Abstract
Transcriptional regulation of the Mg2+-citrate transporter, CitM, the main citrate uptake system of Bacillus subtilis, was studied during growth in rich medium. Citrate in the growth medium was required for induction under all growth conditions. In Luria-Bertani medium containing citrate, citM expression was completely repressed during the exponential growth phase, marginally expressed in the transition phase, and highly expressed in the stationary growth phase. The repression was relieved when the cells were grown in spent Luria-Bertani medium. The addition of a mixture of 18 amino acids restored repression. l-Arginine in the mixture appeared to be solely responsible for the repression, and ornithine appeared to be an equally potent repressor of citM expression. Studies of mutant strains deficient in RocR and SigL, proteins required for the expression of the enzymes of the arginase pathway, confirmed that uptake into the cell and, most likely, conversion of arginine to ornithine were required for repression. Arginine-mediated repression was independent of a functional CcpA, the global regulator protein in carbon catabolite repression (CCR). Nevertheless, CCR-mediated repression was the major mechanism controlling the expression during exponential growth, while the newly described, CcpA-independent arginine-mediated repression was specifically apparent during the transition phase of growth.
The Mg2+-citrate transport protein, CitM, is believed to be the predominant citrate uptake system in Bacillus subtilis under aerobic growth conditions (30). Its ability to transport citrate is dependent on the presence of a well-defined set of divalent metal ions, Mg2+, Mn2+, Zn2+, Ni2+, and Co2+. The divalent metal ions are transported in complex with citrate into the cell (15). More recently, it was demonstrated that d-isocitrate, like citrate, is transported by CitM when complexed to the same set of divalent metal ions (30). The metal ion specificity of the complex renders B. subtilis extremely sensitive to the toxic ions Zn2+, Ni2+, and Co2+ in the presence of citrate or isocitrate when CitM is present in the membrane (14). Uptake of the metal citrate complex catalyzed by CitM is driven by the electrochemical gradient of protons that is maintained across the cell membrane (proton motive force). Translocation was shown to be electrogenic, i.e., CitM couples the uptake of the monovalent Me2+:citrate3− complex to the uptake of at least two protons (3).
Expression of the Mg2+-citrate transporter of Bacillus subtilis has been studied extensively and was shown to be regulated at the transcriptional level. In minimal medium, expression of the citM gene requires the presence of citrate or isocitrate in the medium. Induction is mediated by the two-component system CitST (34), of which the sensor kinase CitS recognizes external citrate or isocitrate and the response regulator CitT works as a transcription activator, by binding to the promoter region of citM. Furthermore, citM expression is repressed by more-favorable carbon sources like glucose via the carbon catabolite repression (CCR) system. In addition to that by glucose, CCR-mediated repression of citM expression by glycerol and inositol and by a combination of the nonsugars succinate and glutamate (29) has been reported. Major players in CCR in gram-positive organisms are the global regulator CcpA and its coeffectors HPr and Crh (reviewed in reference 27). CcpA is a member of the LacI-GalR family of regulatory proteins (12) that binds to its cognate operator site, the so-called cre (catabolite-responsive element) site (13), where it acts as a repressor or activator of transcription (28). cre sites are located within or near the promoter region of the targeted genes (20). Binding of CcpA to these palindromic DNA sequences is driven by complex formation with the Ser46-phosphorylated forms of HPr, the general phosphocarrier protein of the phosphoenolpyruvate-dependent sugar uptake system (phosphotransferase system), or Crh, a protein homologous to HPr (8, 16), which, however, has no function in the phosphotransferase system (9, 17). Mutants deficient in CcpA, HPr, and/or Crh have been shown to result in (partial) relief of repression or activation of several genes (6, 9, 27, 28), including the citM gene (29).
