Abstract
The tyrosine decarboxylase operon of Lactobacillus brevis IOEB9809 contains, adjacent to the tyrosine decarboxylase gene, a gene for TyrP, a putative tyrosine transporter. The two genes potentially form a proton motive tyrosine decarboxylation pathway. The putative tyrosine transporter gene of L. brevis was expressed in Lactococcus lactis and functionally characterized using right-side-out membranes. The transporter very efficiently catalyzes homologous tyrosine-tyrosine exchange and heterologous exchange between tyrosine and its decarboxylation product tyramine. Tyrosine-tyramine exchange was shown to be electrogenic. In addition to the exchange mode, the transporter catalyzes tyrosine uniport but at a much lower rate. Analysis of the substrate specificity of the transporter by use of a set of 19 different tyrosine substrate analogues showed that the main interactions between the protein and the substrates involve the amino group and the phenyl ring with the para hydroxyl group. The carboxylate group that is removed in the decarboxylation reaction does not seem to contribute to the affinity of the protein for the substrates significantly. The properties of the TyrP protein are those typical for precursor-product exchangers that operate in proton motive decarboxylation pathways. It is proposed that tyrosine decarboxylation in L. brevis results in proton motive force generation by an indirect proton pumping mechanism.
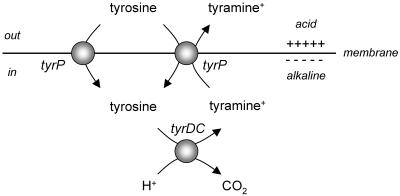
Secondary transporters use the free energy stored in ion and/or solute gradients across the membrane to drive transport. Symporters and antiporters couple the translocation of the substrate to the translocation of protons or sodium ions. The electrochemical gradients of H+ and Na+ across the membrane, or proton motive force (pmf) and sodium ion-motive force, respectively, are directed inwards and are maintained by the action of primary pumps that use the free energy released in chemical reactions (or light) to pump H+ and Na+ across the membrane out of the cell. Well-known examples are the aerobic respiratory chain and F-type ATPases. As a consequence, transport catalyzed by secondary transporters is a metabolic energy-requiring process. In some (facultative) anaerobic bacteria where metabolic energy is usually scarce, alternative energy-generating pathways have evolved that involve secondary transporters that generate rather than dissipate metabolic energy (for reviews, see references 16 and 26). The transporters termed precursor-product exchangers couple the uptake of a substrate (the precursor) to the excretion of the end product of a metabolic pathway (4, 24). Membrane potential is generated during turnover because of a charge difference between the two substrates. The pathways are driven by decarboxylation of the precursor in the cytoplasm yielding the product. The decarboxylation reaction consumes a cytoplasmic proton, thereby generating a pH gradient across the membrane. Consequently, the pathway consisting of a transporter and a decarboxylase performs as an indirect proton pump (see Fig. 1).
FIG. 1.
Secondary metabolic energy generation. The schematic shows the proton motive tyrosine decarboxylation pathway in Lactobacillus brevis that is demonstrated in this paper. The transporter TyrP catalyzes electrogenic tyrosine-tyramine exchange. The decarboxylase TyrDC catalyzes the decarboxylation of tyrosine to tyramine, which results in alkalinization of the cytoplasm. The combined actions of the transporter and decarboxylase result in a proton motive force. Tyrosine uniport (shown at the left) is an additional capacity of the tyrosine transporter.
The substrates of the secondary metabolic energy generating pathways are either di- or tricarboxylates or amino acids. Transporters in the first group, including the oxalate-formate exchanger OxlT of Oxalobacter formigenes (3, 4) and the malate-lactate and citrate-lactate exchangers MleP and CitP, respectively, found in lactic acid bacteria (5, 6, 7, 21), have been studied in detail. Transporters in the second group include the aspartate-alanine exchanger AspT (1, 2) and the histidine-histamine exchanger HisP (20, 22) from lactic acid bacteria and the lysine-cadaverine exchanger CadB of Escherichia coli (27). A similar pathway of the second group involving a glutamate-γ-aminobutyrate exchanger was proposed for a Lactobacillus strain on the basis of the formation of intracellular ATP coupled to glutamate metabolism (12). Precursor-product exchangers do not form a separate family of secondary transporters but are members of different families that also contain H+ and Na+ symporters, antiporters, and uniporters. They are believed to be “normal” secondary transporters that have been optimized to catalyze exchange of structurally related compounds.
Recently, the genes coding for bacterial tyrosine decarboxylases (tyrDC) were identified in the lactic acid bacteria Enterococcus faecalis, Lactobacillus brevis, and Lactococcus lactis (9, 11, 18, 19). In Lactobacillus brevis and Lactococcus lactis, the gene was contained in an operon containing four genes. The tyrDC gene was preceded by a gene homologous to tyrosyl-tRNA synthetases and followed by two genes coding for secondary transporters, a putative tyrosine transporter (tyrP) and a putative Na+/H+ antiporter. The combination of the tyrosine decarboxylase and tyrosine transporter genes could code for a proton motive pathway converting tyrosine into tyramine provided that the transporter gene codes for an electrogenic precursor-product exchanger (Fig. 1). In this study, we have examined the properties of the tyrP gene product. It is demonstrated that the transporter catalyzes tyrosine-tyramine exchange with high efficiency and that a net positive charge is translocated across the membrane during exchange.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and cloning of TyrP.
L. lactis strains were grown at 30°C in half-strength M17 broth (28) containing 0.5% glucose and 5 μg/ml chloramphenicol when appropriate. Strain NZ9000 harboring plasmid pNZtyrP or pNZ8048 (see below) were routinely grown until reaching an optical density of 0.6 measured at 660 nm followed by induction by addition of a 0.1% (vol/vol) supernatant of an overnight culture of the nisin A-producing strain L. lactis NZ9700 (13, 15). Subsequently, cells were allowed to grow for another hour, harvested by centrifugation, and washed once with 50 mM KPi (pH 6) buffer.
