Abstract
Fis is a nucleoid-associated protein that interacts with poorly related DNA sequences with a high degree of specificity. A difference of more than 3 orders of magnitude in apparent Kd values was observed between specific (Kd, ∼1 to 4 nM) and nonspecific (Kd, ∼4 μM) DNA binding. To examine the contributions of Fis residues to the high-affinity binding at different DNA sequences, 13 alanine substitutions were generated in or near the Fis helix-turn-helix DNA binding motif, and the resulting proteins were purified. In vitro binding assays at three different Fis sites (fis P II, hin distal, and λ attR) revealed that R85, T87, R89, K90, and K91 played major roles in high-affinity DNA binding and that R85, T87, and K90 were consistently vital for binding to all three sites. Other residues made variable contributions to binding, depending on the binding site. N84 was required only for binding to the λ attR Fis site, and the role of R89 was dramatically altered by the λ attR DNA flanking sequence. The effects of Fis mutations on fis P II or hin distal site binding in vitro generally correlated with their abilities to mediate fis P repression or DNA inversion in vivo, demonstrating that the in vitro DNA-binding effects are relevant in vivo. The results suggest that while Fis is able to recognize a minimal common set of DNA sequence determinants at different binding sites, it is also equipped with a number of residues that contribute to the binding strength, some of which play variable roles.
Fis (factor for inversion stimulation) is the most abundant nucleoid-associated protein during the logarithmic growth phase in rapidly growing Escherichia coli cells (3, 6). However, during mid- to late-logarithmic growth, the intracellular levels of Fis decrease over 500-fold and become nearly imperceptible during the stationary phase. The impact of Fis on cell physiology is widespread. As a nucleoid-associated protein, it is able to interact with a large number of DNA sites to alter DNA topology (66, 67). Numerous genes are subject to positive or negative regulation by Fis directly or indirectly (6, 10, 19, 22, 30, 49, 56, 61, 73-75). In the case of the promoters rrnB P1 and proP P2, Fis binding to sites centered at positions −71 and −41, respectively, directly stimulates transcription by contacting the C-terminal domain of the RNA polymerase α subunit (α-CTD) (9, 43). In the case of the fis promoter (fis P), Fis binding to sites I and II, centered at positions +25 and −44, respectively, negatively autoregulates the fis operon by hindering RNA polymerase binding (6, 50, 58). As its name suggests, Fis also stimulates site-specific DNA inversion involving the Hin, Gin, and Cin family of recombinases (24, 32, 35). During Hin-mediated DNA inversion, Fis binds to two high-affinity DNA sites (hin-proximal and -distal sites) in a 60-bp recombinational enhancer region and interacts with two DNA-bound Hin recombinases to form a nucleoprotein complex intermediate required for efficient DNA strand cleavage and inversion (25, 33). Bacteriophage λ DNA excision from the E. coli chromosome is also stimulated by Fis by a mechanistically different process involving a high-affinity Fis binding site within λ attR (5, 51, 53, 71). In this case, Fis appears to play an architectural role by contributing to a higher-order nucleoprotein complex that facilitates DNA cleavage and excision (40). Thus, Fis participates in a variety of mechanistically different cellular functions in different nucleoprotein contexts.
A fundamental property of Fis is its ability to bind and bend DNA. However, it is not yet known how Fis attains the specificity with the wide array of DNA-binding sequences that has been reported for this protein. Comparisons of a number of DNA sequences that are specifically bound by Fis have led to proposals for several consensus sequences (16, 17, 26, 28). However, these consensus sequences are somewhat degenerate, which permits substantial sequence variation. Previously, random mutagenesis showed that residues in the C-terminal helix-turn-helix (HTH) DNA binding motif were required for DNA binding because mutations in these residues reduced or abolished this function (38, 53). However, because many of the mutations resulted in changes that could potentially alter the local protein structure or interfere with DNA binding, the precise contributions of the mutated residues to DNA binding were unclear. Although crystal structures have been solved for Fis and for a number of Fis variants (12, 39, 63, 77, 78), a Fis-DNA cocrystal structure remains to be resolved. Several useful docking models have been proposed to explain the specific interactions between Fis and DNA (16, 55, 65, 72), but these models have been based on partial knowledge of residues mediating DNA binding and of the DNA sequence contributing to the binding affinity.
In this work we present a more definitive characterization of the contribution of Fis residues to the binding affinity at different Fis binding sites. To this end, we created alanine substitutions at 13 locations in or near the HTH region and purified each of the mutant proteins. We determined the effects of these substitutions on the protein stability, the binding affinities to three different DNA sites in vitro, and the ability of Fis to function in vivo. These experiments gave both expected and unexpected results, which together showed that some Fis residues are crucial in achieving high-affinity binding to multiple DNA sequences, while others make variable contributions to DNA binding.
MATERIALS AND METHODS
Chemicals, enzymes, and growth media.
Chemicals were purchased from Sigma-Aldrich, Fisher Scientific, Life Technologies Inc. (Gibco BRL), Pharmacia, or VWR Scientific. Enzymes were obtained from New England BioLabs, Inc., Promega Corp., or Roche Molecular Biochemicals. Radioisotopes were obtained from Amersham Biosciences. Oligonucleotides were generated with a Perkin-Elmer automated DNA synthesizer operated in the Department of Biological Sciences, State University of New York at Albany. Bacterial culture media were obtained from Difco Laboratories. Cultures were grown at 37°C in Luria-Bertani (LB) medium or were plated on LB agar or NZCYM medium as required (64). To select for drug resistance, 100 μg of ampicillin per ml, 50 μg of kanamycin per ml, and 75 μg of spectinomycin per ml were added to the growth medium as appropriate for each strain.
Bacterial strains and plasmids.
Strains used in this work are listed in Table 1. fis P repression activity was assayed in vivo using the λ lysogenic strain RO1046. To construct this strain, the fis promoter region from position −83 to position +5 (relative to the primary transcription start site) was fused to lacZ in plasmid pLF413, transformed into JM101, and recombined in λRS45 as described previously (70). The recombinant phage was purified and used to lysogenize RJ1561 in order to make RO1046. The λ excision assay was performed with strain RJ1765, which is a thermosensitive lysogen of λ phage. The Hin-mediated DNA inversion assay was performed with E. coli strain RJ2539, which carries the hin invertible DNA segment from Salmonella enterica serovar Typhimurium fused to lacZ on the chromosome in the “OFF” orientation. Induction of fis from pET11c was performed in RO820, which is a λDE3 lysogen (Novagen from EMD Biosciences) made in RJ1561.
TABLE 1.
Strains used in this study
| Strain | Description | Reference |
|---|---|---|
| JM101 | Δ(pro-lac)U169 supE thi F′ (traD36 proAB+lacIqΔM15) | 45 |
| RJ1561 | F−ara Δ(lac pro) thi str recA56 srl fis::kan | 31 |
| RJ1765 | ara Δ(lac pro) rpsL fis::kan λ cI857 S7 F′ proAB lacIsqZU118 | 53 |
| RJ2539 | ara Δ(lac pro) recA56 srl rpsL fis::kan λ fla406 ‘off' F′ proAB lacIsqZU118/pKH66 | 53 |
| RO820 | RJ1561 λ (DE3) lysogen/pMS421 | This study |
| RO1046 | RJ1561 fis P::lacZ (positions −83 to +5) lysogen | This study |
The plasmids used in this work are listed in Table 2. Fis mutants were engineered as described below and were used to replace the wild-type (WT) fis gene in pRJ807 in order to make plasmid pRJ950 (53), as well as plasmids pLF209, pLF252, pLF254, pLF256, pLF257, pLF259, pLF271, pLF307, pLF308, pLF352, pLF386, and pLF387. The various fis genes cloned in pRJ807-based plasmids were amplified by PCR and cloned into the T7 promoter-based pET11c expression vector (15) between the NdeI and BamHI sites to make pLF317, pLF318, pLF320, pLF321, pLF322, pLF326, pLF327, pLF328, pLF329, pLF330, pLF354, pLF355, pLF407, and pLF408. Three 23-bp DNA fragments containing 15-bp sequences for specific Fis binding sites were obtained by annealing complementary oligonucleotides and were cloned in the XbaI and SacI sites of pCY4 to create pLF234, pLF235, and pLF236. pRJ903 contains the λ attR Fis site immediately flanked on each side by 5 bp of natural sequence cloned into the BglII and SacI sites of pCY7 (53). Using pRJ1028 as the template, the fis P region from position −83 to position +5 was amplified by PCR and cloned in the SmaI site of pRJ800 to make pLF309 or in the EcoRI and SmaI sites of plasmid pRS415 (70) to make pLF413.
TABLE 2.
Plasmids used in this study
| Plasmid | Descriptiona | Reference or sourceb |
|---|---|---|
| pCY4 | pBR322-based plasmid containing a 375-bp direct repeat; Ampr | 59 |
| pCY7 | Similar to pCY4 but with an inverted polylinker region; Ampr | 59 |
| pET11c | IPTG-inducible, phage T7 promoter-based expression vector; Ampr | 15 |
| pJS482 | pCY4 carrying a 17-bp sequence in XbaI and SacI sites lacking a Fis site | |
| pKH66 | pSC101 (lacIq Ptac hin Strr Spcr) | 29 |
| pLF209 | pRJ807 derivative expressing K90A Fis | |
| pLF234 | pCY4 carrying the 15-bp hin distal Fis binding site in XbaI and SacI sites | |
| pLF235 | pCY4 carrying the 15-bp λ attR Fis binding site in XbaI and SacI sites | |
| pLF236 | pCY4 carrying the 15-bp fis P II Fis binding site in XbaI and SacI sites | |
| pLF252 | pRJ807 derivative expressing R89A Fis | |
| pLF254 | pRJ807 derivative expressing N84A Fis | |
| pLF256 | pRJ807 derivative expressing K93A Fis | |
| pLF257 | pRJ807 derivative expressing N98A Fis | |
| pLF259 | pRJ807 derivative expressing Q74A Fis | |
| pLF271 | pRJ807 derivative expressing N73A Fis | |
| pLF307 | pRJ807 derivative expressing T75A Fis | |
| pLF308 | pRJ807 derivative expressing R76A Fis | |
| pLF309 | pRJ800 carrying fis P from position −83 to position +5 in EcoRI and HindIII sites | |
| pLF317 | pET11c derivative expressing R76A Fis | |
| pLF318 | pET11c derivative expressing N84A Fis | |
| pLF320 | pET11c derivative expressing T87A Fis | |
| pLF321 | pET11c derivative expressing R89A Fis | |
| pLF322 | pET11c derivative expressing K90A Fis | |
| pLF326 | pET11c derivative expressing K93A Fis | |
| pLF327 | pET11c derivative expressing N98A Fis | |
| pLF328 | pET11c derivative expressing N73A Fis | |
| pLF329 | pET11c derivative expressing Q74A Fis | |
| pLF330 | pET11c derivative expressing wild-type Fis | |
| pLF352 | pRJ807 derivative expressing R85A Fis | |
| pLF354 | pET11c derivative expressing R85A Fis | |
| pLF355 | pET11c derivative expressing T75A Fis | |
| pLF386 | pRJ807 derivative expressing K91A Fis | |
| pLF387 | pRJ807 derivative expressing K94A Fis | |
| pLF407 | pET11c derivative expressing K91A Fis | |
| pLF408 | pET11c derivative expressing K94A Fis | |
| pLF413 | pRS415 containing the fis P region from position −83 to position +5 in the EcoRI and BamHI sites | |
| pMS421 | pSC101 lacIq | M. Susskind |
| pRJ800 | pBR322-based plasmid carrying a pUC18 multicloning region preceding a W-200 trp-lacZ fusion; Ampr | 6 |
| pRJ807 | pKK223-3 (Ptac fisWT Ampr Kanr) | 53 |
| pRJ903 | pCY7 carrying the λ attR Fis binding site flanked by natural sequences in XbaI and SacI sites | 53 |
| pRJ950 | pRJ807 derivative expressing T87A Fis | 53 |
| pRJ1028 | pRJ800 containing the fis P region from position −375 to position +78 in the HincII site | 58 |
| pRJ1122 | pRJ807 derivative in which the fis gene is excised | 54 |
| pRS415 | pBR322-based plasmid carrying a promoterless lacZ; Ampr | 70 |
Abbreviations: Ampr, ampicillin resistant; Kanr, kanamycin resistant; Strr, streptomycin resistant; Spcr, spectinomycin resistant; fisWT, wild-type fis; fis P, fis promoter.