In this study, the citM expression pattern during growth in rich (Luria-Bertani [LB]) medium was investigated. It is shown that citM expression follows a complex pattern in which CCR plays a major role during the exponential growth phase, after which a new repression mechanism involving arginine metabolism takes over in the transition phase of growth. It is demonstrated that CcpA, the global regulator of CCR, was not involved in the newly described arginine-potentiated repression mechanism.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. subtilis 168-derived strains used in this study are listed in Table 1. Strain CM002 containing the PcitM-lacZ promoter fusion is referred to in this study as the wild type. Precultures of B. subtilis wild-type and mutant strains were grown overnight at 37°C in LB medium. The precultures were diluted 100 times in fresh or spent LB medium supplemented with 10 mM trisodium citrate (LBC and spent LBC, respectively). In addition, the auxotrophic requirement tryptophan was added to spent LB medium. Spent LB medium was prepared by growing the wild-type strain for 6.5 h in LB medium, after which cells were removed by centrifugation for 5 min at 10,000 × g and at 4°C. The supernatant was centrifuged once again under the same conditions and, subsequently, filter sterilized and stored at 4°C until use. Since, quantitatively, the results with the different batches of spent medium were variable, batch numbers are given in the Results section.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotypea | Source or reference |
|---|---|---|
| 168 | trpC2 | This laboratory |
| CM001 | trpC2 amyE::(lacZ cat) | 29 |
| CM002 | trpC2 amyE::(PcitM-lacZ cat) | 29 |
| CM008 | trpC2 ptsH1 crh::aphA3 amyE::(PcitM-lacZ cat) | 29 |
| CM010 | trpC2 ccpA::Tn917 spc amyE::(PcitM-lacZ cat) | 29 |
| QB5407 | trpC2 ccpA::spc | 21 |
| QB5533 | trpC2 rocR::aphA3 | 4 |
| QB5505 | trpC2 sigL::aphA3 | 5 |
| QB5619 | trpC2 ahrC::aphA3 amyE::rocD′-′lacZ | 11 |
| CM021 | trpC2 rocR::aphA3 amyE::(PcitM-lacZ cat) | This study |
| CM023 | trpC2 sigL::aphA3 amyE::(PcitM-lacZ cat) | This study |
| CM031 | trpC2 ccpA::Tn917 spc rocR::aphA3 amyE::(PcitM-lacZ cat) | This study |
| SF62 | trpC2 tnrA62::Tn917 erm | 33 |
| HJ31 | trpC2 ΔglnR | A. L. Sonenshein |
| PS252 | trpC2 codY::tet | A. L. Sonenshein |
| CM040 | trpC2 abrB::spc | This laboratory |
| CM041 | trpC2 spo0A::erm | This laboratory |
| CM043 | trpC2 tnrA62::Tn917 erm amyE::(PcitM-lacZ cat) | This study |
| CM044 | trpC2 ΔglnR amyE::(PcitM-lacZ cat) | This study |
| CM045 | trpC2 codY::tet amyE::(PcitM-lacZ cat) | This study |
| CM046 | trpC2 abrB::spc amyE::(PcitM-lacZ cat) | This study |
| CM047 | trpC2 spo0A::erm amyE::(PcitM-lacZ cat) | This study |
Tn917 spc, Tn917 derivative conferring resistance to spectinomycin; aphA3, Enterococcus faecalis kanamycin resistance gene; cat, pC194 chloramphenicol acetyltransferase gene.
The final concentrations of the amino acids in a mixture of 18 amino acids (M18 mixture) that was added to spent LB medium were the same as indicated by the manufacturer in fresh LB: l-alanine, 6.2 mM; l-arginine, 2.6 mM; l-aspartate, 7.1 mM; l-cystine, 0.3 mM; l-glutamate, 16.4 mM; glycine, 4.5 mM; l-histidine, 1.7 mM; l-isoleucine, 4.6 mM; l-leucine, 7.2 mM; l-lysine, 6.3 mM; l-methionine, 2.1 mM; l-phenylalanine, 3.0 mM; l-proline, 7.6 mM; l-serine, 5.4 mM; l-threonine, 4.2 mM; l-tryptophan, 0.7 mM; l-tyrosine, 1.1 mM; and l-valine, 5.9 mM. The M5 mixture contained l-arginine, l-aspartate, l-glutamate, l-histidine, and l-proline at the same concentrations. Other growth substrates were added at the same concentration as l-arginine, 2.6 mM.
All cells were grown in flasks at 37°C under continuous shaking at 150 rpm. Growth was monitored by measuring the optical density at 660 nm (OD660). When appropriate, the following antibiotics were added at the indicated concentrations: chloramphenicol, 5 μg/ml; kanamycin, 5 μg/ml; spectinomycin, 100 μg/ml; erythromycin, 1 μg/ml; and tetracycline, 10 μg/ml.
Construction of mutant strains.
Mutants CM021 and CM023 were constructed by transformation of chromosomal DNA extracted from the rocR and sigL mutant strains, respectively, to strain CM002, containing the PcitM-lacZ promoter fusion in the amyE site (29). Successful recombinations were selected for by resistance against chloramphenicol and kanamycin. The double mutant CM031 was constructed by transformation of chromosomal DNA extracted from the rocR mutant strain to strain CM010, a CcpA-deficient strain, and was selected for by resistance against the same two antibiotics plus spectinomycin.
β-Galactosidase assay.
