Abstract
We have found that alternative localization of two types of L31 ribosomal protein, RpmE and YtiA, is controlled by the intracellular concentration of zinc in Bacillus subtilis. The detailed mechanisms for the alternation of L31 proteins under zinc-deficient conditions were previously unknown. To obtain further information about this regulatory mechanism, we have studied the stability of RpmE in vivo and the binding affinity of these proteins to ribosomes in vitro, and we have found that liberation of RpmE from ribosomes is triggered by the expression of ytiA, which is induced by the derepression of Zur under zinc-deficient conditions.
The prokaryotic ribosome, a molecular machine for protein biosynthesis, is composed of 3 rRNAs (16S, 23S, and 5S rRNA) and more than 50 ribosomal proteins. Since the genes for most ribosomal proteins are highly conserved in many prokaryotes, it was believed that most ribosomal protein genes were indispensable for cell viability, and they were assigned as single-copy genes in the genomes of many bacteria. However, it has been reported that several paralogous pairs of ribosomal protein genes are present in many bacterial genomes (10). Although it remains unclear why many bacteria possess these duplications in their genomes, it has been reported that the duplications of L31, L33, L36, and S14 found in several bacterial genomes can be classified into two types: one type contains a zinc binding motif (CxxC motif; designated C+), whereas the other does not (designated C−) (10). From these findings, it was predicted that an ancient duplication of the C+ forms took place before the divergence of major bacterial lineages. Subsequently, loss of the C+ form or loss of the CxxC motif after the duplication generated the C− form (10).
In the genome of Bacillus subtilis, one of the best-characterized gram-positive bacteria, genes encoding L31, L33, and S14 proteins are duplicated and are present in both the C+ and C− forms (9, 10). Our previous report showed that two types of L31 protein can be detected in ribosomes prepared from cells at different growth phases in zinc-limiting minimal medium (12). We reported that RpmE (C+ form) is stable when it contains a zinc ion bound to its CxxC motif and that the expression of ytiA, coding for the C− form of the L31 protein, is repressed by Zur, a zinc-specific transcriptional repressor that controls zinc transport operons (2, 3). From these results, we proposed that alternation of two types of L31 protein in the ribosome is triggered by changes in the concentration of zinc in the environment. Although a similar hypothesis was proposed based on in silico analyses of Zur-regulated genes in several bacteria (16), our study provided the first experimental evidence to support this model (12).
The detailed mechanisms explaining the alternation of L31 proteins under zinc-deficient conditions remain to be defined. In particular, clarification is necessary as to whether liberation of RpmE from ribosomes is due to (i) inability of RpmE to bind to a zinc ion because the ion is not available, (ii) incorporation of YtiA into ribosomes instead of RpmE, or (iii) active displacement of RpmE from ribosomes by newly synthesized YtiA. If the first model is true, RpmE will not be associated with the ribosome under zinc-deficient conditions even in the absence of YtiA. Thus, the expression of YtiA and the destabilization of RpmE under zinc-deficient conditions would not be directly correlated with each other. If the second model is true, YtiA will be preferentially incorporated into ribosomes and thus RpmE will be unable to bind. Finally, the third model predicts that RpmE will be ejected from the ribosome once YtiA is synthesized. This model might also allow for the modulation of intracellular concentrations of zinc in the cell under zinc-deficient conditions (16). To obtain further information about this regulatory mechanism, we have studied the stability of RpmE in vivo and the relative binding affinities of these proteins to the ribosomes in vitro.
Involvement of YtiA in the stability of RpmE in vivo.