Plasmids were constructed during this work unless indicated otherwise.
Site-directed mutagenesis.
Site-directed mutagenesis was performed using a two-step PCR megaprimer technique, as described previously (6). The first step consisted of a PCR performed with an oligonucleotide (5′-CGGAATTCATGTTCGAACAACGCG-3′; EcoRI site underlined) that annealed to the beginning of the fis gene, a downstream primer that annealed to fis and contained the desired fis mutation, and pRJ807 as the template. The resulting PCR product was used as a megaprimer in a second PCR along with another primer annealing downstream of fis (5′-CCCAAGCTTGCGGCACTGGAGATCG; HindIII site underlined) to generate the full-length fis gene plus an additional 390 bp of downstream sequence. The PCR products were purified, digested with EcoRI and HindIII, and cloned into the same sites in pRJ807 to replace the wild-type fis gene with a mutated variant. Each cloned fis gene was verified by DNA sequencing using Sequenase 2.0 and dideoxy nucleotides, as described by the supplier (USB Corp.).
Purification of Fis mutant proteins.
RO820 strains carrying the wild-type or mutant fis genes on pET11c-based plasmids were grown in LB medium at 37°C until the optical density at 600 nm was between 0.6 and 0.7, and then they were induced with 0.6 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h at 37°C. Fis purification was performed by using a previously described procedure (53), which essentially involved phosphocellulose liquid chromatography of the crude cell lysate, ammonium sulfate precipitation, and precipitation by dialysis against low-salt buffer. For purification of Fis mutants R76A, R85A, R89A, K91A, K93A, K94A, and N98A, the NaCl concentration used to load the proteins on the cellulose phosphate column was decreased from 0.3 M (the concentration used for the wild-type Fis) to 0.2 or 0.1 M as needed to ensure column retention. K90A was not retained even at 0.1 M NaCl and was instead collected in the flowthrough. A subsequent Sephadex G-100 size exclusion chromatography column removed the bulk of the remaining contaminants from K90A Fis. The final preparation was dialyzed against storage buffer (500 mM NaCl, 20 mM Tris-HCl [pH 7.5], 0.1 mM EDTA, 50% glycerol) and stored at −20°C.
Protein concentrations were determined using the Bio-Rad protein assay dye reagent (Bio-Rad Laboratories) with bovine serum albumin as the standard. Fis purity was determined by electrophoresis in 15% sodium dodecyl sulfate (SDS)-polyacrylamide-bisacrylamide (29:1) gels that were stained with SYPRO Red (Molecular Probes, Inc.). Gels were analyzed using a STORM 860 PhosphorImager set to the red fluorescence mode with a photomultiplier voltage of 850, and the relative intensities of bands within each lane were quantified using the ImageQuant software (Molecular Dynamics, Inc., Sunnyvale, CA). The Fis purity was factored into determination of the Fis concentration in each preparation.
Thermal denaturation analysis.
Fis protein samples (0.1 mg/ml in 0.1 M NaCl-20 mM NaPO4, pH 7.0) were subjected to various temperatures, at which the circular dichroism signal at 222 nm (far-UV circular dichroism) was collected, and the baseline value at 255 nm was subtracted. The ellipticity was measured as a function of temperature in at least two assays, and the experiments were individually fitted with a model of the heat-induced D2→2U equilibrium denaturation pathway using Kaleidagraph (Synergy Software) (44). The average midpoint denaturation temperatures (Tm) derived from the fits were used as indicators of relative protein stability.
Gel electrophoretic mobility shift assays (GEMSA).
DNA fragments (42 to 53 bp) carrying a specific Fis binding site were obtained by cleaving plasmids pLF234, pLF235, pLF236, pRJ903, and pJS482 with EcoRI and BamHI and labeling with [α-32P]dATP using the end-fill reaction, and they were purified by polyacrylamide gel electrophoresis (64). Various amounts of purified Fis were mixed with approximately 1 fmol 32P-labeled DNA in 20 μl binding buffer (20 mM Tris-HCl [pH 7.5], 10 mM EDTA, 80 mM NaCl), incubated at room temperature for 10 min, and combined with 5 μl of loading buffer (20 mM Tris-HCl [pH 7.5], 10 mM EDTA, 80 mM NaCl, 100 μg/ml sonicated salmon sperm DNA, 7.5% Ficoll, 0.1% bromophenol blue). Samples were then loaded onto an 8% polyacrylamide-bisacrylamide (60:1) gel in TBE buffer (0.089 M Tris-borate [pH 8.3], 2.5 mM EDTA) with a 15-mA conducting current. The relative signal intensities were quantified by phosphorimaging. The percentage of bound DNA was plotted against the Fis concentration, and the concentration required to achieve 50% binding (which was not less than a 40-fold molar excess over the DNA concentration) was considered an experimental approximation of the Kd (apparent Kd). Since a fragment lacking a defined Fis binding site and two of the mutant Fis proteins (R85A and K90A) failed to produce a discrete bound complex, we measured the disappearance of the unbound DNA as a function of the Fis concentration to estimate Kd in these cases. A maximum concentration of 2.2 μM was used for several of the mutant Fis preparations since higher concentrations resulted in potential salt interference. This was not the case for WT Fis, which was purified and stored at much higher concentrations.
To examine the effects of the flanking sequences on Fis-induced DNA bending at the λ attR site, pLF235 and pRJ903 were cleaved with BamHI or NheI to place the corresponding Fis binding sites near the end (BamHI) or near the middle (NheI) of a 413-bp (from pLF235) or a 424-bp (from pRJ903) DNA fragment and labeled with 32P as described above.
fis P repression assay.
RO1046 contains a lacZ fusion to the fis promoter region from position −83 to position +5 on the chromosome, which includes a single fis P II centered at position −44. RO1046 carrying pMS421 was transformed with pRJ807-based plasmids containing the wild-type or mutated fis genes and grown in LB medium in the presence of ampicillin and spectinomycin. Saturated cultures were diluted 75-fold in LB medium and grown for 90 min at 37°C, and then β-galactosidase assays were performed as described previously (46). In these cells, uninduced Fis levels were sufficient to achieve about 4.5-fold repression of fis P. Repression activity was calculated using the following formula: 100 × (MUfis − MUMut Fis)/(MUfis − MUWT Fis), where MUfis is the β-galactosidase activity (700 U) in the absence of Fis (RO1046 transformed with pRJ1122), MUWT Fis is the activity (154 U) in the presence of wild-type Fis (RO1046 transformed with pRJ807), and MUMut Fis is the activity in the presence of the mutant Fis.
Hin-mediated DNA inversion assay.
A lacZ fusion to the hin invertible DNA region was present in the E. coli chromosome of RJ2539 such that the promoter located in the invertible region was transcribed away from lacZ (“OFF” orientation) (29, 53). This strain expressed hin from plasmid pKH66 but lacked a functional fis gene, so that inversion was highly inefficient. In the presence of wild-type Fis expressed from pRJ807, DNA inversion occurred, the invertible region switched to the “ON” orientation, and lacZ was expressed. RJ2539 was transformed with pRJ807 or pRJ807-derived plasmids expressing one of the mutant fis genes and was grown in LB medium containing ampicillin and spectinomycin. Cultures were periodically plated on LB agar and LB agar containing ampicillin and spectinomycin to monitor the growth of total and transformed cells, respectively. Cultures reaching a concentration of about 2 × 109 cells/ml were diluted in the same fresh medium to continue growth. After 52 to 55 generations, samples were withdrawn for β-galactosidase assays. Relative inversion activity was calculated using the following formula: 100 × (MUMut Fis − MUfis)/(MUWT Fis − MUfis), where MUWT Fis is the β-galactosidase activity in the presence of wild-type Fis (from pRJ807), MUfis is the activity in the absence of Fis, and MUMut Fis is the activity in the presence of the mutant Fis.
λ phage DNA excision assay.
The pRJ807-based plasmids containing the wild-type or mutated fis genes were transformed into the λ cI857 lysogenic strain RJ1765. Thermoinduction of λ cI857 lysogens was performed as described previously (5, 53). Saturated cultures were diluted 30-fold in 6 ml of LB medium containing ampicillin and were grown in a shaking water bath at 32°C to an optical density at 600 nm of 0.6. The cultures were then shifted to 42°C for 30 min with shaking and then to 37°C for 2 h with constant aeration. After addition of 0.5 ml chloroform and vigorous vortexing, samples were centrifuged at 5,000 rpm for 10 min, and the supernatants were immediately titrated to determine the number of PFU per ml. Relative excision activities were obtained using the following formula: 100 × (PFU/mlMut Fis − PFU/mlfis)/(PFU/mlWT Fis − PFU/mlfis), where PFU/mlWT Fis is the number of PFU/ml in the presence of WT Fis (4.35 × 1010 PFU/ml), PFU/mlfis is the number of the PFU/ml in the absence of Fis (1.84 × 108 PFU/ml), and PFU/mlMut Fis is the number of PFU/ml in the presence of a Fis mutant.
RESULTS
Specificity of binding.
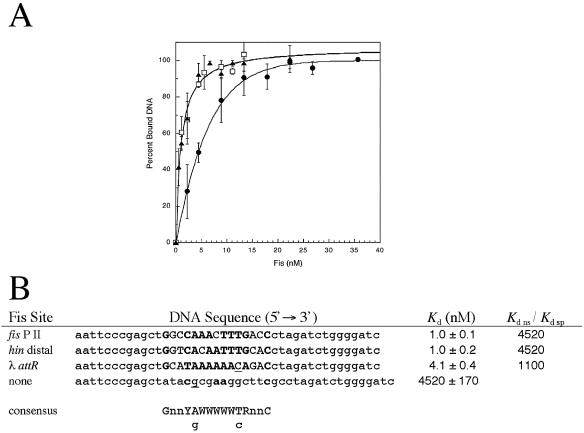
Numerous DNA footprinting experiments (4, 6, 7, 11, 42, 54, 71, 77) demonstrated that Fis has definite binding preferences for specific DNA sites even when their sequences are weakly related (17, 26, 28). With increasing concentrations, Fis interacts with an increasing number of weaker DNA sites until binding becomes nonspecific (8, 67). However, distinctions between specific Fis-DNA interactions of various strengths and truly nonspecific interactions (i.e., interactions at random sites) have seemed vague. Here we examined the ability of Fis to interact with three known Fis sites (fis P II [6, 50, 58], hin distal [11, 33], and λ attR [5, 71]) and a nonspecific sequence (Fig. 1). Each of the Fis sites (15 bp) was placed at the center of a 42- to 44-bp DNA fragment and used in gel electrophoretic mobility shift assays with a range of Fis concentrations. The small DNA fragment minimized the potential for concurrent occupancy of alternative “weaker” Fis binding sites, which may be observed with much longer fragments. The results showed that binding to the DNA fragments carrying the fis P II or hin distal Fis sites occurred with equivalent apparent Kd values (Kd app) of about 1.0 nM, while binding to the fragment carrying the λ attR site occurred with a Kd app of about 4.1 nM. However, binding to the DNA fragment having flanking sequences similar to those of the previous three fragments but with a scrambled central sequence occurred with a Kd app of about 4.5 μM. Other 44-bp DNA fragments carrying no recognizable Fis binding sites have resulted in similarly high Kd app values (Shao and Osuna, unpublished results). Such a large difference in Kd app values, over 3 orders of magnitude (Kd ns/Kd sp), indicates that there is very high binding specificity at the fis P II, hin distal, and λ attR sites. The fourfold increase in the Fis concentration required for binding the λ attR site compared to the concentrations required for binding the fis P II and hin distal sites is far from the range observed for nonspecific binding and thus also represents highly specific binding.