β-Galactosidase activity was determined at 28°C by the method of Miller using o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate (19). Cells from 2 ml of culture were harvested by centrifugation, frozen, and stored at −20°C. The cell pellet was resuspended in a buffer containing 60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, and 1 mM 1,4-dithiothreitol, pH 7.0, and the cells were lysed by treatment with lysozyme in the presence of 10 μM DNase. The assay was started by the addition of 0.1 ml of ONPG (4.5 mg/ml) to the cell extract and stopped by the addition of 0.15 ml of 1.2 M Na2CO3. After a brief spin to remove cell debris, the absorption of the sample was measured at 420 nm. Specific β-galactosidase activities were expressed as the o-nitrophenol released per minute per cell density at 28°C (Miller units). The values reported are averages of at least two independent measurements. Background activities were measured in B. subtilis strain 168 containing plasmid pJM116 integrated in the chromosome (CM001) and amounted to 0.3 to 0.9 Miller unit.
RESULTS
Expression of the citM gene in rich medium.
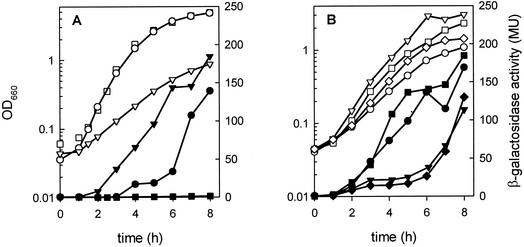
B. subtilis strain CM002 contains the gene encoding β-galactosidase fused behind the promoter region of the citM gene that codes for an Mg2+-citrate transporter (PcitM-lacZ fusion) integrated in the chromosome (Table 1). Growth of the strain in LB medium in the presence or absence of 10 mM citrate revealed the same growth characteristics (Fig. 1A). For the following discussion, three phases will be distinguished in the growth curves: (i) the exponential growth phase (approximately between 1 and 3 h), (ii) the transition phase (between 3 and 6 h), and (iii) the stationary growth phase (following 6 h). Clearly, in the absence of citrate no citM promoter activity could be detected in any of the growth phases, as evidenced by the lack of β-galactosidase activity of the cells (Fig. 1A). This is in agreement with earlier observations obtained with minimal media that showed that citrate is needed for induction of expression of the gene (29, 32, 34). Surprisingly, in the presence of citrate, no promoter activity was observed in the exponential growth phase as well. Only when the cells entered the transition phase was a low level of activity observed, which subsequently increased further in the stationary phase (Fig. 1A).
FIG. 1.
Expression of citM in B. subtilis CM002 grown in fresh and spent LBC medium. β-Galactosidase activity was determined in cells grown in LB medium (▪ and □), LB medium plus 10 mM citrate (• and ○), and spent LB medium plus 10 mM citrate (▾ and ▿) (A) and in spent LBC without further additions (• and ○) and supplemented with an M18 mixture (▾ and ▿), with the same mixture without l-arginine (▪ and □), and with 2.6 mM l-arginine (♦ and ⋄) (B). Samples were taken at the indicated time points to monitor β-galactosidase activity in Miller units (MU) (solid symbols) and growth (open symbols), measured as OD660.
One explanation for the observed expression pattern in the presence of citrate might be that some component(s) in the medium represses citM expression but is gradually depleted during growth, thereby relieving the repression. To explore this possibility, spent medium was prepared by growing B. subtilis for 6.5 h in LB without citrate followed by removal of the cells (see Materials and Methods for details). Strain CM002 grew exponentially for about 5 to 6 h in spent LB medium prepared in this way and supplemented with 10 mM citrate (spent LBC). The growth rate was about twofold lower than that in fresh medium (Fig. 1A). More importantly, expression of citM occurred from the beginning of exponential growth, suggesting that the repression observed in fresh medium was relieved in spent medium (Fig. 1A).
Repression of citM expression by l-arginine.
LB medium is known for its nitrogen-source-rich composition and primarily contains amino acids and vitamins. A mixture of 18 amino acids (M18 mixture) was added to spent medium at the same concentration as that present in fresh LB medium. The β-galactosidase activity of the cells harvested after 5 h of growth was reduced sixfold compared to that of cells grown in spent LBC alone (Table 2), suggesting repression by one or more of the amino acids. To narrow down the search for the repressor(s), a mixture of five amino acids (M5 mixture) that are metabolically linked to the tricarboxylic acid cycle in B. subtilis (l-glutamate, l-proline, l-arginine, l-histidine, and l-aspartate) was added to spent LBC. The mixture was equally potent in repressing the citM promoter activity. Subsequently, a separate addition of the five amino acids to spent medium showed that only l-arginine resulted in reduced levels of expression, while the others did not. Leaving out l-arginine from the M18 and M5 mixtures resulted in specific β-galactosidase activities similar to those observed after growth in spent LBC alone, indicating that arginine was solely responsible for the repression in the M18 and M5 mixtures.