The gene encoding TyrP was amplified by PCR using genomic DNA of L. brevis IOEB 9809 as the template following a standard protocol. The forward primer introduced an NcoI site around the initiation codon of the tyrP gene, and the backward primer introduced an XbaI site downstream of the stop codon. The PCR product was digested with the two restriction enzymes and ligated into the corresponding restriction sites of vector pNZ8048 (15). The resulting plasmid, named pNZtyrP, codes for TyrP extended with a 10-histidine tag at the N terminus. The sequence of the insert was confirmed (BioMedical Technology Centre, University of Groningen, Groningen, The Netherlands), and the plasmid was subsequently introduced into the L. lactis strain NZ9000, which allows expression of genes under control of the tightly regulated nisA promoter (10).
Preparation of RSO membrane vesicles.
Right-side-out (RSO) membrane vesicles of L. lactis NZ9000 were prepared by the osmotic shock procedure as described previously (23) with modifications. Cells were resuspended to a final optical density at 660 nm of 120 in 17.5 ml of 100 mM potassium phosphate buffer (pH 7.0) containing 10 mM MgSO4 and 20 mg/ml lysozyme followed by incubation for 60 min at 30°C. Under conditions of continuous stirring, 16 ml of K2SO4 was added slowly to reach a final concentration of 0.36 M. Subsequently, the suspension was poured slowly into 120 ml 100 mM potassium phosphate buffer (pH 7) containing 50 μg/ml of RNase and DNase. The suspension was left for 20 min at 30°C under conditions of continuous stirring, after which 3 ml of K-EDTA (pH 7.5) was added, yielding a final concentration of 20 mM. After 10 min 4 ml of MgSO4 was added, yielding a final concentration of 25 mM. The suspension was centrifuged for 30 min at 750 × g at 4°C, and the supernatant was centrifuged for 30 min at 48,200 × g at 4°C. The pellet was resuspended in 30 ml of 50 mM potassium phosphate (pH 6) and centrifuged for 60 min at 750 × g at 4°C. The RSO membranes were collected from the supernatant by centrifugation for 30 min at 48,200 × g at 4°C, and the pellet was resuspended in 2 ml of 50 mM potassium phosphate (pH 6.0). Aliquots of 0.1 ml were rapidly frozen in liquid nitrogen and stored at −80°C until use. The protein concentration was determined using a DC protein assay kit (Bio-Rad).
SDS-PAGE and immunoblotting.
Membrane proteins were separated on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred onto a polyvinylidene difluoride membrane (Roche) by semidry electroblotting. His-tagged proteins were detected with a primary anti-His antibody (Amersham Biosciences) and a secondary anti-mouse antibody coupled to alkaline phosphatase (Sigma), followed by chemiluminescent detection with CDP-Star (Roche).
Transport assays in whole cells.
L. lactis cells were washed once with ice-cold 50 mM KPi (pH 6) and resuspended to an optical density of 2.0 at 660 nm. Following the addition of 0.2% glucose, 100-μl samples were incubated for 5 min at 20°C under conditions of constant stirring. At time 0, l-[U-14C]tyrosine was added to achieve a final concentration of 1.5 μM. Uptake was stopped at the indicated times by the addition of 2 ml of ice-cold 0.1 M LiCl solution immediately followed by filtering through a 0.45-μm-pore-size nitrocellulose filter (BA 85; Schleicher & Schuell GmbH). The filter was washed once with 2 ml of ice-cold 0.1 M LiCl and submerged in Emulsifier Scintillator Plus scintillation fluid (Packard BioScience), and the retained radioactivity was counted in a Tri-Carb 2000CA liquid scintillation analyzer (Packard Instrumentation). The background was estimated by adding the radiolabeled substrate to the cell suspension immediately after the addition of 2 ml of ice-cold LiCl, followed by filtering. In the chase experiments, tyramine, tyrosine, or buffer alone was added after 60 s at a final concentration of 0.5 mM.
Transport assays in RSO membranes.
Concentrated RSO membranes were loaded with the appropriate buffer by incubation for 1 h at room temperature. Aliquots of 2 μl of concentrated membranes were diluted 100-fold into 50 mM phosphate buffer containing labeled or unlabeled substrates. The final protein concentration in the samples was between 20 and 90 μg/ml. Transport was quenched, and the samples were processed as described above for cells.
For pmf-driven uptake, membranes were preloaded with 50 mM KPi (pH 6) containing 100 mM potassium acetate in the presence of 150 μM valinomycin (29). The membranes were diluted into NaPi (pH 6) containing 50 mM Na2SO4 and 11.8 μM l-[U-14C]tyrosine, 11.8 μM [1-14C]tyramine, or 1.55 μM l-[U-14C]leucine. For efflux, membranes were preloaded with 50 mM KPi (pH 6) containing, unless otherwise stated, 77.5 μM of l-[U-14C]tyrosine or [1-14C]tyramine in the presence of 150 μM valinomycin and 75 μM nigericin. For exchange or counterflow experiments, membranes were loaded with the same buffer containing 1 mM of labeled or unlabeled l-tyrosine, respectively. In the exchange assays, the membranes were diluted into buffer containing 1 mM of l-tyrosine, tyramine, or one of the substrate analogues. In the counterflow assays, the membranes were diluted into buffer containing 0.388 μM l-[U-14C]tyrosine and 1 mM of the substrate analogues when indicated.
The exchange experiments were analyzed by fitting the release of radiolabel from the membranes versus time to an exponential function. The first-order rate constant of the exchange catalyzed by TyrP was estimated from the difference between the rate constant obtained with the pNZtyrP membranes and that obtained with the pNZ8048 control membranes. Experiments were usually performed in triplicate but were performed at least in duplicate. The results shown represent typical experiments.
Materials.
l-[U-14C]tyrosine (434 mCi/mmol) and l-[U-14C]leucine (290 mCi/mmol) were purchased from Amersham Pharmacia (Roosendaal, The Netherlands). [1-14C]tyramine (50 mCi/mmol) was purchased from American Radiolabeled Chemicals Inc. (St. Louis, Missouri). All other compounds were obtained from commercial sources.
RESULTS
Functional expression of TyrP in Lactococcus lactis.