FIG. 1.
Relative Fis-DNA binding affinities. (A) Wild-type Fis protein binding to 42-bp DNA fragments containing fis P II (▴), hin distal (□), and λ attR (•) Fis sites was measured using gel mobility shift assays. The results are averages of three experiments, and the error bars indicate standard deviations. (B) Sequences of the three DNA fragments used for panel A together with the sequence of a 44-bp DNA fragment containing no recognizable Fis site. The central 15-bp core sequences of the three specific Fis binding sites are in uppercase letters and are flanked by plasmid-derived sequences. At the bottom is a consensus sequence (26) (Y = pyrimidine; R = purine; W = A or T; n = any nucleotide; lowercase g and c represent a weak preference for guanine and cytosine). Matches with the consensus sequence in the Fis binding sites are indicated by boldface type, and matches with relatively weaker preferences are underlined. The apparent Kd for Fis binding at each of the DNA fragments derived from the plots in panel A is the average ± standard deviation for three experiments. No specific bound complex was observed with the negative control DNA in gel mobility shift assays, and binding activity in this case was monitored by measuring the disappearance of the unbound DNA with increasing Fis concentrations. The ratios of Kd ns to Kd sp, obtained by dividing the Kd of the negative control DNA by the Kd of each of the three Fis binding sites, were considered a measure of binding specificity.
Generation, purification, and stability of Fis mutants.
Alanine is an excellent helix former (52) that is not generally expected to cause significant disruptions in local structure or interfere with DNA binding. Thus, 13 residues in or near the HTH DNA binding motif were replaced with alanine, and the resulting mutants were purified (Fig. 2A and B). These residues were chosen on the basis of three criteria: (i) the results of previous random mutagenesis that pinpointed residues potentially involved in DNA binding (38, 53), (ii) examination of the Fis crystal structure (39, 63, 77) for solvent-exposed residues in the HTH region (Fig. 2A), and (iii) consideration of several proposed Fis-DNA docking models (16, 65, 72). On the basis of SDS-polyacrylamide gel electrophoresis, 10 of the proteins (including wild-type Fis) were purified to 95% purity or greater, and four (T75A, R85A, R89A, and K90A) were purified to within 70 to 90% purity (Fig. 2C). The three preparations with the lowest levels of purity (R85A, R89A, and K90A) resulted in poor or undetectable DNA binding to three different Fis sites (as discussed below) so that concerns about potential contributions to DNA binding by contaminants in these preparations were largely mitigated. In addition, when mixed with WT Fis, these preparations did not inhibit DNA binding (not shown).
FIG. 2.
Alanine substitutions in or near the Fis helix-turn-helix DNA binding motif. (A) Ribbon model of the crystal structure of Fis (39, 63, 77). The diagram on the left represents the Fis homodimer. The amino-terminal β-loop followed by four α-helices, α-A, α-B, α-C, and α-D, is indicated for one of the subunits. The α-B, α-C, and α-D helices of a single subunit are shown in the diagram on the right, which indicates the positions of the side chains for the residues in or near the helix-turn-helix DNA binding motif (comprised of α-C and α-D) that were replaced with alanine. (B) Generation of alanine mutations. The DNA sequence of E. coli fis is expressed as triplet codons, and the deduced amino acid sequence is shown above the DNA sequence. The positions of alanine substitutions (Ala) are indicated above the amino acid sequence, and the corresponding DNA mutations are indicated beneath the DNA sequence. The arrows indicate the regions that form β-strands (β-1 and β-2), and the boxes indicate the regions that form the four α-helices. The first and last residues of helices α-C (positions 74 and 81) and α-D (positions 85 and 94) are indicated. (C) SDS-polyacrylamide gel electrophoresis of the purified Fis proteins stained with Coomassie blue. The estimated purities of the various Fis proteins are indicated below the lanes.
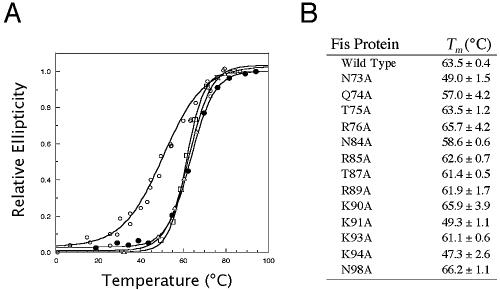
Circular dichroism-monitored thermal denaturation of WT Fis showed that the average midpoint temperature of denaturation was 63.5°C (Fig. 3), demonstrating that Fis is relatively stable at high temperatures. Mutations that significantly perturb intra- or intermolecular interactions can result in alterations in protein conformation or stability that can be detected by a change in the Tm. The Tm for Fis mutants Q74A, T75A, R76A, N84A, R85A, T87A, R89A, K90A, K93A, and N98A were within 6.5°C of that of WT Fis, suggesting that these mutations caused minimal disturbance to the local structure and stability. However, the Tm for mutants N73A, K91A, and K94A were about 14 to 16°C lower than that of the WT, suggesting that there were appreciable alterations in the local structure or stability.
FIG. 3.
Relative stabilities of the WT and mutant Fis proteins. (A) Representative thermal denaturation curves for the wild-type (•), R85A (▵), R89A (□), and K91A (○) Fis proteins. The extent of protein unfolding was measured by determining the change in the UV circular dichroism signal at 220 nM and is expressed as relative ellipticity on a scale from 0 to 1.0. (B) Midpoint denaturation temperatures of WT and mutant proteins. The values are averages ± standard deviations for two or three experiments.
Fis residues required for binding to the fis P II site.
Using GEMSA, we compared the mutant proteins to WT Fis in terms of the ability to bind the DNA fragment containing fis P II (Table 3). We found that Q74A, N84A, and N98A had a less-than-twofold effect on the binding affinity compared to WT Fis, suggesting that these residues are largely dispensable for binding this site in vitro. Moderate decreases in relative binding were seen with T75A (8-fold), R76A (10-fold), and K93A (20-fold). Greater effects were observed with N73A (47-fold) and K94A (37-fold). Even greater effects (>200-fold) were observed with R89A and K91A, which were located near the middle of the α-D helix. The largest effects (>1,000-fold) were observed with R85A, T87A, and K90A, suggesting that these residues play the most critical roles in DNA binding. Of the five last mutants, only K91A exhibited a significant decrease in thermal stability (Fig. 3), suggesting that the drastic decreases in binding for the other four mutants could not be attributed to significant changes in protein structure or stability.
TABLE 3.
Effects of Fis mutations on DNA binding
| Fis protein | fis P II Kd (nM)a | hin distal Kd (nM)a | λ attR Kd (nM)a |
|---|---|---|---|
| WT | 1.0 ± 0.1 | 1.0 ± 0.2 | 4.1 ± 0.4 |
| N73A | 47 ± 8 | 65 ± 7 | 91 ± 39 |
| Q74A | 1.7 ± 2 | 3.8 ± 0.5 | 6 ± 1.1 |
| T75A | 8 ± 1 | 6.3 ± 0.7 | 29 ± 13 |
| R76A | 10 ± 1 | 6 ± 0.9 | 37 ± 10b |
| N84A | 1.5 ± 0.2 | 1.9 ± 2.1 | 157 ± 15 |
| R85Ac | >2,200d | >2,200d | >2,200d |
| T87A | 1,100 ± 316 | >2,200d | 1,300 ± 545 |
| R89A | 232 ± 59 | >2,200d | 466 ± 271 |
| K90Ac | >2,200d | >2,200d | >2,200d |
| K91A | 24 ± 18 | >2,200d | >2,200d |
| K93A | 20 ± 2 | 9 ± 4 | 46 ± 15 |
| K94A | 37 ± 8 | 6.4 ± 1.4 | 18 ± 11 |
| N98A | 1.0 ± 0.1 | 1.2 ± 0.6 | 2.7 ± 0.7 |
Gel mobility shift assays were performed as described in the legend to Fig. 1 with 42-bp DNA fragments carrying the fis P II, hin distal, or λ attR sequences. The percentage of bound DNA at equilibrium was plotted against the Fis concentration used, as shown in Fig. 1A, and the concentrations required to achieve 50% binding were considered approximate Kd values for the binding reactions. The data are averages ± standard deviations for three experiments unless indicated otherwise.
The data are the average ± standard deviation for two experiments.
No discrete bound complexes were observed with this mutant.
The maximum Fis concentration used in the assays was 2.2 μM, at which less than 50% binding was observed.
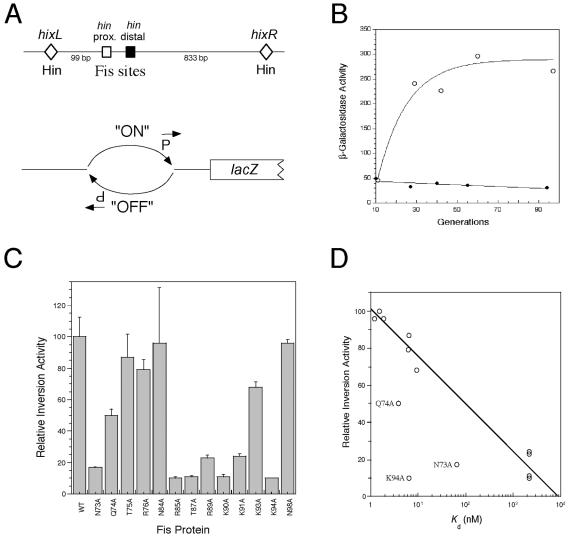
We developed a repression assay using a fis P::lacZ fusion that relied on the ability of Fis to bind the fis P II site in vivo (Fig. 4A). A Western blot analysis of three separate cultures (data not shown) indicated that the levels of the Fis mutants were within twofold of the levels of WT Fis when they were expressed from pRJ807-derived plasmids. Only N73A and K94A fell outside this range, with about 10-fold decreases in protein levels compared to WT Fis. We compared the mutant Fis proteins to the WT in terms of their ability to repress fis P and expressed the results as percentages of repression (Fig. 4B). Overall, there seemed to be a good correspondence between the effects of the mutations on binding affinities to fis P II in vitro and fis P II-mediated repression activity in vivo. Indeed, if N73A and K94A were excluded, there was an exponential correlation (R = 0.98) between the two relative activities (Fig. 4C), demonstrating that the in vivo repression assay is sensitive to differences in apparent Kd values observed in vitro within 3 orders of magnitude.