TABLE 2.
β-Galactosidase activity in B. subtilis CM002 grown in spent LBC medium supplemented with amino acids
| Additiona | Batchb | β-Galactosidase activity (Miller units)c |
|---|---|---|
| None | I | 153 |
| None | II | 99 |
| M18 mixture | I | 25 |
| M5 mixture | I | 22 |
| l-Arginine | II | 17 |
| l-Aspartate | II | 116 |
| l-Glutamate | II | 99 |
| l-Histidine | II | 140 |
| l-Proline | II | 112 |
| M5 mixture-l-arginine | I | 108 |
| M18 mixture-l-arginine | I | 136 |
Amino acids were added at concentrations as present in fresh LB medium (see Materials and Methods).
Two batches of spent LB that differed slightly in their properties were used.
Activity was measured at t = 5 h.
The steady-state level of expression of a gene product in growing cells depends both on the promoter activity and the growth rate (31). A fair comparison of expression levels measured at a single time point in terms of promoter activities, as done above, is valid only if the growth rate under the different growth conditions is similar. Figure 1B shows that the addition of various amino acids increased the growth rates by at most 30%, not enough to account for the observed decrease in β-galactosidase activity brought about by l-arginine. The complete expression pattern during growth on spent LBC supplemented with l-arginine showed characteristics similar to those seen during growth on fresh LBC. Expression was low during the initial stages of growth, to increase drastically in the late exponential phase (compare Fig. 1A and B). Again, the difference in expression patterns during growth in the M18 mixture and the same mixture without l-arginine revealed that in the mixture l-arginine was fully responsible for the repression.
Repression of citM gene expression requires arginine metabolism.
In B. subtilis, the genes located in the roc operons rocABC, rocDEF, and rocG are involved in arginine metabolism (1, 4, 10, 11). Deficiencies in RocR, a transcriptional activator needed for expression of the roc genes, and SigL, a σ54 factor required for the transcription of the arginine catabolic operons, render B. subtilis unable to utilize arginine (4, 5). The role of metabolism in the repression of citM gene expression by arginine in spent LBC medium was investigated by introducing the PcitM-lacZ promoter fusion in strains deficient in RocR and SigL, yielding strains CM021 and CM023, respectively (Table 1). The strains grew with similar growth rates in fresh and spent LBC (data not shown).
Strains CM021 and CM023, deficient in RocR and SigL, respectively, were grown in spent LBC in the presence and absence of l-arginine. Both the rocR and sigL mutants brought about elevated citM gene expression in the presence of l-arginine relative to the wild-type strain (Table 3), indicating that for l-arginine to repress citM expression, uptake and/or metabolism seemed to be essential. Table 3 further analyzes intermediates of arginine degradation pathways and some related products for their ability to repress citM expression in spent LBC. Clearly, the addition of citrulline, agmatine, putrescine, urea, α-ketoglutarate, or ammonia was without effect. However, ornithine resulted in a repression similar to that seen for arginine, suggesting that the arginine breakdown route leading to ornithine is involved in the repression. Similar to the repression by l-arginine, the repression by ornithine was lifted in the RocR and SigL mutant strains, indicating that repression by ornithine requires uptake and/or metabolism.
TABLE 3.
β-Galactosidase activity in B. subtilis CM002 grown in spent LBC medium supplemented with various nitrogen sources or arginine degradation products
| Additiona | Batch | β-Galactosidase activity (Miller units)b
|
||
|---|---|---|---|---|
| CM002 (wild type) | CM021 (rocR::aphA3) | CM023 (sigL::aphA3) | ||
| None | III | 101 | NDc | ND |
| None | IV | 131 | 142 | 108 |
| l-Arginine | III | 37 | ND | ND |
| l-Arginine | IV | 46 | 106 | 80 |
| Citrulline | III | 91 | ND | ND |
| Ornithine | III | 41 | 145 | 111 |
| Urea | III | 103 | ND | ND |
| Ammonium | III | 80 | ND | ND |
| α-Ketoglutarate | IV | 122 | ND | ND |
| Agmatine | IV | 98 | ND | ND |
| Putrescine | IV | 128 | ND | ND |
Nitrogen sources were added at concentrations of 2.6 mM.
Activity was measured at t = 5 h.