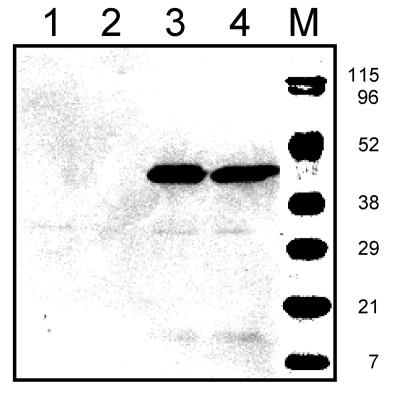
The putative tyrosine transporter gene located in the tyrosine decarboxylase operon of Lactobacillus brevis IOEB 9809, termed tyrP, was amplified by PCR using total DNA as the template and ligated into vector pNZ8048 downstream of the nisin-inducible promoter (NICE system). The resulting plasmid, pNZtyrP, codes for the tyrP gene product extended with a 10-histidine tag at the N terminus. The plasmid and the empty vector were introduced in the expression strain L. lactis NZ9000 (10, 15). The cells were grown in the presence of a range of concentrations of the inducer nisin, after which membranes were isolated from the cells. SDS-PAGE followed by immunoblotting using an antibody directed at the His tag detected a single protein with an apparent molecular mass of 44 kDa in the cells harboring pNZtyrP that was not present in the cells harboring the control vector (Fig. 2). Integral membrane proteins are known to have a higher mobility on SDS-PAGE, which explains the discrepancy between the apparent molecular mass of the band and the calculated molecular mass of the TyrP construct of 54 kDa. The expression levels of TyrP were more or less constant over a 20-fold concentration range of nisin in the medium (Fig. 2; compare lanes 3 and 4).
FIG. 2.
Expression of TyrP in Lactococcus lactis. An immunoblot of RSO membrane vesicles (10 μg of protein per lane) prepared from L. lactis NZ9000 cells harboring the vector pNZ8048 (lanes 1 and 2) or pNZtyrP (lanes 3 and 4) induced by 0.005% (lanes 1 and 3) and 0.1% (lanes 2 and 4) nisin containing supernatant (see Materials and Methods) is shown. Lane M shows an overlay of prestained protein markers. The molecular masses of the markers are indicated on the right in kilodaltons.
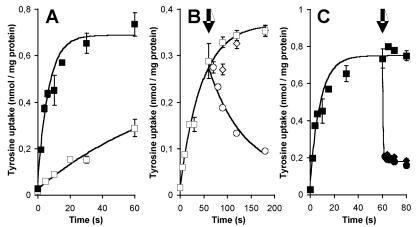
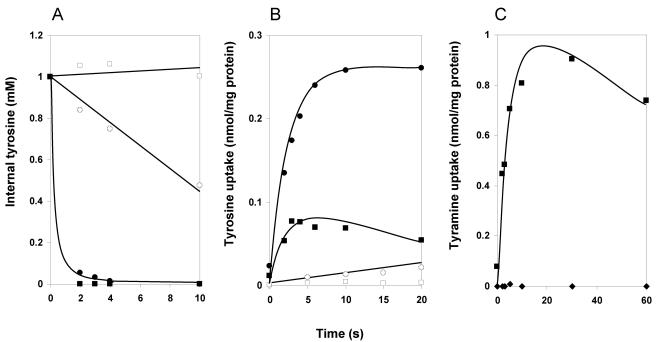
Tyrosine transport by TyrP was demonstrated by comparing the uptake of l-[U-14C]tyrosine in resting cells of L. lactis NZ9000 containing either pNZtyrP or the control vector pNZ8048 grown in the presence of nisin. At a concentration of 1.5 μM l-[U-14C]tyrosine, the control cells took up tyrosine at an initial rate of 0.36 nmol/min · mg of cell protein (Fig. 3A), demonstrating the presence of an endogenous tyrosine transporter in the membrane of L. lactis NZ9000. Cells expressing TyrP took up tyrosine at an initial rate that was about 15 times higher (5.3 nmol/min · mg of cell protein), demonstrating functional expression of the tyrP gene and the ability of TyrP to transport tyrosine. Subsequent chase experiments demonstrated that tyramine also was a substrate of TyrP. Cells were allowed to take up l-[U-14C]tyrosine for 1 min, after which an excess (500 μM) of unlabeled tyrosine or tyramine was added to the cell suspension. The endogenous L. lactis tyrosine transporter catalyzed tyrosine-tyrosine exchange, as evidenced by the release of radiolabel from the cells upon the addition of tyrosine (Fig. 3B). Addition of tyramine did not affect the uptake, indicating that the endogenous transporter has no affinity for this substrate. Addition of both tyrosine and tyramine to cells expressing TyrP of L. brevis resulted in the immediate release of previously accumulated l-[U-14C]tyrosine (Fig. 3C). It follows that TyrP very efficiently catalyzes homologous tyrosine-tyrosine and heterologous tyrosine-tyramine exchange.
FIG.3.
Tyrosine uptake in whole cells. A. Uptake of l-[U-14C]tyrosine by resting cells of L. lactis NZ9000 harboring either pNZ8048 (open squares) or pNZtyrP (solid squares). The initial tyrosine concentration was 1.5 μM. B and C. Cells harboring pNZ8048 (B) and pNZtyrP (C) were allowed to take up l-[U-14C]tyrosine for 60 s. At the arrows, unlabeled tyrosine (circles) and tyramine (diamonds) were added to achieve a final concentration of 500 μM or the same volume of buffer was added (squares).
Unidirectional transport modes catalyzed by TyrP.