FIG. 4.
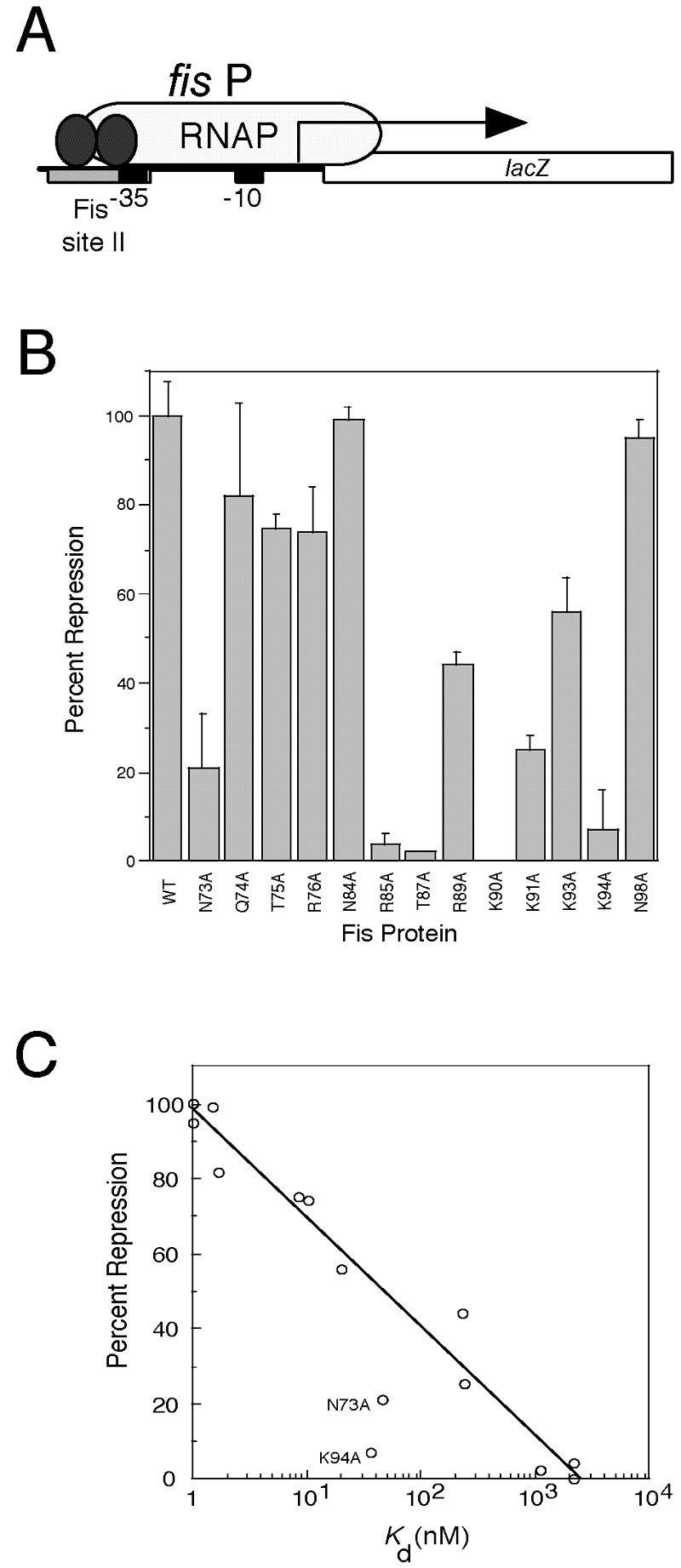
Effect of Fis mutations on fis P II site-mediated repression in vivo. (A) Schematic diagram of the fis promoter::lacZ fusion used in the repression assay. The fis promoter (fis P) region from position −83 to position +5, which carries the fis P II Fis site centered at position −44 (gray rectangle), the fis P −35 and −10 regions (black boxes), and the fis P transcription initiation region (arrow), is fused to lacZ and placed as a single copy in the chromosome. Fis (two ellipses) binds to the single fis P II site to hinder RNA polymerase (RNAP) binding and cause transcription repression. (B) Relative fis P repression activities of the Fis mutants used in this study. The percentages of repression are calculated from β-galactosidase activity measurements and are averages for at least three independent assays; the error bars indicate standard deviations. (C) Correlation (R = 0.98) between the percent repression and the logarithm of Kd app from the in vitro binding to fis P site II for the majority of the Fis proteins. The exceptions were the values for N73A and N94A, which deviated significantly from the curve and are labeled.
Fis residues required for binding at hin distal and λ attR sites.
The 15-bp core sequences of the hin distal and λ attR sites differ from each other at nine positions and from fis P II at 5 and 10 positions, respectively (Fig. 1B). Given the high binding affinity of WT Fis at these sites, we asked whether DNA binding contributions made by the Fis residues examined in this work were similar at the three sites or if they varied substantially. Thus, we performed GEMSA with DNA fragments containing the hin distal or λ attR 15-bp core sequences and each of the 13 Fis mutants. While the effects of many of the Fis mutants on relative binding to the two sequences were roughly similar to the effects observed at fis P II within about a twofold range, some of the mutants exhibited greater variation (Table 3). Q74A had less than a twofold effect at fis P II and λ attR compared to WT Fis and about a 3.8-fold effect at the hin distal site; N98A had less than a twofold effect at all three sites. Moderate losses of DNA binding were observed with T75A (∼6- to 8-fold), R76A (∼6- to 10-fold), and K93A (∼9- to 20-fold). Larger and more variable effects were seen with N73A (22- to 65-fold), N84A (∼1.5- to 38-fold), and K94A (∼4- to 37-fold). The variable effects of N84A were particularly notable because they seemed to be negligible at the fis P II (Kd app, 1.5 nM) and hin distal (Kd app, 1.9 nM) sites but were substantial at the λ attR site (Kd app, 157 nM). The most severe effects were generally observed with R85A, T87A, R89A, K90A, and K91A, with R85A, T87A, and K90A consistently requiring concentrations in the range required for nonspecific binding at all three sites. The effect of R89A was more severe at the hin distal site (Kd, >2,200 nM) than at the fis P II site (Kd, 232 nM) or the λ attR site (Kd, 466 nM), and the effect of K91A was more severe at the hin distal and λ attR sites (Kd, >2,200 nM) than at the fis P II site (Kd, 247 nM).
Effects of various flanking sequences.
Because the DNA fragments carrying the fis P II, hin distal, and λ attR Fis sites all contained the same plasmid-derived flanking sequences (Fig. 1B), differences in their binding activities could be attributed to differences in their central 15-bp core sequences. However, it has been observed that flanking sequences can affect the extent of Fis-induced DNA bending angles by as much as 50° and, in some cases, can also alter the binding affinity by as much as threefold (55). We found that WT Fis exhibited a 12-fold increase in binding affinity at the λ attR Fis site when the core sequence was immediately flanked on each side by 5 bp of naturally occurring sequence followed by a plasmid-derived sequence (λ attRnat), in contrast to an exclusively plasmid-derived sequence (λ attRpd) (Table 4). GEMSA using circularly permuted DNA fragments greater in length than 400 bp showed that the Fis-λ attRnat complex caused a greater shift in electrophoretic mobility than the Fis-λ attRpd complex when the binding sites were near the center of the fragment but not when they where near the ends (Fig. 5). This suggests that the flanking sequences of λ attRnat are more conducive to a Fis-induced DNA bend than the sequences flanking λ attRpd.
TABLE 4.
Effects of λ attR flanking sequences on binding of wild-type and mutant Fis
| Fis protein | λ attRpdKd (nM)a | λ attRnatKd (nM)a |
|---|---|---|
| WT | 4.1 ± 0.4 | 0.33 ± 0.05 |
| N73A | 91 ± 39 | 239 ± 29 |
| Q74A | 6 ± 1 | 1.2 ± 0.0 |
| T75A | 29 ± 13 | 54 ± 7 |
| R76A | 37 ± 10b | 30 ± 1 |
| N84A | 157 ± 15 | 169 ± 41 |
| R85A | >2,200 | >2,200 |
| T87A | 1,300 ± 545 | 1,420 ± 30 |
| R89A | 466 ± 271 | 12 ± 0.1 |
| K90A | >2,200 | >2,200 |
| K91A | >2,200 | 416 ± 20 |
| K93A | 46 ± 15 | 100 ± 16 |
| K94A | 18 ± 11 | 16 ± 2 |
| N98A | 2.7 ± 0.7 | 0.7 ± 0.3 |
Gel mobility shift assays were performed as described in the legend to Fig. 1 with a 42-bp DNA fragment carrying λ attRpd, the λ attR 15-bp core sequence flanked by plasmid-derived sequences, as shown in Fig. 1B, or with a 53-bp DNA fragment carrying λ attRnat, the λ attR sequence flanked on each side by 5-bp natural sequences. The apparent Kd values are averages ± standard deviations for three binding assays, unless indicated otherwise.
The data are the average ± standard deviation for two experiments.
FIG. 5.
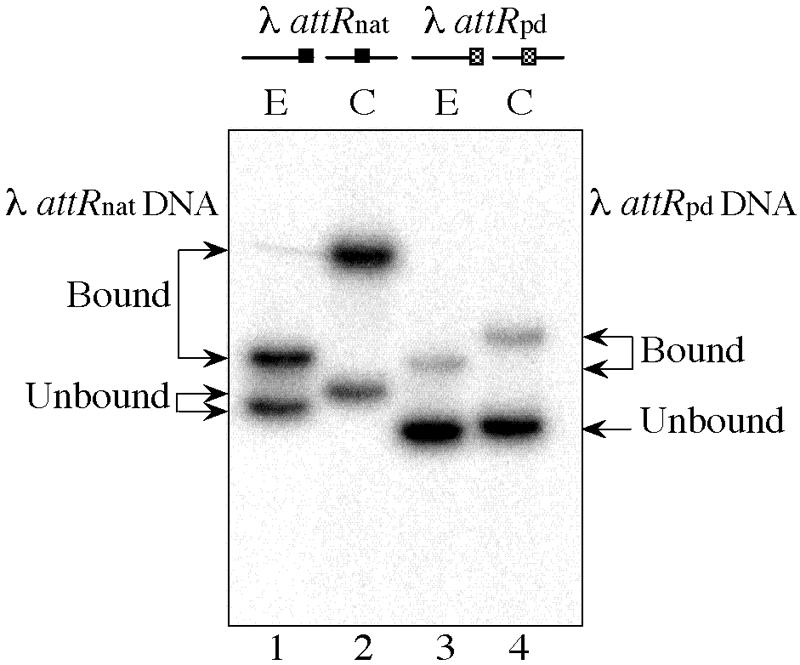
Effect of the λ attR flanking sequence on Fis-induced DNA bending. Gel mobility shift assays were performed with 0.5 nM Fis and either a 424-bp DNA fragment carrying the λ attR site immediately flanked on either side by 5 bp of natural sequence (λ attRnat) (lanes 1 and 2) or a 413-bp DNA fragment carrying the λ attR site flanked by a plasmid-derived sequence (λ attRpd) (lanes 3 and 4). Fis sites were placed near the end (E) (lanes 1 and 3) or near the center (C) (lanes 2 and 4) of the DNA fragments. The positions of unbound and bound DNA are indicated by arrows on the left and right for λ attRnat and λ attRpd, respectively.