ND, not determined.
Arginine metabolism-linked repression in the transition phase of growth.
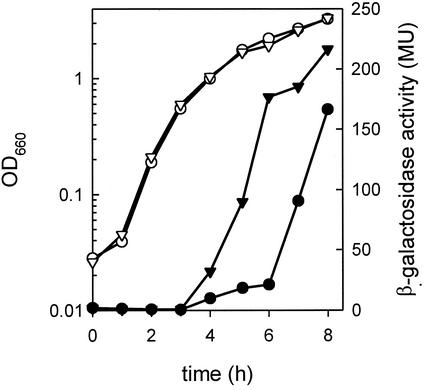
The lack of repression by arginine and ornithine in the RocR mutant allowed for the evaluation of citM expression during growth in fresh LBC in the absence of arginine repression (Fig. 2). The results indicated that arginine repression was exerted specifically during the transition phase of growth. In the exponential growth phase no expression of citM was observed in either the wild-type strain or the RocR mutant strain. In the transition phase, the RocR-deficient strain showed rapidly increasing levels of expression, while the levels in the wild type remained low.
FIG. 2.
Expression of citM in RocR-deficient cells grown in LBC medium. Strains CM002 (• and ○) and CM021 (▾ and ▿) were grown in LB medium containing 10 mM citrate. Samples were withdrawn at the indicated time points to monitor β-galactosidase activity in Miller units (MU) (solid symbols) and growth (open symbols), measured as the OD660.
Involvement of CCR in citM expression in rich medium.
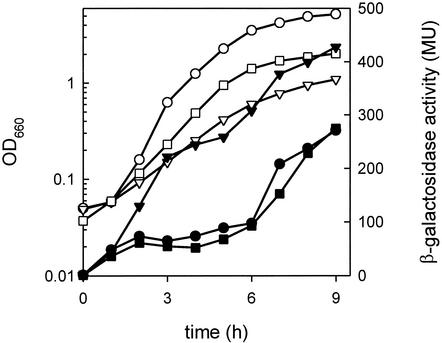
citM gene expression is subject to CCR during growth in minimal medium. Repression was relieved in mutant strain CM010, which is deficient in CcpA, a global regulator in CCR (see Table 1). Strain CM010 grown in LBC medium showed significant β-galactosidase activity during the exponential growth phase (between 1 and 3 h), whereas the wild-type strain CM002 showed no activity (compare Fig. 1 and 3), suggesting a role for the CCR system in repression during this growth phase. Interestingly, no change in expression level was observed during the transition phase, suggesting no role of the CCR system in arginine repression. To exclude the possibility that the expression in the exponential growth phase of the CcpA mutant would be an unspecific effect of the construction of the strain itself, not related to CCR, expression was measured under the same conditions in strain CM008, which contains the PcitM-lacZ promoter fusion in a ptsH1 crh double mutant, lacking functional HPr and Crh, which prevents repression by CCR at a different level (27). The results were similar to those observed for the CcpA mutant (data not shown). In conclusion, citM expression in the wild-type strain is repressed during exponential growth in LBC medium, a repression that is CcpA and HPr/Crh mediated, i.e., CCR mediated.
FIG. 3.
Expression of citM in CcpA-deficient cells grown in fresh and spent LBC medium. Strain CM010 was grown in fresh LBC medium (• and ○), spent LBC medium (▾ and ▿), and spent LBC medium plus 2.6 mM l-arginine (▪ and □). Samples were taken at the indicated time points to monitor β-galactosidase activity in Miller units (MU) (solid symbols) and growth (open symbols), measured as OD660.
More direct evidence for the lack of involvement of CcpA in arginine repression was obtained by growing the CM010 strain in spent LBC in the presence and absence of l-arginine. In the absence of l-arginine, the expression pattern was similar to that observed for the wild-type strain CM002 but the expression levels were approximately twofold higher over the whole growth curve (compare Fig. 1 and 3). In the presence of l-arginine, repression was exerted to the same level as that observed in fresh LBC medium, showing that arginine-mediated repression is still functional in the CcpA mutant.