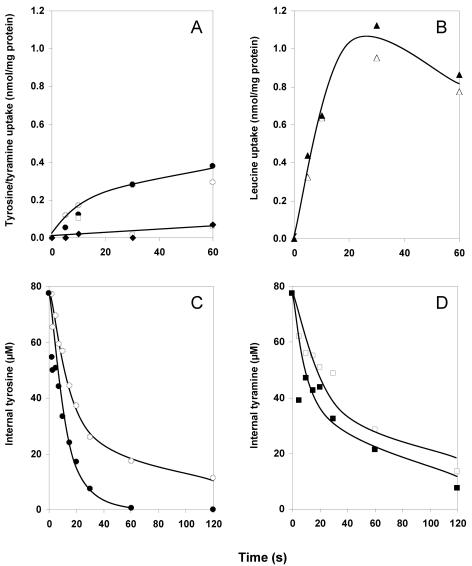
RSO membrane vesicles provide a well-defined experimental system to study capacities of secondary transporters as uniport, symport, and antiport. RSO membranes prepared from L. lactis harboring the control vector pNZ8048 accumulated l-[U-14C]tyrosine to low but significant levels in the presence of an artificially imposed pmf (29), suggesting that the endogenous L. lactis transporter is a proton symporter (Fig. 4A). In the absence of a pmf, no uptake was observed (not shown). Under the same conditions, the membranes did not take up any significant amounts of [1-14C]tyramine in the presence of a pmf, in agreement with the inability of tyramine to chase accumulated tyrosine from the cells containing the control vector (Fig. 3B). The results with RSO membranes containing TyrP of L. brevis were quantitatively the same (Fig. 4A). No significant pmf-driven accumulation of either tyrosine or tyramine was found above the levels that were observed in the membranes devoid of TyrP. Control experiments measuring [14C]leucine accumulation revealed similar levels of uptake in both membrane preparations, demonstrating that the energetic state and membrane integrity were comparable (Fig. 3B). It follows that TyrP does not catalyze accumulation of tyrosine or tyramine in response to a pmf and, apparently, does not couple the translocation of the substrates to the translocation of a proton. The TyrP-dependent uptake in whole cells (Fig. 3A) is likely to be the consequence of exchange with an internal pool of tyrosine. In agreement, accumulation in whole cells was not inhibited in the presence of protonophores (not shown). L. lactis is known to retain significant pools of amino acids following growth on rich media (14).
FIG.4.
Unidirectional transport modes of TyrP. RSO membranes were derived from L. lactis NZ9000 harboring pNZtyrP (closed symbols) or the control vector pNZ8048 (open symbols). A and B. pmf-driven uptake of 11.8 μM l-[U-14C]tyrosine (circles), 11.8 μM [1-14C]tyramine (diamonds, pNZtyrP; squares, pNZ8048), and 1.55 μM l-[14C]leucine (triangles). The final protein concentration was 90 μg/ml. C and D. Efflux of 77.5 μM l-[U-14C]tyrosine (C; circles) and 77.5 μM of [1-14C]tyramine (D; squares). The final protein concentration was 50 μg/ml.
The endogenous tyrosine transporter of L. lactis catalyzed downhill transport at a considerable rate, as evidenced by the release of radiolabel upon 100-fold dilution of RSO membranes preloaded with 77.5 μM l-[U-14C]tyrosine into buffer (Fig. 4C). The presence of the combination of the ionophores valinomycin and nigericin prevents the formation of a proton gradient in the experiment. A significant higher rate of tyrosine efflux from RSO membranes containing TyrP was observed, demonstrating the ability of TyrP to catalyze unidirectional transport of tyrosine driven by a tyrosine gradient. The same experiments with tyramine rather than tyrosine as the substrate were hampered by the relatively high passive permeability of the former. Tyramine is a weak base that is uncharged in the unprotonated state and easily crosses the phospholipid bilayer. Under the conditions of the experiments, the amount of the unprotonated fraction of tyramine is large enough to produce a rapid release of label from the membranes not containing TyrP (Fig. 4D). Some enhancement of the efflux rate by TyrP may be observed; however, the difference is hardly significant. It is concluded that TyrP catalyzes tyrosine uniport (Fig. 1) whereas uniport of tyramine is obscured by the passive permeability of the substrate.
Exchange modes catalyzed by TyrP.
The chemical identity and concentration of the internalized substrate in the chase experiments demonstrated in Fig. 3B and C are unknown. RSO membranes preequilibrated with a known concentration of substrate provide a well-defined system for measurement of the exchange capacity of a transporter. Dilution of RSO membranes preloaded with 1 mM of l-[U-14C]tyrosine in buffer containing the same concentrations of unlabeled tyrosine and tyramine confirmed the observations made with the chase experiments. External tyrosine and tyramine resulted in a very rapid release of internal l-[U-14C]tyrosine from RSO membranes prepared from cells expressing TyrP (Fig. 5A). In fact, essentially all tyrosine was released within 2 s, which is the first reliable time point in the assay. The estimated first-order rate constant of the exchange was >1 mM s−1. In contrast, external tyrosine resulted in the release of internal tyrosine from RSO membranes prepared from the control cells with a half-time of about 10 s, while external tyramine did not result in significant release during the first 10 s (Fig. 5A). Please note the higher internal concentration in these experiments relative to the efflux experiments discussed above (1 mM versus 77.5 μM; Fig. 4C and D); this higher internal concentration lowers the relative efflux rate. Membranes loaded with 1 mM l-[U-14C]tyrosine and diluted in buffer without any added substrate revealed essentially the same lack of efflux as those diluted into 1 mM tyramine (not shown). The rate enhancement by TyrP observed for homologous tyrosine-tyrosine exchange (Fig. 5A) was much higher than that observed for tyrosine efflux (Fig. 4C), indicating that TyrP is especially efficient in catalyzing exchange.
FIG. 5.
Exchange catalyzed by TyrP. RSO membranes were derived from L. lactis NZ9000 harboring pNZtyrP (closed symbols) or the control vector pNZ8048 (open symbols). A. Exchange. RSO membrane vesicles preloaded with 1 mM l-[U-14C]tyrosine were diluted into buffer containing 1 mM tyrosine (circles) or tyramine (squares). The final protein concentration was 20 μg/ml. B. Counterflow. RSO membranes preloaded with 1 mM l-tyrosine (circles) or tyramine (squares) were diluted in buffer containing 0.78 μM l-[U-14C]tyrosine. The final protein concentration was 20 μg/ml. C. ΔΨ-driven tyrosine-tyramine exchange. RSO membrane vesicles containing TyrP were preloaded with 1 mM of l-tyrosine in 100 mM KPi (pH 6) buffer in the presence of 150 μM of valinomycin. The membranes were diluted 100 times into 100 mM KPi (pH 6) (diamonds) or 100 mM NaPi (pH 6) (squares) containing 1 mM of l-tyrosine and 3.6 μM of [1-14C]tyramine (circles). The final protein concentration in the assays was 35 μg/ml.
