Abstract
1 The LD50 in the rat and the mouse is about 1600 mg/kg (oral administration) and 75 mg/kg (rat) and 50 mg/kg (mouse) on intravenous administration.
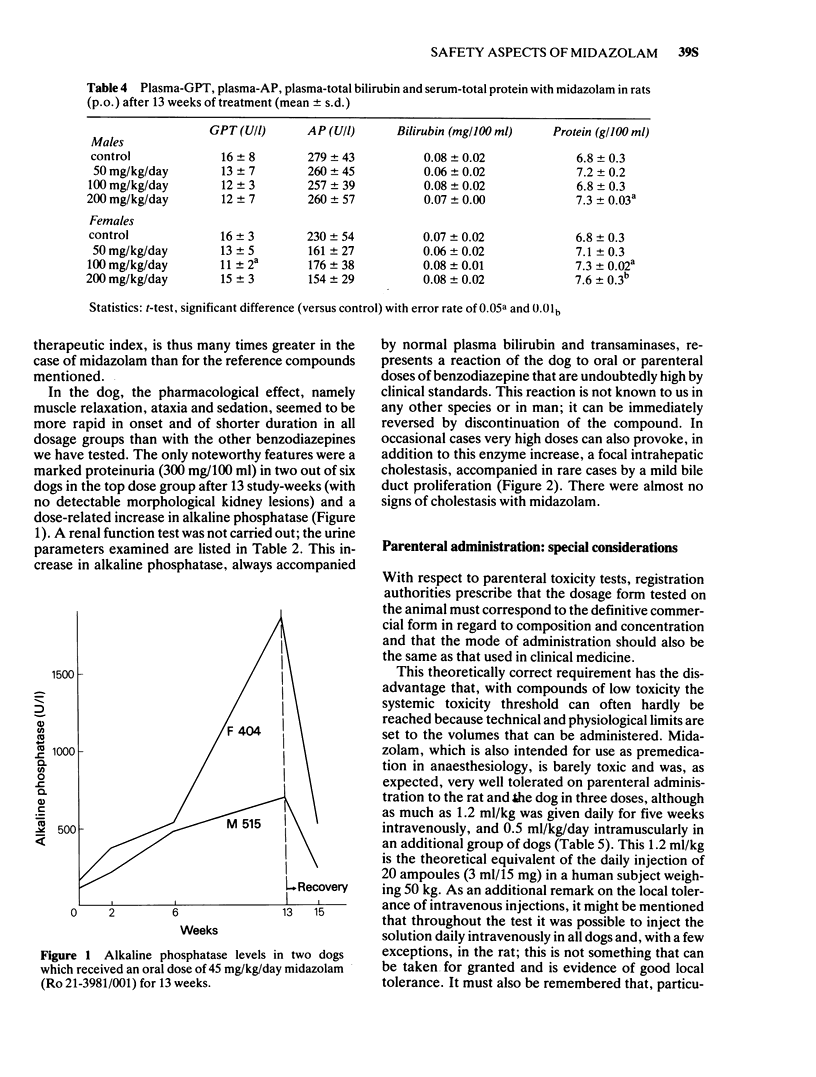
2 Subchronic oral studies over 13 weeks in doses of 5, 15 and 45 mg/kg/day in the dog and 50, 100 and 200 mg/kg/day in the rat have demonstrated minimal toxicity for midazolam, as for other benzodiazepines. High doses produced increased liver weight in the rat and the expected increases in alkaline phosphatase in the dog (species-specific reaction). Detailed blood and urine analyses as well as histological examination of organs produced no indication of changes relevant for man.
3 Subchronic parenteral studies (i.v. and i.m. for five weeks) using up to 6 mg/kg/day in dogs and rats showed the compound to be not only systemically, but also locally, extremely well tolerated.
4 Reproduction toxicology studies have shown that midazolam is neither embryotoxic nor teratogenic and that it has no effect on the fertility and post-natal development of animals.
5 In the AMES test and the fluctuation test, midazolam had no mutagenic effect.
Full text
PDF