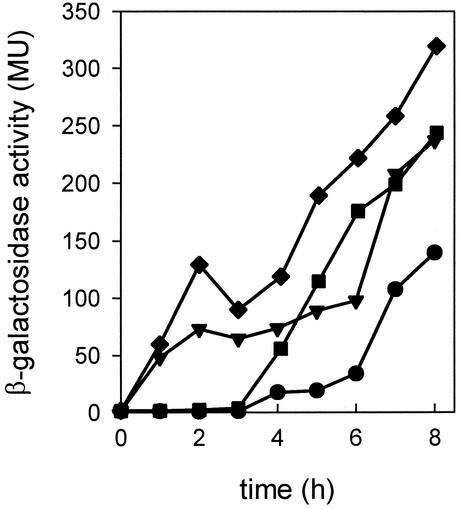
A mutant strain, CM031, was constructed in which both CcpA and RocR were inactivated. Figure 4 compares the expression patterns during growth in LBC medium of the ccpA rocR double mutant, the RocR-deficient mutant, the CcpA-deficient mutant, and the wild-type strain CM002. It follows that the CCR-mediated repression and l-arginine repression are more or less additive processes (see also Discussion).
FIG. 4.
Overview of expression of citM in repression-deficient mutants during growth in LBC medium. β-Galactosidase activity of strain CM002 (wild type) (•), CM010 (CcpA deficient) (▾), CM021 (RocR deficient) (▪), and CM031 (CcpA/RocR deficient) (♦) grown in fresh LB plus 10 mM citrate. MU, Miller units.
Several other global regulatory proteins involved in gene expression are known in B. subtilis, including CodY, GlnR, and TnrA, which play a role in nitrogen regulation (2, 23, 24, 33), and AbrB and Spo0A, two transcription factors involved in regulation of gene expression in the transition into the stationary growth phase (25, 26). The PcitM-lacZ promoter fusion was introduced in strains deficient in these proteins, and the resulting strains were assayed for involvement in the repression of the citM gene by l-arginine as described for the RocR-deficient strain (Fig. 2). None of the regulators could be shown to be involved in the l-arginine-mediated repression (data not shown).
DISCUSSION
Expression of the Mg2+-citrate transporter, CitM, of B. subtilis during growth in minimal media is induced by the presence of citrate or isocitrate in the medium and repressed by preferable carbon sources like glucose, glycerol, and inositol but also by the combination of the nonsugars succinate and glutamate (29). Repression is at the level of transcription and mediated by the CCR system, involving the global regulatory protein CcpA. In this study, expression of citM was studied in rich medium from which a complex regulation emerges. As in minimal medium, expression requires induction by citrate, but in addition, two different mechanisms of repression seem to be operative: CCR and a newly described, CcpA-independent repression involving arginine metabolism that is manifested in the transition phase of growth. The regulation of transcription of the citM gene during the exponential, transition, and stationary growth phases in LBC medium is summarized in Fig. 4. The B. subtilis wild-type strain showed no expression in the exponential growth phase, a low level of expression in the transition phase, and rapidly increasing expression in the stationary phase. Experiments with a ccpA-null mutant conferred elevated expression in the exponential growth phase, indicating that repression in the wild-type was CCR mediated. A rocR-null mutant, deficient in the enzymes of the arginine metabolic pathway, showed elevated levels of expression specifically in the transition phase of growth. Experiments using spent medium revealed that the wild-type strain was repressed by the presence of l-arginine in the medium, a repression that required the arginine metabolic enzymes. In the exponential growth phase in LBC medium, repression was not relieved in the RocR-deficient strain, indicating that CCR-mediated repression was still present. Either arginine-mediated repression is not operative in the exponential growth phase or it is overruled by CCR-mediated repression. Expression in the transition phase was not enhanced relative to the exponential growth phase in the CcpA mutant, indicating that expression was still repressed by l-arginine, consistent with the lack of relief of repression by l-arginine in the ccpA mutant strain grown in spent LBC. A double mutant deficient in both CcpA and RocR showed the highest levels of expression throughout the three growth phases, suggesting that the two repression mechanisms are independent. Expression of citM increased significantly in the stationary growth phase in all strains tested, most likely as a result of the decreased growth rate in this growth phase (31).
Transcription of citM is down-regulated by the global regulatory protein CcpA in response to the availability of glucose, glycerol, inositol, and succinate and/or glutamate in the medium (29). The main constituents of LB medium are amino acids, which were shown not to result in repression or CcpA-dependent repression (l-arginine) when added to spent LBC. According to the composition supplied by the manufacturer (Difco manual, 11th ed.), LB broth contains a low concentration of inositol (117 μM). The addition of inositol at the same concentration to spent LBC medium did not repress citM promoter activity (data not shown). No further attempts were made to identify the effector(s) of CcpA-mediated repression during the exponential growth phase. Possibly, the observed repression is the result of a mixture of effectors, all present at low concentrations.