Dilution of RSO membranes containing TyrP preloaded with 1 mM of either unlabeled tyrosine or tyramine in buffer containing a low concentration of labeled tyrosine (0.4 μM) resulted in the rapid accumulation of labeled substrate in the membranes. The accumulation is driven by an exchange of internal unlabeled substrate and external labeled substrate (counterflow; Fig. 5B). The initial rates of uptake of l-[U-14C]tyrosine with 1 mM tyrosine and 1 mM tyramine as the internal substrate were in the same order of magnitude, with 4.7 and 2.8 nmol/min · mg membrane protein, respectively. The level of accumulation with internal tyramine was significantly lower and the accumulation more transient, which is a consequence of the higher passive permeability of tyramine relative to tyrosine. The initial phase of accumulation in a counterflow experiment is followed by release of the labeled substrate when the net efflux of unlabeled substrate diminishes the concentration gradient. Under identical conditions, the initial rate of tyrosine uptake was approximately 70 times faster in the membranes containing TyrP loaded with 1 mM tyrosine than that observed with the control membranes (Fig. 5B). As anticipated, control membranes loaded with tyramine did not accumulate any labeled tyrosine; tyramine is not a substrate of the endogenous L. lactis transporter.
In the physiological pH range, tyramine is positively charged, while tyrosine bears no net charge. Therefore, exchange of the two substrates in the absence of any cotransported ions is electrogenic, and this is the proposed function of TyrP in the tyrosine decarboxylation pathway of L. brevis. The electrogenicity of the tyrosine-tyramine exchange reaction catalyzed by TyrP was demonstrated by the following experiment. RSO membranes containing TyrP were loaded with 1 mM unlabeled tyrosine in a buffer containing 150 mM K+ ions. The membranes were treated with valinomycin, a K+ ionophore. A 100-fold dilution of the membranes in exactly the same buffer (1 mM tyrosine, 150 mM K+) with an additional low concentration of 3.6 μM [1-14C]tyramine showed no accumulation of the latter in the membranes, since no significant concentration gradient of exchangeable substrates across the membrane exists (Fig. 5C). Dilution of the membranes in the same buffer but containing Na+ rather than K+ results in the generation of a membrane potential of −120 mV as a result of the imposed K+ gradient. Under these conditions, a rapid accumulation of [1-14C]tyramine in the membranes was observed, clearly demonstrating that heterologous tyramine-tyrosine exchange catalyzed by TyrP is electrogenic (Fig. 1).
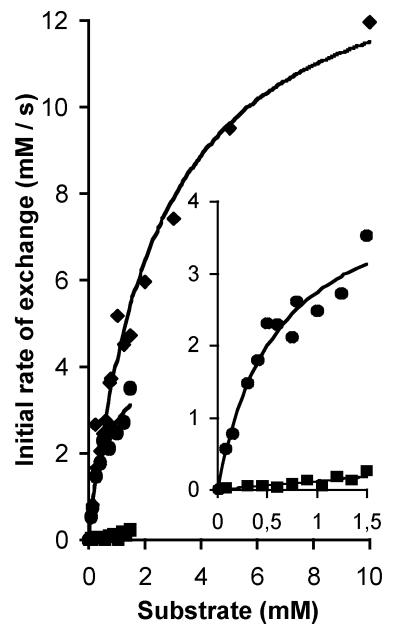
Estimation of the kinetic parameters for the exchange reaction catalyzed by TyrP of L. brevis was problematic for the following reasons: (i) at high levels of expression of the transporter, the exchange rate was too high to measure initial rates, (ii) at low levels of expression, the background activity of the endogenous tyrosine transporter of L. lactis was interfering, and (iii) the low solubility of tyrosine limited the concentration range to a maximum of 1.5 mM. Best results were obtained when RSO membranes containing a high level of expression of TyrP were loaded with a high concentration of [1-14C]tyramine (9.6 mM) to slow down the relative rate as much as possible. Moreover, tyramine is not a substrate of the endogenous tyrosine transporter and has better solubility than tyrosine. The membranes were diluted 250 times in a buffer containing a concentration range of unlabeled tyrosine or tyramine (Fig. 6). For both l-tyrosine and tyramine Michaelis-Menten-type kinetics was observed, with a four-times-higher affinity for l-tyrosine than for tyramine (Km of 0.59 ± 0.16 mM and 2.45 ± 0.32 mM, respectively). In contrast, the maximal rate was approximately three times higher with tyramine than with tyrosine (Vmax of 14.3 ± 0.89 mM/s and 4.33 ± 0.51 mM/s, respectively). Consequently, at low concentrations, the rates of exchange are more or less the same for the two substrates. TyrP of L. brevis is highly stereo specific, as the exchange rates with d-tyrosine were significantly lower (Fig. 6). The low activity observed in the possible concentration range of up to 1.5 mM did not allow the estimation of the kinetic parameters for d-tyrosine. At 1.5 mM the rate was 18 times lower with d-tyrosine than observed with l-tyrosine.
FIG. 6.
Kinetic analysis of TyrP catalyzed exchange with l-tyrosine, d-tyrosine, and tyramine as substrates. RSO membrane vesicles of L. lactis NZ9000 expressing pNZtyrP were preloaded with 9.6 mM [1-14C]tyramine and diluted 250-fold in buffer containing l-tyrosine concentrations in a range of 0.075 to 1.5 mM (circles), d-tyrosine in a range between 0.075 and 1.5 mM (squares), or tyramine in a range from 0.15 to 10 mM (diamonds). The final protein concentration in the assays was 30 μg/ml. The insert shows a concentration range of up to 1.5 mM for l- and d-tyrosine. The data were fitted to a Michaelis-Menten curve after calculation of the initial rate of exchange from the first-order rate constants.