A greater DNA bend provoked by the flanking sequence may allow the DNA to wrap around the sides of Fis in order to facilitate additional Fis-DNA contacts in the flanking region. Contacts in the core-binding region might also be altered by conformational changes inherent in the highly bent complex. Thus, we examined the binding of each of the Fis mutants to DNA fragments containing λ attRpd or λ attRnat (Table 4). Relatively minor differences in binding affinities were observed between the two sites when R76A, N84A, R85A, T87A, K90A, or K94A was used in these assays. It is worth noting that N84A caused a substantial decrease in DNA binding at λ attR irrespective of the flanking sequence and that the DNA binding with R85A, T87A, and K90A was severely reduced, suggesting that the roles of these residues were not altered by the flanking sequence. N73A, T75A, and K93A exhibited close to twofold decreases in binding to λ attRnat compared to λ attRpd, despite the improved WT Fis binding at λ attRnat. Several improvements in the binding affinities were observed at λ attRnat compared to λ attRpd, the most dramatic of which was with R89A, which resulted in an apparent Kd of about 466 nM at λ attRpd and in an apparent Kd of about 12 nM at λ attRnat. Thus, it appears that the conformational change produced by the flanking sequence alters the DNA binding contributions of a subset of the Fis residues.
Effect of Fis mutations on stimulation of DNA inversion and λ DNA excision in vivo.
To evaluate the in vivo significance of the relative in vitro binding affinities of the various Fis mutants at the hin distal and λ attR sites, we examined their abilities to promote Hin-mediated DNA inversion and λ excision in vivo. We adapted a previously developed qualitative Hin-mediated DNA inversion assay (29, 53) (Fig. 6A) to obtain a quantitative β-galactosidase-based assay that allowed us to measure the relative effects of the various Fis mutants on this process. The β-galactosidase activity in RJ2539 transformed with pRJ807 increased in a growing culture until after about 50 generations, after which nearly maximal β-galactosidase activity was observed (Fig. 6B). RJ2539 was then transformed with plasmids expressing each of the Fis mutants, grown for 52 to 56 generations, and analyzed for β-galactosidase activity, which was considered a measure of the relative DNA inversion activity (Fig. 6C). In general, the results showed that there was good agreement between the effects on relative DNA inversion activity in vivo and the relative binding affinities to the hin distal site in vitro. With the exception of N73A, Q74A, and K94A Fis, a logarithmic correlation (R = 0.98) could be fitted to these relative effects (Fig. 6D), suggesting that the relative differences in DNA inversion activity could be largely attributed to the effects of Fis binding to the hin distal site (and probably also to the hin proximal site).
FIG. 6.
Effect of Fis mutations on stimulation of hin-mediated DNA inversion in vivo. (A) Schematic diagrams of the hin inversion region. The top diagram shows the relative positions of the hixL and hixR Hin binding sites (diamonds) and the proximal (open square) and distal (solid square) Fis binding sites. Distances (in bp) between hixL and the proximal Fis site and between hixR and the distal Fis site are indicated. The lower diagram shows the λ fla406 construct on the E. coli chromosome used in the in vivo Hin-mediated DNA inversion assay. The promoter located within the invertible DNA segment is initially in the “OFF” position. In the presence of Hin and functional Fis, the DNA is inverted to the “ON” position and lacZ is expressed. (B) Effect of Fis on Hin-mediated DNA inversion in vivo. E. coli strain RJ2539 (λ fla406 ‘off’) was transformed with either pRJ807 (○) or pRJ1122 (•) and grown for up to 96 generations. The results of β-galactosidase assays performed at various times after growth were plotted against the number of generations. (C) Relative DNA inversion stimulation by WT and mutant Fis. The DNA inversion activity relative to WT Fis after 52 to 56 generations was calculated as described in Material and Methods. The results are averages from three experiments, and the error bars indicate standard deviations. (D) Correlation (R = 0.98) between the relative inversion activity and the logarithm of Kd from the in vitro binding activity to the hin distal Fis site for the majority of the Fis mutants. The exceptions are the values for N73A, N94A, and Q74A, which deviated significantly from the curve and are labeled.
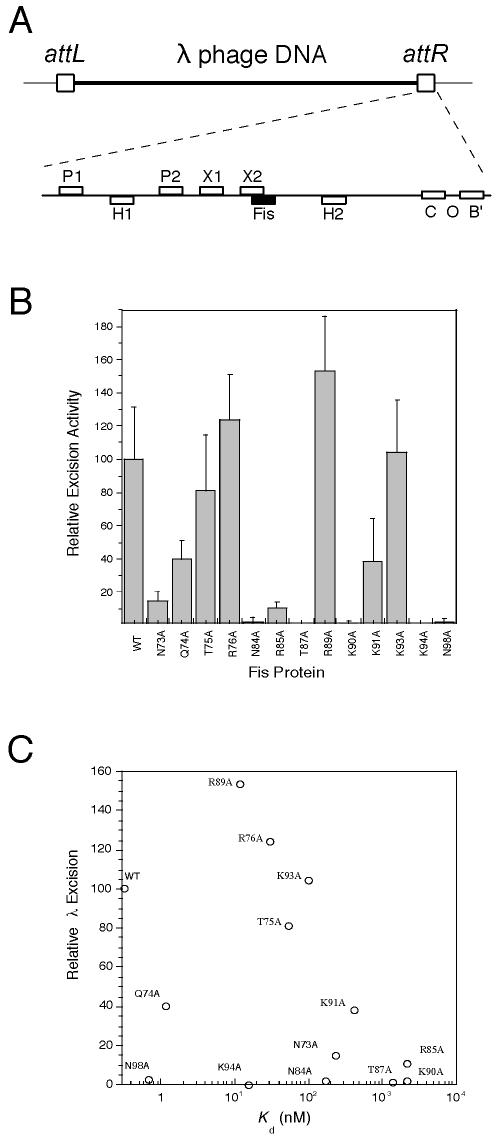
Fis binding to a single site within λ attR (Fig. 7A) results in cooperative interactions with λ excisionase (Xis) bound at the X1 site (40) and works together with Xis, integration host factor (IHF), and integrase (Int) to stimulate excision of λ phage DNA from the chromosome (5, 37). Thermal induction of λ phage excision from the chromosome has been shown to generate a >200-fold increase in the resulting phage titer in the presence of Fis compared to the titer in its absence (5, 53), an effect that is abolished when the λ attR Fis site is mutated. Thus, we used the phage titer assay as an indicator of relative λ DNA excision frequencies stimulated by WT or mutant Fis.
FIG. 7.
Effect of Fis mutations on stimulation of λ phage DNA excision. (A) Schematic diagrams of the λ phage attR region. The top diagram shows the λ phage DNA (thick line) flanked by attL and attR (boxes) and inserted into the E. coli chromosome (thin lines). The attR region is expanded beneath this diagram to illustrate the relative positions of the integrase core region (C and B′) and attR arm binding sites (P1 and P2), as well as the IHF (H1 and H2), Xis (X1 and X2), and Fis (solid box) binding sites. (B) Stimulation of λ phage DNA excision by WT or mutant Fis. The data are based on PFU/ml values obtained from RJ1765 in the presence of WT or mutant Fis and are averages from at least three independent assays; the error bars indicate standard deviations. (C) There was not a general correlation between the relative λ phage DNA excision activities and Kd values obtained from in vitro binding to the λ attRnat site. Use of Kd values for the λ attRpd site did not improve the correlation (not shown).
Thermoinduction of lysogenic strain RJ1765 resulted in about a 240-fold increase in the phage titer in the presence of WT Fis. When the same cells were transformed with the various pRJ807-related plasmids expressing our Fis mutants, a wide range of relative λ excision activities were observed (Fig. 7B). In this case, no overall correlation between the relative λ excision activities and relative λ attR site binding affinities could be clearly delineated (Fig. 7C). This may have been due in part to the cooperative interactions between Fis and Xis, which may have compensated for some of the DNA binding defects in Fis. Nonetheless, the effects of many of the Fis mutants on λ excision were compatible with their relative binding affinities to λ attR. For instance, the poor excision activities of N84A, R85A, T87A, and K90A were anticipated from their relatively poor DNA binding activities in vitro. T75A and K91A exhibited decreasing λ excision activities, which roughly mirrored their relative effects on λ attR binding. Because N73A and K94A were poorly expressed in vivo compared to the remainder of the Fis proteins, we cannot unequivocally interpret their seemingly poor abilities to stimulate λ excision. R89A resulted in phage titers that were higher than those of the WT. This effect may have been facilitated to a large degree by the substantial increase in R89A binding affinity to λ attR that was observed in the context of the naturally occurring flanking sequence (Table 4). R76A and K93A resulted in wild type-levels of excision activity despite the measurable reductions in DNA binding activities in vitro (Kd, 30 nM and 100 nM, respectively), suggesting that the λ excision reaction in vivo is able to accommodate certain deficiencies in DNA binding observed in vitro. However, Q74A and N98A resulted in significant reductions in excision activity despite their near-WT levels of DNA binding affinity and in vivo expression levels, suggesting that they play important roles in stimulating λ excision by providing a critical function other than DNA binding.
DISCUSSION
Specificity of Fis-DNA binding.
Consistent with its role as a nucleoid-associated protein, Fis is able to interact with a wide range of DNA sequences (17). Poorly related sequences can be recognized with a remarkable degree of specificity. As observed in this work, Fis binds several different sites with Kd app values between 3 × 10−10 and 4 × 10−9 M, which are comparable to the values reported for other specific DNA-binding proteins, such as IHF, λ repressor, 434 repressor, and Fur (18, 41, 48, 76). When the match to the consensus sequence was obliterated, binding occurred with a Kd app of about 4.5 × 10−6 M, and the resulting bound complexes failed to form discrete bands during GEMSA, suggesting that nonspecific weak occupancy of DNA occurred at the high Fis concentrations. In addition, discrete bound complexes were not observed with R85A or K90A, and only a small fraction of bound complexes were observed with T87A even when these proteins were present at micromolar concentrations, suggesting that binding specificity was severely reduced in these mutants. Moreover, the in vivo repression activities were negligible, suggesting that specific recognition of fis P II in vivo was impaired. Hence, a difference in Fis concentration of more than 3 orders of magnitude distinguishes highly specific DNA binding from nonspecific DNA binding.
We noted that highly specific DNA binding could easily be observed within a 12-fold range of binding affinity, such as that when WT Fis interacts with λ attRnat or λ attRpd. Moreover, K91A and R89A, which bind the fis P II site with Kd app values of 247 and 232 nM, respectively, to produce discrete band shifts similar to those of WT Fis, still exhibit more than a 10-fold increase in DNA binding affinity compared to nonspecific binding. K91A and R89A are capable of generating 30 to 50% of the maximum fis P repression activity in vivo (Fig. 4), a function that relies on specific binding to the fis P II site (50, 58). Thus, appreciable DNA selectivity may still occur in a range of DNA binding affinities of more than 2 orders in magnitude (e.g., with Kd app values ranging from 0.33 to 247 nM). This implies that multiple Fis-DNA complexes observed during GEMSA when large DNA fragments are bound with a range of Fis concentrations do not necessarily represent random DNA binding if the affinities are within 1 or 2 orders of magnitude of that for a highly specific Fis binding site.