The major pathway for the degradation of l-arginine in B. subtilis is the arginase pathway. The genes involved are organized in the roc regulon containing the rocABC, rocDEF, and rocG operons (1, 10, 11). Expression of the regulon requires the presence of ornithine (11) and is mediated by the positive regulatory protein RocR, a member of the NtrC/NifA family of regulators. RocR acts together with a σ54 factor, SigL (4, 5). citM promoter activity was reduced by the presence of both arginine and ornithine in the growth medium (Tables 2 and 3). Since both rocR- and sigL-null mutants were able to overcome the repression of citM transcription by arginine and ornithine, it seems fair to conclude that the arginase pathway is involved in the regulation. It is likely that both arginine and ornithine have to be transported into the cell and that arginine has to be converted into ornithine to exert repression, which would imply that ornithine is the true repressor as it is the true activator for roc regulon activation (11).
The citM and citST (the two-component system responsible for induction of CitM) promoter regions do not contain the known σ54 consensus sequence (18) or RocR binding site (4), making a putative link between roc regulon activation and citM gene deactivation indirect. Several other regulatory proteins in B. subtilis are known to be involved in gene expression in response to amino acid availability. CodY-dependent repression occurs in cells growing rapidly in media containing amino acids (7), and GlnR and TnrA are involved during growth on amino acids, like glutamine, when used as a nitrogen source (22, 33). None of these regulators appeared to be involved in the arginine-mediated repression of citM. Since the repression seemed to be important, especially in the transition phase of growth, the involvement of the regulatory proteins AbrB and Spo0A, which are known to operate postexponentially (25, 26), was investigated, but these were also not involved in the repression.
Acknowledgments
We thank A. L. Sonenshein and M. Débarbouillé for kindly providing some of the strains used in this study.
This work was supported by grants from the Ministry of Economic Affairs of The Netherlands, in the framework of the IOP Milieutechnologie/Zware Metalen, project IZW97404.
REFERENCES
- 1.Belitsky, B. R., and A. L. Sonenshein. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 180:6298-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belitsky, B. R., L. V. Wray, Jr., S. H. Fisher, D. E. Bohannon, and A. L. Sonenshein. 2000. Role of TnrA in nitrogen source-dependent repression of Bacillus subtilis glutamate synthase gene expression. J. Bacteriol. 182:5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boorsma, A., M. E. van der Rest, J. S. Lolkema, and W. N. Konings. 1996. Secondary transporters for citrate and the Mg2+-citrate complex in Bacillus subtilis are homologous proteins. J. Bacteriol. 178:6216-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calogero, S., R. Gardan, P. Glaser, J. Schweizer, G. Rapoport, and M. Débarbouillé. 1994. RocR, a novel regulatory protein controlling arginine utilization in Bacillus subtilis, belongs to the NtrC/NifA family of transcriptional activators. J. Bacteriol. 176:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Débarbouillé, M., I. Martin-Verstraete, F. Kunst, and G. Rapoport. 1991. The Bacillus subtilis sigL gene encodes an equivalent of σ54 from Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 88:9092-9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutscher, J., J. Reizer, C. Fischer, A. Galinier, M. H. J. Saier, and M. Steinmetz. 1994. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J. Bacteriol. 176:3336-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher, S. H., K. Rohrer, and A. E. Ferson. 1996. Role of CodY in regulation of the Bacillus subtilis hut operon. J. Bacteriol. 178:3779-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galinier, A., J. Deutscher, and I. Martin-Verstraete. 1999. Phosphorylation of either Crh or HPr mediates binding of CcpA to the Bacillus subtilis xyn cre and catabolite repression of the xyn operon. J. Mol. Biol. 286:307-314. [DOI] [PubMed] [Google Scholar]
- 9.Galinier, A., J. Haiech, M. C. Kilhoffer, M. Jaquinod, J. Stülke, J. Deutscher, and I. Martin-Verstraete. 1997. The Bacillus subtilis crh gene encodes a HPr-like protein involved in carbon catabolite repression. Proc. Natl. Acad. Sci. USA 94:8439-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardan, R., G. Rapoport, and M. Débarbouillé. 1995. Expression of the rocDEF operon involved in arginine catabolism in Bacillus subtilis. J. Mol. Biol. 249:843-856. [DOI] [PubMed] [Google Scholar]