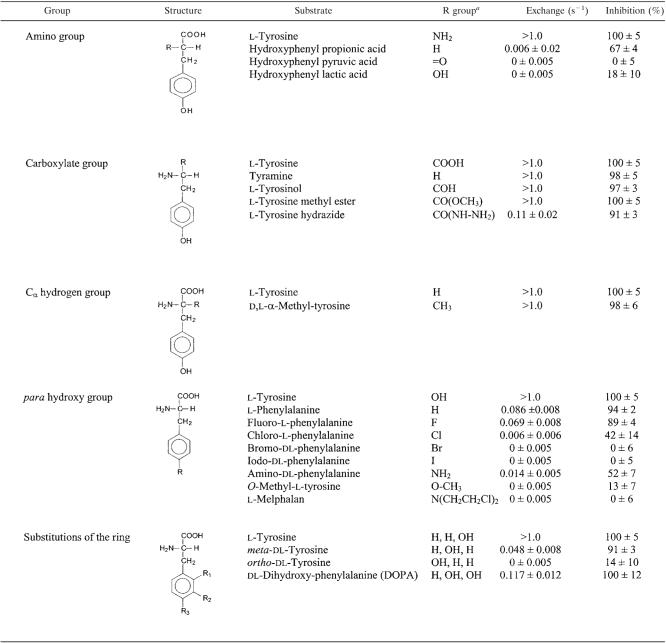
Substrate specificity of TyrP.
A set of 19 commercially available tyrosine analogues modified at different groups of the molecule was used to analyze the interaction between the TyrP protein and the substrates. The compounds were tested for their ability to release preaccumulated radiolabeled tyrosine from RSO membranes in a heterologous exchange reaction (see Fig. 5A for homologous tyrosine-tyrosine exchange) and for their inhibitory effect on the uptake of radiolabeled tyrosine driven by a tyrosine gradient in a counterflow experiment (Fig. 5B). The exchange assay demonstrates that TyrP binds and transports the substrate, while the counterflow assay demonstrates that the transporter binds the substrate but does not necessarily transport it. Table 1 presents the exchange rate constants for the various substrates catalyzed by TyrP (see Materials and Methods). Under the conditions of the experiments, reliable rate constants could be obtained in a range between 0.05 and 1.0 s−1. In the inhibition assay, the contribution of the endogenous tyrosine transporter of L. lactis to the rate of uptake in counterflow was at most 2% and was neglected in the analysis.
TABLE 1.
Activity of TyrP with tyrosine substrate analogues
For substitutions of the ring, the R group is given as R1, R2, and R3.
Modifications of the amino group of the substrates were only poorly tolerated by TyrP (Table 1). Replacement with a hydrogen atom in hydroxyphenyl propionate, with a keto group in hydroxyphenyl pyruvate, or with an alcohol group in hydroxyphenyl lactate resulted in the loss of essentially all exchange activity. Hydroxyphenyl propionate still inhibited the counterflow assay significantly, indicating that the amino group is more critical for the translocation than for the binding of the substrates. Nevertheless, the amino group of the physiological substrates tyrosine and tyramine is clearly important for the interaction with the transporter.
TyrP exchanges tyrosine and its decarboxylation product tyramine with high efficiency (see Fig. 5). Therefore, it may be expected that modifications of the carboxylate group will not affect the interaction with the protein drastically. Accordingly, methylation of the carboxylate (l-tyrosine methyl ester) or reduction to an alcohol group (l-tyrosinol) did not affect the exchange rate or the inhibition of counterflow significantly (Table 1). However, l-tyrosine hydrazide revealed a significantly reduced exchange rate, while the inhibition was only marginally affected. Again, translocation appeared to be more sensitive than binding. Since the volumes of the hydrazide group and the methyl ester group are similar, the defect will most likely not be due to a steric effect; rather, the additional positive charge carried by the protonated hydrazide group may cause the problem. Modification of the Cα hydrogen group by a methyl group in α-methyl-tyrosine was well tolerated by TyrP (Table 1).
Modifications of the hydroxyl group at the para position of the phenyl ring strongly affected the activity of TyrP with the substrates (Table 1D). The exchange rate with l-phenylalanine was reduced by 1 order of magnitude, while the inhibition was not affected. The exchange rate and inhibition decreased when the hydroxyl group was replaced with a halogen atom in the order F → Cl → B → I. The decrease correlates with an increase of atomic radii and a decrease in electronegativity (polarity) of the halogens. The low but significant activity observed with amino-dl-phenylalanine suggests that the latter correlation may be responsible for the reduced interaction with TyrP. Replacement of the para hydroxyl group with the bulkier groups found in O-methyl-l-phenylalanine and l-melphalan resulted in essentially loss of all interaction with the substrates. Clearly, the para hydroxyl group is critical for the interaction between TyrP and its substrates. Also, the position of the hydroxyl on the ring is important, as the exchange activity dropped dramatically when the hydroxyl moved from the para through the meta to the ortho position (Table 1). The lower activity with m-tyrosine and, especially, o-tyrosine in comparison to the activity with phenylalanine shows that the hydroxyl groups at the ortho and meta positions are counterproductive in the interaction. In agreement, the activity with 3,4-dihydroxyphenylalanine (DOPA) was lower than with tyrosine. The hydroxyl group at the meta position did not affect the binding of the ring very drastically, as both m-tyrosine and DOPA still inhibited the counterflow reaction essentially completely.