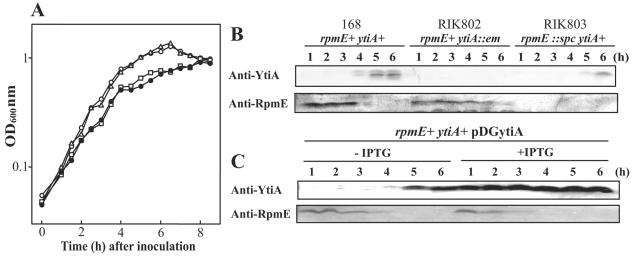
To study the two types of L31, we made rpmE and ytiA single disruptants and an rpmE ytiA double mutant. RIK802, carrying a ytiA::erm mutation, was constructed by replacing the 197-bp sequence from +11 through +207 in the ytiA gene (nucleotide numbers indicate positions relative to the first base of the translation start codon ATG) with the erythromycin resistance gene (erm) derived from pTC3 (formally named pAE41 [7]). RIK803, carrying an rpmE::spc mutation, was obtained by replacing the 149-bp sequence from +11 through +159 in the rpmE gene with the spectinomycin resistance cassette derived from pBEST517A (19). Chromosomal DNA extracted from RIK803 was used to transform RIK802 to generate RIK814 (rpmE::spc ytiA::erm). These strains were precultured on LB agar plates (17) at 28°C for about 16 h, then inoculated into CSM at an optical density at 600 nm of ca. 0.03, and incubated at 37°C with shaking. CSM is a simple semisynthetic competence and sporulation medium, based on Spizizen's minimal glucose medium (11). The composition of CSM is as follows: 6 g of KH2PO4, 14 g of K2HPO4, 2 g of (NH4)2SO4, 1 g of trisodium citrate · 2H2O per liter of deionized water, 0.2% glucose, 0.02% casein acid hydrolysate (Amicase; Sigma), 1 mM MgSO4, 1 mM Ca(NO3)2, 1 μM FeSO4, 10 μM MnCl2, and 50 μg ml−1 of tryptophan. As shown in Fig. 1A, the rpmE single mutant as well as the rpmE ytiA double mutant grew slowly in CSM compared to the wild type. In contrast, the ytiA mutant grew normally in CSM (Fig. 1A). These results suggest that although neither gene is essential, RpmE may function as the main L31 protein.
FIG. 1.
(A) Growth characteristics of L31 mutants in CSM at 37°C. Symbols: ○, 168 (ytiA+ rpmE+); ▵, RIK802 (ytiA::erm); □, RIK803 (rpmE::spc); •, RIK814 (ytiA::erm rpmE::spc). (B and C) Effect of ytiA mutation (B) or overexpression of ytiA (C) on intracellular levels of RpmE. (B) Cells were incubated in CSM at 37°C with shaking and were collected at the indicated times. Crude extracts containing 60 μg of proteins were separated by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (18), and transferred to polyvinylidene difluoride membranes (Millipore). Immunodetection procedures were carried out as described previously (13). An anti-RpmE antibody (12) and an anti-YtiA antibody (which was prepared from a rabbit immunized with purified YtiA protein) were used at a 1:1,000 dilution. (C) RIK812 cells carrying the inducible ytiA expression plasmid, pDGytiA, were grown in CSM at 37°C with or without 1 mM IPTG. Cells were collected at the indicated times after inoculation, and aliquots of extracts containing 60 μg of total proteins were used for Western blot analysis.
To examine whether or not ytiA disruption affects the intracellular level of RpmE, Western blot analysis was carried out using an anti-RpmE antibody. Although RpmE rapidly becomes undetectable in the wild type after the end of exponential growth in CSM (4 h after inoculation), its levels decrease slowly in the ytiA disruptant (Fig. 1B). Northern blot analysis reveals that the transcription level of rpmE is almost the same in the ytiA mutant and wild-type cells. In both cases, transcription of rpmE ceases after the end of exponential growth in CSM (data not shown). The delayed accumulation of YtiA in the rpmE mutant (Fig. 1B) is likely due to the slow growth phenotype of this strain in CSM (12). These results suggest that YtiA protein is needed to destabilize RpmE, perhaps by preventing its incorporation into the ribosome or by actively displacing RpmE from already assembled ribosomes.