- 11.Gardan, R., G. Rapoport, and M. Débarbouillé. 1997. Role of the transcriptional activator RocR in the arginine-degradation pathway of Bacillus subtilis. Mol. Microbiol. 24:825-837. [DOI] [PubMed] [Google Scholar]
- 12.Henkin, T. M., F. J. Grundy, W. L. Nicholson, and G. H. Chambliss. 1991. Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacl and galR repressors. Mol. Microbiol. 5:575-584. [DOI] [PubMed] [Google Scholar]
- 13.Hueck, C. J., and W. Hillen. 1995. Catabolite repression in Bacillus subtilis: a global regulatory mechanism for the Gram-positive bacteria? Mol. Microbiol. 15:395-401. [DOI] [PubMed] [Google Scholar]
- 14.Krom, B. P., H. Huttinga, J. B. Warner, and J. S. Lolkema. 2002. Impact of the Mg2+-citrate transporter CitM on heavy metal toxicity in Bacillus subtilis. Arch. Microbiol. 178:370-375. [DOI] [PubMed] [Google Scholar]
- 15.Krom, B. P., J. B. Warner, W. N. Konings, and J. S. Lolkema. 2000. Complementary metal ion specificity of the metal-citrate transporters CitM and CitH of Bacillus subtilis. J. Bacteriol. 182:6374-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin-Verstraete, I., J. Deutscher, and A. Galinier. 1999. Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signalling pathway for the Bacillus subtilis levanase operon. J. Bacteriol. 181:2966-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Verstraete, I., A. Galinier, E. Darbon, Y. Quentin, M. C. Kilhoffer, V. Charrier, J. Haiech, G. Rapoport, and J. Deutscher. 1999. The Q15H mutation enables Crh, a Bacillus subtilis HPr-like protein, to carry out some regulatory HPr functions, but does not make it an effective phosphocarrier for sugar transport. Microbiology 145:3195-3204. [DOI] [PubMed] [Google Scholar]
- 18.Merrick, M. J. 1993. In a class of its own—the RNA polymerase sigma factor σ54 (σN). Mol. Microbiol. 10:903-909. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Miwa, Y., A. Nakata, A. Ogiwara, M. Yamamoto, and Y. Fujita. 2000. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 28:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Presecan-Siedel, E., A. Galinier, R. Longin, J. Deutscher, A. Danchin, P. Glaser, and I. Martin-Verstraete. 1999. Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis. J. Bacteriol. 181:6889-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreier, H. J., S. W. Brown, K. D. Hirschi, J. F. Nomellini, and A. L. Sonenshein. 1989. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J. Mol. Biol. 210:51-63. [DOI] [PubMed] [Google Scholar]
- 23.Serror, P., and A. L. Sonenshein. 1996. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J. Bacteriol. 178:5910-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slack, F. J., P. Serror, E. Joyce, and A. L. Sonenshein. 1995. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol. Microbiol. 15:689-702. [DOI] [PubMed] [Google Scholar]
- 25.Strauch, M., V. Webb, G. Spiegelman, and J. A. Hoch. 1990. The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc. Natl. Acad. Sci. USA 87:1801-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauch, M. A., and J. A. Hoch. 1993. Transition-state regulators: sentinels of Bacillus subtilis post-exponential gene expression. Mol. Microbiol. 7:337-342. [DOI] [PubMed] [Google Scholar]
- 27.Stülke, J., and W. Hillen. 2000. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 54:849-880. [DOI] [PubMed] [Google Scholar]
- 28.Tobisch, S., D. Zühlke, J. Bernhardt, J. Stülke, and M. Hecker. 1999. Role of CcpA in regulation of the central pathways of carbon catabolism in Bacillus subtilis. J. Bacteriol. 181:6996-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warner, J. B., B. P. Krom, C. Magni, W. N. Konings, and J. S. Lolkema. 2000. Catabolite repression and induction of the Mg2+-citrate transporter CitM of Bacillus subtilis. J. Bacteriol. 182:6099-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner, J. B., and J. S. Lolkema. 2002. Growth of Bacillus subtilis on citrate and isocitrate is supported by the Mg2+-citrate transporter CitM. Microbiology 148:3405-3412. [DOI] [PubMed] [Google Scholar]
- 31.Warner, J. B., and J. S. Lolkema. 2002. LacZ-promoter fusions: the effect of growth. Microbiology 148:1241-1243. [DOI] [PubMed] [Google Scholar]
- 32.Willecke, K., and A. B. Pardee. 1971. Inducible transport of citrate in a Gram-positive bacterium, Bacillus subtilis. J. Biol. Chem. 246:1032-1040. [PubMed] [Google Scholar]
- 33.Wray, L. V., Jr., A. E. Ferson, K. Rohrer, and S. H. Fisher. 1996. TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:8841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto, H., M. Murata, and J. Sekiguchi. 2000. The CitST two-component system regulates the expression of the Mg-citrate transporter in Bacillus subtilis. Mol. Microbiol. 37:898-912. [DOI] [PubMed] [Google Scholar]