DISCUSSION
The transport properties of the tyrP gene product in the tyrosine decarboxylase operon of L. brevis IOEB 9809 support the idea of the proton motive pathway depicted in Fig. 1. (i) Both tyrosine and its decarboxylation product tyramine are substrates of TyrP. (ii) TyrP catalyzes exchange much more efficiently than unidirectional transport. (iii) Tyrosine-tyramine exchange is electrogenic. The pathway consists of the tyrosine-tyramine exchanger TyrP and the tyrosine decarboxylase TyrDC. Tyrosine is taken up from the medium by TyrP and, subsequently, decarboxylated by TyrDC, yielding tyramine that is excreted by TyrP coupled to the uptake of tyrosine. The physiological function of the pathway is generation of pmf at the expense of the free energy released in the decarboxylation reaction. The two components of the pmf, membrane potential and pH gradient, are generated separately in the two steps of the pathway. Turnover of TyrP results in membrane potential of physiological polarity (positive out), since monovalent, positively charged tyramine is exchanged for uncharged tyrosine. The decarboxylation of tyrosine catalyzed by TyrDC consumes a proton which alkalinizes the cytoplasm relative to the external medium. For each turnover of the pathway in which one external tyrosine molecule is converted to one external tyramine molecule, one positive charge is translocated across the membrane and one proton is removed from the cytoplasm, which is equivalent to the pumping of one proton across the membrane. Hence, the pathway is an indirect proton pump. Alternatively, the pathway may play a role in cytoplasmic pH homeostasis and resistance against acid stress by virtue of the alkalinizing effect of the decarboxylation reaction (26).
TyrP catalyzes, in addition to tyrosine-tyramine exchange, tyrosine uniport. Exchange of an internal and external substrate is a partial reaction of a symporter or uniporter, and it is believed that the bacterial precursor-product exchangers are unidirectional transporters that have been optimized to catalyze exchange. Precursor-product exchangers are members of transporter families that, in addition to exchangers, contain symporters, uniporters, and antiporters, indicating that they are structurally and mechanistically similar. Examples are the oxalate-formate exchanger OxlT in the major facilitator superfamily that contains many H+-carbohydrate symporters and H+-drug antiporters, the lysine-cadaverine exchanger CadB in the amino acid-polyamine-choline superfamily that contains many H+- and Na+-coupled amino acid symporters, and the citrate-lactate exchanger CitP and the malate-lactate exchanger MleP in the 2-hydroxycarboxylate transporter family that contains H+- and Na+-coupled di- and tricarboxylate transporters (8, 26). The origin of the bacterial transporters that catalyze the physiological relevant precursor-product exchange reaction may be recognized in the pmf-driven transport modes they catalyze. For example, the lysine-cadaverine CadB of E. coli catalyzes H+-cadaverine symport (27) and the citrate-lactate exchanger CitP of Leuconostoc mesenteroides catalyzes H+-citrate symport (21). Tyrosine accumulation by TyrP of Lactobacillus brevis in response to a pmf could not be demonstrated in this study, but transport down a tyrosine concentration gradient was evident, indicating that TyrP catalyzes tyrosine uniport. The unidirectional transport mode may or may not play a physiological role. In case of CadB, the symport mode is believed to be operational at neutral pH, while the exchange mode would be functional under acidic conditions. Also, a role under pre-steady-state conditions, when a concentration of the internal substrate has to develop to get the exchange reaction going, has been suggested (1). In the case of the tyrosine decarboxylation pathway in L. brevis, such a role seems unlikely, since additional transporters for tyrosine will usually be present in the membrane; otherwise, the highly active proteolytic system of lactic acid bacteria will produce a supply of cytoplasmic tyrosine in the cytoplasm (14). The rapid uptake of radiolabeled tyrosine in L. lactis cells expressing TyrP is likely to be the result of exchange with the internal tyrosine pool.
Precursor-product exchangers have affinity for structurally related compounds. The affinity of TyrP in the exchange reaction was only four times higher for l-tyrosine (Km is 0.59 mM) than for the decarboxylation product tyramine (Km is 2.33 mM). Moreover, the lower affinity of TyrP for tyramine is compensated for by a higher maximal-rate Vmax, making the results with respect to catalytic efficiency (Vmax/Km) at low substrate concentrations more or less the same for tyrosine and tyramine. Consequently, the rates in the lower concentration range are comparable for the two substrates. In agreement, modifications of the carboxylate group that is removed in the decarboxylation reaction were well tolerated by TyrP. Only the more drastic change of the negatively charged carboxylate group to a positively charged hydrazide group reduced the exchange rate significantly. Apparently, no specific interaction between the carboxylate group on the substrate and the TyrP protein exists. This is in marked contrast to what was observed for the citrate-lactate and malate-lactate exchangers of lactic acid bacteria, where the carboxylate group that is removed by the decarboxylase makes the difference between high- and low-affinity binding of the substrates (7). Analysis of the substrate specificity of TyrP in the exchange reaction demonstrates that the amino group and the phenyl ring with the para hydroxyl group are the most important interaction sites of the molecules with the protein. All modifications of these groups resulted in at least a 10-fold reduction of the exchange rate. Remarkably, for many of the modifications the effect on the exchange rate was stronger than on the inhibition of counterflow, suggesting that translocation involves more-critical interactions between substrate and protein than the initial binding step.
The decarboxylation products of amino acids accumulate as biogenic amines in foods such as dairy products and wine, depending on the availability of the precursors and the presence of microorganisms containing specific amino acid decarboxylases (17, 25). The decarboxylation pathways provide metabolic energy to the microorganisms and/or support intracellular pH homeostasis. In foods, biogenic amines are undesirable and are a health threat when present at high concentrations. The decarboxylation product of tyrosine is tyramine, which is the most abundant biogenic amine in dairy products, especially in cheeses (11). Insight into tyramine production by bacteria could help prevent tyramine accumulation in fermented foods.
Acknowledgments
This work was funded by the European Commission; contract QLK1-CT-2002-02388.
