mediterranei, an enzyme of the medium chain dehydrogenase/reductase family. FEMS Microbiol. Lett. 200:221-227. [DOI] [PubMed] [Google Scholar]
- 19.Ried, J. L., and A. Collmer. 1987. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57:239-246. [DOI] [PubMed] [Google Scholar]
- 20.Rosenshine, I., and M. Mevarech. 1989. Isolation and partial characterization of plasmids found in three Halobacterium volcanii isolates. Can. J. Microbiol. 35:92-95. [DOI] [PubMed] [Google Scholar]
- 21.Thompson, D. K., J. R. Palmer, and C. J. Daniels. 1999. Expression and heat-responsive regulation of a TFIIB homologue from the archaeon Haloferax volcanii. Mol. Microbiol. 33:1081-1092. [DOI] [PubMed] [Google Scholar]
- 22.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Wezel, G. P., and M. J. Bibb. 1996. A novel plasmid vector that uses the glucose kinase gene (glkA) for the positive selection of stable gene disruptants in streptomyces. Gene 182:229-230. [DOI] [PubMed] [Google Scholar]
- 24.Woese, C. R., and G. E. Fox. 1977. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc. Natl. Acad. Sci. USA 74:5088-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woods, W. G., and M. L. Dyall-Smith. 1997. Construction and analysis of a recombination-deficient (radA) mutant of Haloferax volcanii. Mol. Microbiol. 23:791-797. [DOI] [PubMed] [Google Scholar]