Cellular Fis protein levels have been estimated to vary from less than 50 to more than 25,000 dimers per cell during the first 75 min of outgrowth from the stationary phase in rich medium (6). Assuming that the average cell volume is about 1 μm3 (47), Fis concentrations can be estimated to vary from less than 80 nM to more than 40 μM during growth phase transitions. Changes in concentrations can be expected to switch the emphasis for the gamut of Fis-mediated processes from those that rely exclusively on highly specific DNA interactions (e.g., regulation of transcription and site-specific DNA recombination) to processes that rely on nonspecific interactions (e.g., alterations in DNA topology). Between these two extremes the chromosome exhibits a number of specific DNA binding sites with various affinities, which may be effectively occupied when proper intracellular Fis concentrations are generated. This provides an excellent opportunity for cells to control processes that rely on specific Fis binding to sites having relatively low or intermediate strength.
Effect of alanine substitutions on protein stability.
While most alanine substitutions had small or negligible effects on the thermal stability, the decreases in the Tm of about 14 to 16°C observed for N73A, K91A, and K94A suggest that these mutations appreciably altered the structure or stability of the protein. There is substantial evidence that there are strong preferences for certain N-cap residues located prior to the first residue in an α helix, which help stabilize the helix by forming hydrogen bonds between their side chains and accessible backbone amines of N-terminal residues in an α helix (14, 60, 69). Asparagine has been recognized as the most favorable N-cap residue, while alanine is a relatively poor one (14). Indeed, in the Fis crystal structure (39, 63, 77), the carbonyl oxygen in the side chain of N73 forms a hydrogen bond with the backbone amine of R76 in the α-C helix, suggesting that N73 is an α-C helix N-cap residue. This obscures interpretation of our finding that N73A results in 47- and 65-fold reductions in binding at the fis P II and hin distal sites, respectively, as these effects may be attributed in part or in whole to its α-C helix stabilizing role. Our observation that N73A results in a more severe reduction in binding to λ attRnat than to λ attRpd may indicate an additional role for N73 in contacting the DNA backbone in the flanking region, particularly when its sequence is more conducive to DNA bending.
The crystal structure shows that the side chains of K91 and K94 in the α-D helix form intramolecular salt bridges with E59 and E52 in the α-B helix, respectively. Thus, replacement of K91 or K94 with alanine should disrupt these putative salt bridges, which should decrease the Tm. Despite the similar effects of N73A, K91A, and K94A on protein stability, K91A resulted in a much greater loss of DNA binding affinity than N73A or K94A (Table 3), suggesting that K91 may also participate directly in DNA binding. In a growing number of DNA binding proteins, it has been observed that salt bridges formed near the DNA binding surface can become disrupted during DNA binding so that the cationic side chain can be free to interact with DNA (23, 57, 62). A similar process may occur with K91 in Fis. However, it is also possible that structural alterations caused by K91A affect the DNA binding capability of the neighboring K90 residue.
Since the in vivo levels of N73A and K94A were considerably lower than those of the other mutant and WT Fis proteins, it was not surprising that their relative in vivo activities were lower than expected based on their relative DNA binding affinities. We previously observed that the P61A mutation, which decreases the stability of the C-terminal region, results in increased susceptibility to proteolytic cleavage (27). Thus, it is possible that structural disturbances caused by N73A and K94A in the C-terminal region also render Fis more susceptible to proteolytic attack in vivo.
Fis residues required for DNA binding.
A qualitative summary of the effects of Fis mutations on the relative binding affinities to the various Fis binding sites analyzed is shown in Table 5. In general, the α-D helix residues R85, T87, R89, K90, and K91 were the most critical residues in achieving highly specific DNA binding. Of these, R85, T87, and K90 were consistently vital for highly specific binding at all sites examined, suggesting that they may have obligatory roles in achieving binding specificity at all Fis sites. A previously proposed docking model placed a hydrogen bond between the R85 side chain and the O6 position of the first G base (G1) in the 15-bp core sequence of the hin distal site and another hydrogen bond between the K90 side chain and N7 of the G base at position 12 (16, 55). This is in good agreement with the findings that (i) loss of R85 or K90 consistently abolished specific DNA binding, (ii) despite the poorly related sequences at Fis binding sites, G1 is the most highly conserved base and a purine (either of which provides the suggested N7 contact for K90) is conserved at position 12, and (iii) other solved protein-DNA structures involving HTH DNA binding proteins, such as lac repressor, λ Cro repressor, and CAP, have side chains at analogous positions in their recognition helices, forming hydrogen bonds with specific bases via the major groove (2, 36, 68). A threonine in the third position of the recognition helix in the lac repressor makes a phosphate contact in the operator region (36). T87 is in an analogous position in Fis and may thus play a similar role.
TABLE 5.
Summary of the effects of Fis mutations on DNA binding
| Fis mutation | Fis binding site
|
Helix locationb | |||
|---|---|---|---|---|---|
| fis P IIa | hin distala | λ attRpda | λ attRnata | ||
| WT | ++++ | ++++ | +++ | ++++ | |
| N73A | ++ | ++ | ++ | + | |
| Q74A | ++++ | ++++ | +++ | ++++ | α-C |
| T75A | +++ | +++ | ++ | ++ | α-C |
| R76A | +++ | +++ | ++ | ++ | α-C |
| N84A | ++++ | ++++ | + | + | |
| R85A | − | − | − | − | α-D |
| T87A | − | − | − | − | α-D |
| R89A | + | − | + | +++ | α-D |
| K90A | − | − | − | − | α-D |
| K91A | + | − | − | + | α-D |
| K93A | +++ | +++ | ++ | + | α-D |
| K94A | ++ | +++ | +++ | +++ | α-D |
| N98A | ++++ | ++++ | ++++ | ++++ | |
The relative DNA binding affinities are qualitatively summarized as follows: ++++, Kdapp of <4 nM; +++, Kdapp of 4 to 20 nM; ++, Kdapp of 20 to 100 nM; +, Kdapp of 100 to 1,000 nM; −, Kdapp of >1,000 nM.
α-C and α-D are the two α-helices that form the HTH motif, as shown in Fig. 2.
Glutamine at the beginning of the HTH region is often found to contact a phosphate on the DNA backbone in other protein-DNA complexes (1, 2, 34), and asparagine at the carboxy-terminal end of Fis appears to come near the DNA backbone in docking models (16, 65, 72). Thus, it was somewhat surprising that Q74A and N98A had minimal effects on the binding affinities at all three sites, suggesting that these residues are not essential for DNA binding. The remainder of the residues examined (N73, T75, R76, N84, K93, and K94) contributed to various degrees to the binding affinity, depending on the residue and the Fis binding site (Table 5). These residues may contribute to the overall binding energy via DNA backbone interactions. The side chains of N73, T75, R76, and K93 extend toward the flanking DNA region or the boundary of the core region in docking models (16, 65, 72) and may be required for stabilizing a Fis-mediated DNA bend through contacts with the outer region of a Fis binding site (Fig. 8A and B).
FIG. 8.
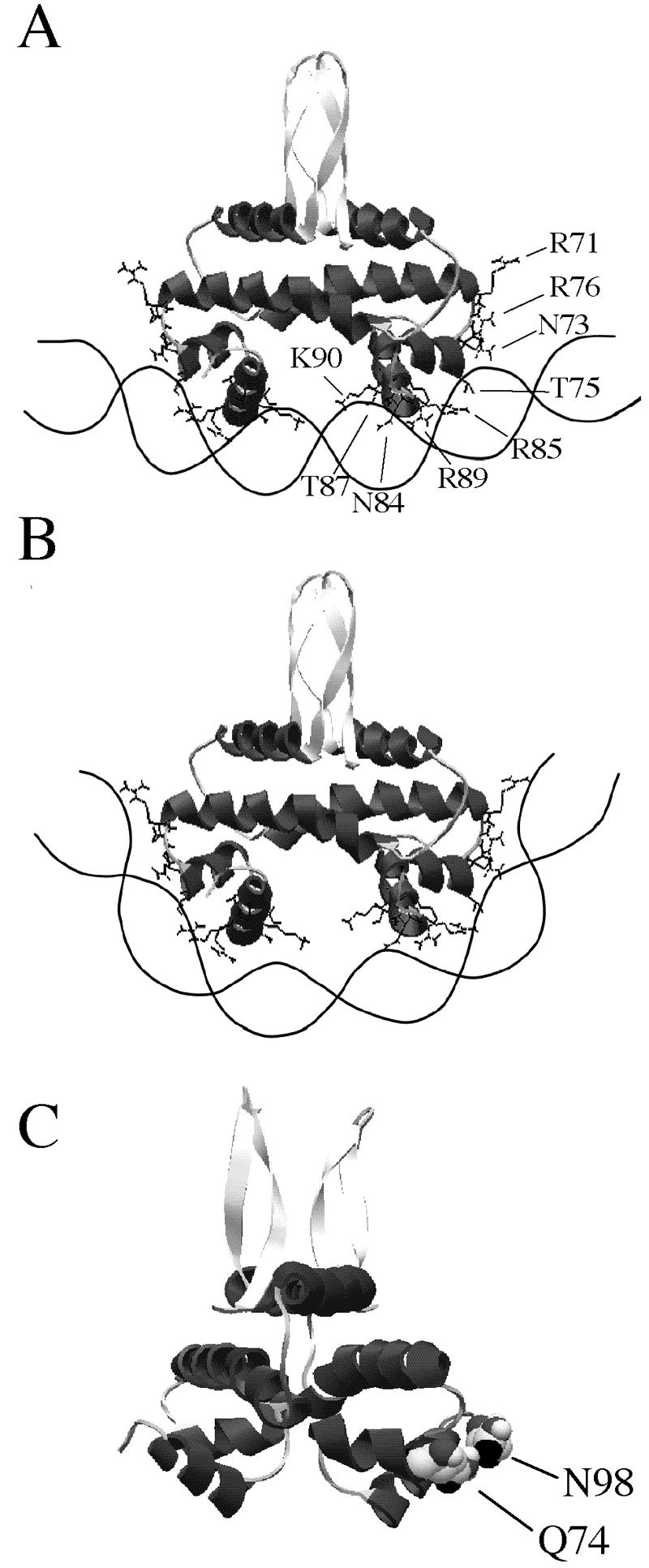
Variable roles of Fis residues. (A) Hypothetical schematic diagram of interactions between Fis and a high-affinity DNA binding site flanked by sequences having relatively low flexibility. Optimal interactions occur between Fis residues in the HTH region and the 15-bp core sequence, while fewer interactions occur with the flanking region. (B) When the Fis binding site is flanked by highly flexible sequences, the DNA is able to wrap around the sides of Fis so that optimal contact is made with the flanking region. This may compensate for possible weakening of interactions with the core region resulting from the conformational changes. (C) Ribbon model of a Fis dimer showing that Q74 and N98 (in a space-filling diagram) are next to each other and connected by a hydrogen bond on the Fis crystal structure. The side chains of these residues extend from the same surface of Fis and are similarly accessible for potential intermolecular interactions.
Variable contributions of N84, R89, and K91.
The effects of N84A, R89A, and K91A differed considerably when different Fis binding sites were used (Table 5). These effects were also seen in vivo since N84A resembled WT Fis in the ability to repress fis P (Fig. 4) and stimulate Hin-mediated DNA inversion (Fig. 6) but was completely unable to stimulate λ DNA excision (Fig. 7). This is a remarkable finding because it suggests that Fis relies on a different subset of residues for contacting the λ attR site than for contacting the fis P II or hin distal sites. We noticed that the central sequence of the λ attR site forms an A6 tract that is not formed in the other Fis sites. This sequence, which has been shown in crystals to adopt a nonbent structure (13, 20, 21), may alter the local structure in the λ attR core region in order to both enable and require N84 contacts with the DNA backbone or a base. This exemplifies the agility of Fis in adjusting to different DNA sequences and structures to achieve high binding specificity.