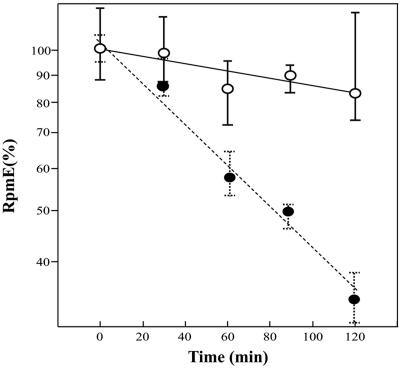
To examine whether or not YtiA is involved in the posttranscriptional regulation of RpmE, we next introduced a multicopy plasmid, pDGytiA, into the wild type and monitored the effect of overexpressed ytiA on the intracellular level of RpmE. pDGytiA carries a ytiA gene controlled by an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible Pspac promoter. The plasmid was constructed as follows. First, the ytiA gene was amplified by means of primers pDGytiA-F (5′-GCCGAAGCTTTTTATTGAAAGGAGATACCC-3′) and pDGytiA-R (5′-GCCTGCCTTATTTCCCCATGT-3′). (The underlined sequence represents a HindIII restriction site.) Second, the terminator region of the rpmE gene was amplified by PCR using primers rpmEter-F (5′-ATATAACATGGGGAAATAAGGCAGGCGTAATAATAGATTTCTCAACAGG-3′) and rpmEter-R (5′-ACGCGTCGACCTGCCAGCTGTGTTTTAGAC-3′). (The underlined sequence represents a SalI restriction site.) These fragments were then used simultaneously as the template for PCR amplification using primers pDGytiA-F and rpmEter-R. The resulting fragment was digested with HindIII and SalI and cloned into pDG148 (8). Using this strain containing pDG148 (RIK812), we found that in the presence of IPTG (1 mM), RpmE protein levels decrease rapidly after the end of exponential growth in CSM (Fig. 1B). Similar results were obtained using a zur disruptant, in which constitutive expression of ytiA is observed (data not shown). From these results, it was hypothesized that YtiA affects the stability of RpmE in the cell. To prove this, we next examined the relative stability of RpmE. As shown in Fig. 2, the half-life of RpmE in cells incubated with IPTG is about 80 min, whereas it is about 470 min in the absence of IPTG (Fig. 2). These results indicate that RpmE protein is destabilized by overexpression of ytiA. CSM contains only about 14 nM (13.6 ± 1.3 nM) of trace zinc ions, whose value was determined by inductively coupled plasma-mass spectrometry (ICP-MS). However, it was expected that cells growing exponentially in CSM would contain enough zinc to repress the expression of ytiA, because the cells were precultured on LB plates which contained 15 μM of trace zinc (11, 12, 15). It is thus likely that destabilization of RpmE is enhanced under zinc-deficient conditions, in which RpmE would not carry a zinc ion.
FIG. 2.
Effect of overexpression of YtiA on the relative stability of RpmE. RIK812 cells were grown in CSM at 37°C with or without 1 mM IPTG. Chloramphenicol (100 μg/ml) and rifampin (10 μg/ml) were added to the culture when the optical density reached ca. 0.2. Time zero was taken as being 5 min after the addition of the antibiotics, and cells were collected at the times indicated. Aliquots of extracts containing 50 μg of protein (○) (without IPTG) or 70 μg (•) (with IPTG) were used for Western blot analysis. Relative RpmE amounts were quantified with ImageQuant 5.2 software (Molecular Dynamics). Each result is the average of three determinations. The ordinate (expressed with a log scale) shows the percentage of RpmE remaining. Error bars, standard deviations.
Binding affinity of RpmE and YtiA proteins to the ribosome.