REFERENCES
- 1.Abe, K., H. Hayashi, and P. C. Maloney. 1996. Exchange of aspartate and alanine. Mechanism for development of a proton-motive force in bacteria. J. Biol. Chem. 271:3079-3084. [DOI] [PubMed] [Google Scholar]
- 2.Abe, K., F. Ohnishi, K. Yagi, T. Nakajima, T. Higuchi, M. Sano, M. Machida, R. I. Sarker, and P. C. Maloney. 2002. Plasmid-encoded asp operon confers a proton motive metabolic cycle catalyzed by an aspartate-alanine exchange reaction. J. Bacteriol. 184:2906-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe, K., Z. S. Ruan, and P. C. Maloney. 1996. Cloning, sequencing, and expression in Escherichia coli of OxlT, the oxalate:formate exchange protein of Oxalobacter formigenes. J. Biol. Chem. 271:6789-6793. [DOI] [PubMed] [Google Scholar]
- 4.Anantharam, V., M. J. Allison, and P. C. Maloney. 1989. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. J. Biol. Chem. 264:7244-7250. [PubMed] [Google Scholar]
- 5.Bandell, M., V. Ansanay, N. Rachidi, S. Dequin, and J. S. Lolkema. 1997. Membrane potential generating malate (MleP) and citrate (CitP) transporters of lactic acid bacteria are homologous proteins. Substrate specificity of the 2-hydroxy-carboxylate transporter family. J. Biol. Chem. 272:18140-18146. [DOI] [PubMed] [Google Scholar]
- 6.Bandell, M., and J. S. Lolkema. 1999. Stereoselectivity of the membrane potential generating citrate (CitP) and malate (MleP) transporters of lactic acid bacteria. Biochemistry 38:10352-10360. [DOI] [PubMed] [Google Scholar]
- 7.Bandell, M., and J. S. Lolkema. 2000. Arg425 of the citrate transporter CitP is responsible for high affinity binding of di- and tricarboxylates. J. Biol. Chem. 275:39130-39136. [DOI] [PubMed] [Google Scholar]
- 8.Busch, W., and M. H. Saier, Jr. 2004. The IUBMB-endorsed transporter classification system. Mol. Biotechnol. 27:253-262. [DOI] [PubMed] [Google Scholar]
- 9.Connil, N., Y. Le Breton, X. Dousset, Y. Auffray, A. Rince, and H. Prevost. 2002. Identification of the Enterococcus faecalis tyrosine decarboxylase operon involved in tyramine production. Appl. Environ. Microbiol. 68:3537-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ruyter, P. G. G. A., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández, M., D. M. Linares, and M. A. Alvarez. 2004. Sequencing of the tyrosine decarboxylase cluster of Lactococcus lactis IPLA 655 and the development of a PCR method for detecting tyrosine decarboxylating lactic acid bacteria. J. Food Prot. 67:2521-2529. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi, T., H. Hayashi, and K. Abe. 1997. Exchange of glutamate and γ-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 179:3362-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuipers, O. P., M. M. Beerthuyzen, R. J. Siezen, and W. M. De Vos. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur. J. Biochem. 216:281-291. [DOI] [PubMed] [Google Scholar]
- 14.Kunji, E. R., I. Mierau, A. Hagting, B. Poolman, and W. N. Konings. 1996. The proteolytic systems of lactic acid bacteria. Antonie Leeuwenhoek 70:187-221. [DOI] [PubMed] [Google Scholar]
- 15.Kunji, E. R. S., D. J. Slotboom, and B. Poolman. 2003. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta 1610:97-108. [DOI] [PubMed] [Google Scholar]
- 16.Lolkema, J. S., B. Poolman, and W. N. Konings. 1996. Secondary transporters and metabolic energy generation in bacteria, p. 229-260. In W. N. Konings, H. R. Kaback, and J. S. Lolkema (ed.), Handbook of biophysics. Elsevier Science, Amsterdam, The Netherlands.
- 17.Lonvaud-Funel, A. 2001. Biogenic amines in wines: role of lactic acid bacteria. FEMS Microbiol. Lett. 199:9-13. [DOI] [PubMed] [Google Scholar]
- 18.Lucas, P., and A. Lonvaud-Funel. 2002. Purification and partial gene sequence of the tyrosine decarboxylase of Lactobacillus brevis IOEB 9809. FEMS Microbiol. Lett. 211:85-89. [DOI] [PubMed] [Google Scholar]
- 19.Lucas, P., J. Landete, M. Coton, E. Coton, and A. Lonvaud-Funel. 2003. The tyrosine decarboxylase operon of Lactobacillus brevis IOEB 9809: characterization and conservation in tyramine-producing bacteria. FEMS Microbiol. Lett. 229:65-71. [DOI] [PubMed] [Google Scholar]
- 20.Lucas, P. M., W. A. Wolken, O. Claisse, J. S. Lolkema, and A. Lonvaud-Funel. 2005. Histamine-producing pathway encoded on an unstable plasmid in Lactobacillus hilgardii 0006. Appl. Environ. Microbiol. 71:1417-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marty-Teysset, C., J. S. Lolkema, P. Schmitt, C. Divies, and W. N. Konings. 1995. Membrane potential generating transport of citrate and malate catalyzed by the citP gene product of Leuconostoc mesenteroides. J. Biol. Chem. 270:25370-25376. [DOI] [PubMed] [Google Scholar]
- 22.Molenaar, D., J. S. Bosscher, B. ten Brink, A. J. Driessen, and W. N. Konings. 1993. Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J. Bacteriol. 175:2864-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto, R., R. G. Lageveen, H. Veldkamp, and W. N. Konings. 1982. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J. Bacteriol. 149:733-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poolman, B., D. Molenaar, E. J. Schmid, T. Ubbink-Kok, T. Abee, P. P. Renault, and W. N. Konings. 1991. Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J. Bacteriol. 173:6030-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silla Santos, M. H. 1996. Biogenic amines: their importance in food. Int. J. Food Microbiol. 29:213-231.8796424 [Google Scholar]
- 26.Sobczak, I., and J. S. Lolkema. 2005. The 2-hydroxycarboxylate transporter family. Microbiol. Mol. Biol. Rev. 69:665-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soksawatmaekhin, W., A. Kuraishi, K. Sakata, K. Kashiwagi, and K. Igarashi. 2004. Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 51:1401-1412. [DOI] [PubMed] [Google Scholar]
- 28.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolner, B., T. Ubbink-Kok, B. Poolman, and W. N. Konings. 1995. Characterization of the proton/glutamate symport protein of Bacillus subtilis and its functional expression in Escherichia coli. J. Bacteriol. 177:2863-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]