R89A was unable to bind to the hin distal or λ attRpd site but was able to bind weakly to the fis P II site and very efficiently to the λ attRnat site (Table 5). The relative effects of R89A in vivo were compatible with its DNA binding behavior: poor stimulation of DNA inversion (Fig. 6), moderate fis P repression (Fig. 4), and excellent stimulation of λ excision (Fig. 7). The latter effect suggests that interactions with λ attRnat more closely resemble interactions at the λ attR site in vivo than interactions with λ attRpd.
Fis-induced DNA bending was enhanced at λ attRnat, where R89 was not essential, compared to λ attRpd, where R89 was essential. Docking models of the hin distal site place the R89 side chain near the boundary between the 15-bp core and the flanking sequence (16, 65, 72). It is possible that contacts between R89 and the core-flanking boundary are more critical when the flanking DNA is less prone to bending than when it is more easily bent. Flexible flanking DNA may come in contact with the sides of Fis to help stabilize the bound complex, even in the absence of R89. However, when the flanking DNA is less prone to bending, interactions with the flanking sequence are less likely and the complex relies strongly on R89-DNA contacts. Thus, while a role for R89 may not be imperative at all Fis binding sites, it may help salvage binding to numerous sites flanked by sequences not amenable to bending. In this sense, R89 may be seen to play an important role in broadening the scope of DNA sequences to which Fis may bind specifically.
Contributions by the 15-bp core site and the flanking DNA sequences.
The most important interactions are those between Fis and the 15-bp core region because sequence replacement in this region had the most severe effects on binding. The flanking sequence can also affect the binding affinity by as much as 12-fold and can affect the extent of Fis-induced DNA bending. A previous study showed that there was a 2.5-fold reduction in Fis binding to the λ attR site when its normal flanking sequence was replaced with that of the hin distal site (55). The λ attR flanking sequence resulted in increased Fis-induced DNA bending at both the λ attR and hin distal sites compared to that at the hin distal flanking sequence, but it did not increase the binding affinity at the hin distal site. Thus, the effects of the flanking sequence on the binding affinity seemed to be more pronounced when the interaction with the core sequence was weaker. We have noticed a similar tendency with other Fis core binding sequences (Shao and Osuna, unpublished results).
In the CAP-DNA structure, two sharp DNA bends are produced by 40° kinks between TG-CA base pairs on each half-site, and the central region has an additional 8° bend that affects the DNA contact by a lysine residue (68). In the case of Fis, we envision a model in which the bound complex is able to achieve considerable stability when the core sequence confers optimal Fis interactions, even if interactions with the flanking region are inefficient (Fig. 8A). When a flexible flanking region facilitates a greater DNA bend, additional backbone interactions are possible in this region (Fig. 8B). The large conformational change can include smaller alterations in the structure of the central binding region that may suffice to alter certain interactions between Fis and the core-binding region. Putative weakening of contacts with the core region may be compensated for by additional contacts in the flanking region with minimal change in the binding affinity. In cases in which interactions with the core region are initially deficient, a flexible flanking region may result in a net increase in the binding affinity. A prediction from this model is that some residues should be essential only under conditions of limited DNA bending, which is what we observed with R89. On the other hand, N73, T75, and K93 stepped up their contributions to the binding affinity under conditions that facilitated DNA bending, an effect that should also be expected.
Roles of Q74 and N98 in vivo.
Q74A and N98A bound the fis P II, hin distal, and λ attR sites with affinities comparable to those of WT Fis. The fis P repression assay confirmed these observations. However, Q74A was considerably less efficient in terms of the ability to stimulate Hin-mediated DNA inversion (50% of wild-type levels) or λ excision (∼40% of wild-type levels). N98A showed normal stimulation of DNA inversion but was completely unable to stimulate λ excision (Fig. 6C and 7B). These results suggest that Q74 and N98 have an important function in vivo other than DNA binding during stimulation of λ excision or DNA inversion (in the case of Q74).
Q74 has been implicated in protein-protein interactions between Fis and the α-CTD of RNA polymerase at proP P2 and rrnB P1 (9, 43). Thus, it is plausible that Q74 may participate in contacting other proteins as well, such as the Hin recombinase during DNA inversion or Xis during λ DNA excision. Interestingly, N98 is located next to Q74 in the crystal structure (Fig. 8C). Therefore, N98 is similarly poised to participate in putative intermolecular interactions during λ DNA excision. Xis is an attractive candidate because it has been shown to bind cooperatively with Fis in the λ attR region (40, 71). Other possibilities that cannot be excluded at present include a role for one or both of these residues in achieving a sufficient degree of DNA bending required in these processes and a role in DNA binding required in the context of the chromosomal DNA, which can be altered by DNA supercoiling or DNA looping.
Concluding remarks.
The fact that most of the recognized sequences can be described by a degenerate consensus sequence implies that there is a minimally defined set of sequences that are recognized by Fis. This is mirrored by a minimally defined set of Fis residues (R85, T87, and K90) that are consistently critical in DNA recognition. The stability of the bound complex ultimately relies on the establishment of numerous additional DNA contacts. If, as imagined, the majority of these contacts are with the DNA backbone, then variations in binding affinity are highly influenced by the structure or flexibility of the core and flanking sequences. However, within limits, Fis is able to handle variations in DNA sequence or structure. The combinatorial effects of all these factors should contribute substantially to the variability of sequences recognized by Fis.
Acknowledgments
We are grateful to Sara Boswell and Liye Zhang for assisting with thermal denaturation experiments. We also thank Meranda D. Bradley for helpful suggestions.
This work was supported by Public Health Service grant GM52051 from the National Institutes of Health to R.O. and by National Science Foundation grant MCB 9984983 to W.C.
REFERENCES
- 1.Aggarwal, A. K., D. W. Rodgers, M. Drottar, M. Ptashne, and S. C. Harrison. 1988. Recognition of a DNA operator by the repressor of phage 434: a view at high resolution. Science 242:899-907. [DOI] [PubMed] [Google Scholar]
- 2.Albright, R. A., and B. W. Matthews. 1998. Crystal structure of lambda-Cro bound to a consensus operator at 3.0 Å resolution. J. Mol. Biol. 280:137-151. [DOI] [PubMed] [Google Scholar]
- 3.Ali Azam, T., A. Iwata, A. Nishimura, S. Ueda, and A. Ishihama. 1999. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 181:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustin, L. B., B. A. Jacobson, and J. A. Fuchs. 1994. Escherichia coli Fis and DnaA proteins bind specifically to the nrd promoter region and affect expression of an nrd-lac fusion. J. Bacteriol. 176:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball, C. A., and R. C. Johnson. 1991. Efficient excision of phage lambda from the Escherichia coli chromosome requires the Fis protein. J. Bacteriol. 173:4027-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball, C. A., R. Osuna, K. C. Ferguson, and R. C. Johnson. 1992. Dramatic changes in Fis levels upon nutrient upshift in Escherichia coli. J. Bacteriol. 174:8043-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beach, M. B., and R. Osuna. 1998. Identification and characterization of the fis operon in enteric bacteria. J. Bacteriol. 180:5932-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betermier, M., D. J. Galas, and M. Chandler. 1994. Interaction of Fis protein with DNA: bending and specificity of binding. Biochimie 76:958-967. [DOI] [PubMed] [Google Scholar]
- 9.Bokal, A. J., W. Ross, T. Gaal, R. C. Johnson, and R. L. Gourse. 1997. Molecular anatomy of a transcription activation patch: FIS-RNA polymerase interactions at the Escherichia coli rrnB P1 promoter. EMBO J. 16:154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosch, L., L. Nilsson, E. Vijgenboom, and H. Verbeek. 1990. FIS-dependent trans-activation of tRNA and rRNA operons of Escherichia coli. Biochim. Biophys. Acta 1050:293-301. [DOI] [PubMed] [Google Scholar]
- 11.Bruist, M. F., A. C. Glasgow, R. C. Johnson, and M. I. Simon. 1987. Fis binding to the recombinational enhancer of the Hin DNA inversion system. Genes Dev. 1:762-772. [DOI] [PubMed] [Google Scholar]
- 12.Cheng, Y. S., W. Z. Yang, R. C. Johnson, and H. S. Yuan. 2000. Structural analysis of the transcriptional activation on Fis: crystal structures of six Fis mutants with different activation properties. J. Mol. Biol. 302:1139-1151. [DOI] [PubMed] [Google Scholar]
- 13.Dickerson, R. E., D. S. Goodsell, and S. Neidle. 1994. “The tyranny of the lattice…” Proc. Natl. Acad. Sci. USA 91:3579-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doig, A. J., and R. L. Baldwin. 1995. N- and C-capping preferences for all 20 amino acids in alpha-helical peptides. Protein Sci. 4:1325-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubendorff, J. W., and F. W. Studier. 1991. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J. Mol. Biol. 219:45-59. [DOI] [PubMed] [Google Scholar]
- 16.Feng, J.-A., H. A. Yuan, S. E. Finkel, R. C. Johnson, M. Kaczor-Grzeskowiak, and R. E. Dickerson. 1992. The interaction of Fis protein with its DNA-binding sequences, p. 1-9. In R. H. Sarma and M. H. Sarma (ed.), Structure & function, vol. 2. Adenine Press, Schenectady, NY. [Google Scholar]
- 17.Finkel, S. E., and R. C. Johnson. 1992. The Fis protein: it's not just for DNA inversion anymore. Mol. Microbiol. 6:3257-3265. [DOI] [PubMed] [Google Scholar]
- 18.Fromknecht, K., P. D. Vogel, and J. G. Wise. 2003. Combinatorial redesign of the DNA binding specificity of a prokaryotic helix-turn-helix repressor. J. Bacteriol. 185:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Gil, G., P. Bringmann, and R. Kahmann. 1996. FIS is a regulator of metabolism in Escherichia coli. Mol. Microbiol. 22:21-29. [DOI] [PubMed] [Google Scholar]
- 20.Goodsell, D. S., and R. E. Dickerson. 1994. Bending and curvature calculations in B-DNA. Nucleic Acids Res. 22:5497-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodsell, D. S., M. Kaczor-Grzeskowiak, and R. E. Dickerson. 1994. The crystal structure of C-C-A-T-T-A-A-T-G-G. Implications for bending of B-DNA at T-A steps. J. Mol. Biol. 239:79-96. [DOI] [PubMed] [Google Scholar]
- 22.Green, J., M. F. Anjum, and J. R. Guest. 1996. The ndh-binding protein (Nbp) regulates the ndh gene of Escherichia coli in response to growth phase and is identical to Fis. Mol. Microbiol. 20:1043-1055. [DOI] [PubMed] [Google Scholar]
- 23.Grove, A. 2003. Surface salt bridges modulate DNA wrapping by the type II DNA-binding protein TF1. Biochemistry 42:8739-8747. [DOI] [PubMed] [Google Scholar]
- 24.Haffter, P., and T. A. Bickle. 1987. Purification and DNA-binding properties of FIS and Cin, two proteins required for the bacteriophage P1 site-specific recombination system, Cin. J. Mol. Biol. 198:579-587. [DOI] [PubMed] [Google Scholar]
- 25.Heichman, K. A., and R. C. Johnson. 1990. The Hin invertasome: protein-mediated joining of distant recombination sites at the enhancer. Science 249:511-517. [DOI] [PubMed] [Google Scholar]
- 26.Hengen, P. N., S. L. Bartram, L. E. Stewart, and T. D. Schneider. 1997. Information analysis of Fis binding sites. Nucleic Acids Res. 25:4994-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobart, S. A., D. W. Meinhold, R. Osuna, and W. Colon. 2002. From two-state to three-state: the effect of the P61A mutation on the dynamics and stability of the factor for inversion stimulation results in an altered equilibrium denaturation mechanism. Biochemistry 41:13744-13754. [DOI] [PubMed] [Google Scholar]
- 28.Hubner, P., P. Haffter, S. Iida, and W. Arber. 1989. Bent DNA is needed for recombinational enhancer activity in the site-specific recombination system Cin of bacteriophage P1. The role of FIS protein. J. Mol. Biol. 205:493-500. [DOI] [PubMed] [Google Scholar]
- 29.Hughes, K. T., P. Youderian, and M. I. Simon. 1988. Phase variation in Salmonella: analysis of Hin recombinase and hix recombination site interaction in vivo. Genes Dev. 2:937-948. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson, B. A., and J. A. Fuchs. 1998. Multiple cis-acting sites positively regulate Escherichia coli nrd expression. Mol. Microbiol. 28:1315-1322. [DOI] [PubMed] [Google Scholar]
- 31.Johnson, R. C., C. A. Ball, D. Pfeffer, and M. I. Simon. 1988. Isolation of the gene encoding the Hin recombinational enhancer binding protein. Proc. Natl. Acad. Sci. USA 85:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson, R. C., M. F. Bruist, and M. I. Simon. 1986. Host protein requirements for in vitro site-specific DNA inversion. Cell 46:531-539. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, R. C., and M. I. Simon. 1985. Hin-mediated site-specific recombination requires two 26 bp recombination sites and a 60 bp recombinational enhancer. Cell 41:781-791. [DOI] [PubMed] [Google Scholar]
- 34.Jordan, S. R., and C. O. Pabo. 1988. Structure of the lambda complex at 2.5 Å resolution: details of the repressor-operator interactions. Science 242:893-899. [DOI] [PubMed] [Google Scholar]
- 35.Kahmann, R., F. Rudt, C. Koch, and G. Mertens. 1985. G inversion in bacteriophage Mu DNA is stimulated by a site within the invertase gene and a host factor. Cell 41:771-780. [DOI] [PubMed] [Google Scholar]
- 36.Kalodimos, C. G., A. M. Bonvin, R. K. Salinas, R. Wechselberger, R. Boelens, and R. Kaptein. 2002. Plasticity in protein-DNA recognition: lac repressor interacts with its natural operator 01 through alternative conformations of its DNA-binding domain. EMBO J. 21:2866-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim, S., and A. Landy. 1992. Lambda Int protein bridges between higher order complexes at two distant chromosomal loci, attL and. attR. Science 256:198-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch, C., O. Ninnemann, H. Fuss, and R. Kahmann. 1991. The N-terminal part of the E. coli DNA binding protein FIS is essential for stimulating site-specific DNA inversion but is not required for specific DNA binding. Nucleic Acids Res. 19:5915-5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kostrewa, D., J. Granzin, D. Stock, H. W. Choe, J. Labahn, and W. Saenger. 1992. Crystal structure of the factor for inversion stimulation FIS at 2.0 Å resolution. J. Mol. Biol. 226:209-226. [DOI] [PubMed] [Google Scholar]
- 40.Landy, A. 1989. Dynamic, structural, and regulatory aspects of λ site-specific recombination. Annu. Rev. Biochem. 58:913-949. [DOI] [PubMed] [Google Scholar]
- 41.Lavrrar, J. L., and M. A. McIntosh. 2003. Architecture of a fur binding site: a comparative analysis. J. Bacteriol. 185:2194-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin, R. G., and J. L. Rosner. 1997. Fis, an accessorial factor for transcriptional activation of the mar (multiple antibiotic resistance) promoter of Escherichia coli in the presence of the activator MarA, SoxS, or Rob. J. Bacteriol. 179:7410-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLeod, S. M., S. E. Aiyar, R. L. Gourse, and R. C. Johnson. 2002. The C-terminal domains of the RNA polymerase alpha subunits: contact site with Fis and localization during co-activation with CRP at the Escherichia coli proP P2 promoter. J. Mol. Biol. 316:517-529. [DOI] [PubMed] [Google Scholar]
- 44.Meinhold, D., S. Boswell, and W. Colón. 2005. P61A mutation in the factor for inversion stimulation results in a thermostable dimeric intermediate. Biochemistry 44:14715-14724. [DOI] [PubMed] [Google Scholar]
- 45.Messing, J., R. Crea, and P. H. Seeburg. 1981. A system for shotgun DNA sequencing. Nucleic Acids Res. 9:309-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller, J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 47.Neidhardt, F. C. 1987. Chemical composition of Escherichia coli. American Society for Microbiology, Washington, DC.
- 48.Nelson, H. C., and R. T. Sauer. 1985. Lambda repressor mutations that increase the affinity and specificity of operator binding. Cell 42:549-558. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson, L., A. Vanet, E. Vijgenboom, and L. Bosch. 1990. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 9:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ninnemann, O., C. Koch, and R. Kahmann. 1992. The E. coli fis promoter is subject to stringent control and autoregulation. EMBO J. 11:1075-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Numrych, T. E., R. I. Gumport, and J. F. Gardner. 1991. A genetic analysis of Xis and Fis interactions with their binding sites in bacteriophage lambda. J. Bacteriol. 173:5954-5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Neil, K. T., and W. F. DeGrado. 1990. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science 250:646-651. [DOI] [PubMed] [Google Scholar]
- 53.Osuna, R., S. E. Finkel, and R. C. Johnson. 1991. Identification of two functional regions in Fis: the N-terminus is required to promote Hin-mediated DNA inversion but not lambda excision. EMBO J. 10:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osuna, R., D. Lienau, K. T. Hughes, and R. C. Johnson. 1995. Sequence, regulation, and functions of fis in Salmonella typhimurium. J. Bacteriol. 177:2021-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pan, C. Q., S. E. Finkel, S. E. Cramton, J. A. Feng, D. S. Sigman, and R. C. Johnson. 1996. Variable structures of Fis-DNA complexes determined by flanking DNA-protein contacts. J. Mol. Biol. 264:675-695. [DOI] [PubMed] [Google Scholar]
- 56.Pease, A. J., B. R. Roa, W. Luo, and M. E. Winkler. 2002. Positive growth rate-dependent regulation of the pdxA, ksgA, and pdxB genes of Escherichia coli K-12. J. Bacteriol. 184:1359-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pervushin, K., M. Billeter, G. Siegal, and K. Wuthrich. 1996. Structural role of a buried salt bridge in the 434 repressor DNA-binding domain. J. Mol. Biol. 264:1002-1012. [DOI] [PubMed] [Google Scholar]
- 58.Pratt, T. S., T. Steiner, L. S. Feldman, K. A. Walker, and R. Osuna. 1997. Deletion analysis of the fis promoter region in Escherichia coli: antagonistic effects of integration host factor and Fis. J. Bacteriol. 179:6367-6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prentki, P., M. H. Pham, and D. J. Galas. 1987. Plasmid permutation vectors to monitor DNA bending. Nucleic Acids Res. 15:10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson, J. S., and D. C. Richardson. 1988. Amino acid preferences for specific locations at the ends of alpha helices. Science 240:1648-1652. [DOI] [PubMed] [Google Scholar]
- 61.Ross, W., J. F. Thompson, J. T. Newlands, and R. L. Gourse. 1990. E. coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 9:3733-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saecker, R. M., and M. T. Record, Jr. 2002. Protein surface salt bridges and paths for DNA wrapping. Curr. Opin. Struct. Biol. 12:311-319. [DOI] [PubMed] [Google Scholar]
- 63.Safo, M. K., W. Z. Yang, L. Corselli, S. E. Cramton, H. S. Yuan, and R. C. Johnson. 1997. The transactivation region of the Fis protein that controls site-specific DNA inversion contains extended mobile beta-hairpin arms. EMBO J. 16:6860-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 65.Sandmann, C., F. Cordes, and W. Saenger. 1996. Structure model of a complex between the factor for inversion stimulation (FIS) and DNA: modeling protein-DNA complexes with dyad symmetry and known protein structures. Proteins 25:486-500. [DOI] [PubMed] [Google Scholar]
- 66.Schneider, R., R. Lurz, G. Luder, C. Tolksdorf, A. Travers, and G. Muskhelishvili. 2001. An architectural role of the Escherichia coli chromatin protein FIS in organizing DNA. Nucleic Acids Res. 29:5107-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider, R., A. Travers, and G. Muskhelishvili. 1997. FIS modulates growth phase-dependent topological transitions of DNA in Escherichia coli. Mol. Microbiol. 26:519-530. [DOI] [PubMed] [Google Scholar]
- 68.Schultz, S. C., G. C. Shields, and T. A. Steitz. 1991. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science 253:1001-1007. [DOI] [PubMed] [Google Scholar]
- 69.Serrano, L., J. Sancho, M. Hirshberg, and A. R. Fersht. 1992. Alpha-helix stability in proteins. I. Empirical correlations concerning substitution of side-chains at the N and C-caps and the replacement of alanine by glycine or serine at solvent-exposed surfaces. J. Mol. Biol. 227:544-559. [DOI] [PubMed] [Google Scholar]
- 70.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 71.Thompson, J. F., L. Moitoso de Vargas, C. Koch, R. Kahmann, and A. Landy. 1987. Cellular factors couple recombination with growth phase: characterization of a new component in the lambda site-specific recombination pathway. Cell 50:901-908. [DOI] [PubMed] [Google Scholar]
- 72.Tzou, W. S., and M. J. Hwang. 1999. Modeling helix-turn-helix protein-induced DNA bending with knowledge-based distance restraints. Biophys. J. 77:1191-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson, R. L., S. J. Libby, A. M. Freet, J. D. Boddicker, T. F. Fahlen, and B. D. Jones. 2001. Fis, a DNA nucleoid-associated protein, is involved in Salmonella typhimurium SPI-1 invasion gene expression. Mol. Microbiol. 39:79-88. [DOI] [PubMed] [Google Scholar]
- 74.Xu, J., and R. C. Johnson. 1995. Fis activates the RpoS-dependent stationary-phase expression of proP in Escherichia coli. J. Bacteriol. 177:5222-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu, J., and R. C. Johnson. 1995. Identification of genes negatively regulated by Fis: Fis and RpoS comodulate growth-phase-dependent gene expression in Escherichia coli. J. Bacteriol. 177:938-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang, S. W., and H. A. Nash. 1995. Comparison of protein binding to DNA in vivo and in vitro: defining an effective intracellular target. EMBO J. 14:6292-6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuan, H. S., S. E. Finkel, J. A. Feng, M. Kaczor-Grzeskowiak, R. C. Johnson, and R. E. Dickerson. 1991. The molecular structure of wild-type and a mutant Fis protein: relationship between mutational changes and recombinational enhancer function or DNA binding. Proc. Natl. Acad. Sci. USA 88:9558-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuan, H. S., S. S. Wang, W. Z. Yang, S. E. Finkel, and R. C. Johnson. 1994. The structure of Fis mutant Pro61Ala illustrates that the kink within the long alpha-helix is not due to the presence of the proline residue. J. Biol. Chem. 269:28947-28954. [DOI] [PubMed] [Google Scholar]