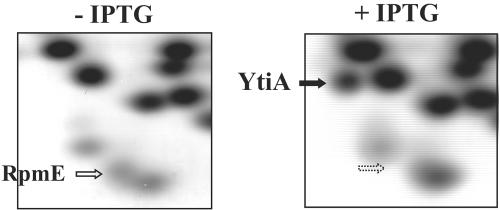
The results described above strongly suggest that YtiA is either directly or indirectly involved in the stability of RpmE. Previous studies indicate that L31 protein is not essential for the formation of RI*50 (1), which is the essential product of the early assembly of the 50S subunit (6, 14). It has also been shown that the association of L31 with the ribosome is highly variable (1). In fact, L31 was not found in crystals of the 70S ribosome from Thermus thermophilus (20), although the gene coding for L31 is found in the genome (5). From these results, it appears likely that the L31 protein is not tightly associated with the ribosome and is thus easily released from the ribosome without causing any significant damage to the 50S subunit. Therefore, we have postulated that RpmE is released from the ribosome and destabilized when newly synthesized YtiA is efficiently incorporated into the ribosome, thereby displacing RpmE. To prove this possibility, we have determined the type of L31 protein actually incorporated into the ribosome in vivo when YtiA is overexpressed during exponential growth in CSM. Crude ribosomes used for the examinations in this study were prepared as previously described (12). Briefly, the crude S-100 fraction, obtained by ultracentrifugation for 100 min at 180,000 × g, was dissolved in buffer II (20 mM Tris-HCl [pH 7.6], 15 mM magnesium acetate, 1 M ammonium acetate, 6 mM β-mercaptoethanol, and 2 mM phenylmethylsulfonyl fluoride [PMSF]). After centrifugation at 30,000 × g for 60 min in the RPS 50-2 rotor, 2-ml aliquots of supernatant were layered onto 2 ml of buffer II containing a 30% (wt/vol) sucrose bed and were centrifuged at 180,000 × g for 3.5 h in the RPS 50-2 rotor. The crude ribosomes were prepared by the acetic acid method (4) and dialysis against 1% acetic acid. As shown in Fig. 3, only YtiA was detected in ribosomes prepared from cells grown with IPTG, though RpmE was found in the crude ribosome fraction prepared from RIK812 cells grown without IPTG. These results evidently support our idea stated above that RpmE is replaced by newly synthesized YtiA. However, these results do not rule out the possibility that the amount of YtiA is much larger than that of RpmE in the cell.
FIG. 3.
RFHR 2D gel electrophoresis of ribosomes prepared from YtiA-overproducing cells. RIK812 cells were grown in CSM at 37°C with shaking with or without 1 mM IPTG and were harvested 2 h after inoculation. Crude ribosomes were then purified and analyzed by RFHR 2-dimensional electrophoresis, which was carried out as described previously (12). PhastGel Blue R (Amersham) was used to stain the gels.
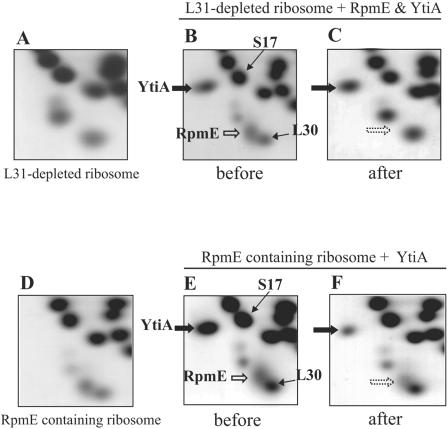
Therefore, we next set about to determine which type of L31 protein is preferentially incorporated into the ribosome in vitro, as follows. First, we purified RpmE and YtiA proteins using the IMPACT system (New England Biolabs) as described previously (12). We confirmed that the purified RpmE contained about 0.8 atoms of zinc per molecule of protein, as judged by ICP-MS analysis (12). Next, 30 A260 units of crude ribosome prepared from RIK814 (rpmE::spc ytiA::erm) cells was mixed with purified RpmE and/or YtiA proteins (2.8 nmol each) in 200 μl of buffer IV (10 mM Tris-HCl [pH 7.6], 15 mM magnesium acetate, 100 mM ammonium acetate, 6 mM β-mercaptoethanol, 2 mM PMSF) and incubated at 37°C for 20 min with 1 mM ZnSO4. Aliquots (100 μl) were stored as a control and used for radical-free and highly reducing (RFHR) 2-dimensional (2D) gel electrophoresis (Fig. 4B and E). The remainder, containing 1.4 nmol of purified RpmE and YtiA, was diluted with 4.9 ml of buffer IV and centrifuged for 100 min at 180,000 × g at 4°C in a HITACHI P50ST rotor. The pellet was dissolved in buffer III (50 mM Tris-HCl [pH 8.0], 6 mM β-mercaptoethanol, and 2 mM PMSF) and then analyzed by RFHR 2D gel electrophoresis. The staining intensities of YtiA and RpmE on the 2-dimensional gels were normalized using the values of S17 (for YtiA) and L30 (for RpmE), respectively. Relative amounts of YtiA and RpmE were calculated by ImageQuant 5.2 software (Molecular Dynamics). We also confirmed that purified RpmE protein was copelleted with the crude ribosomes lacking both L31 proteins (data not shown). We found that the staining intensities of RpmE and L30 in the 2D gel showed equally when 1.4 nmol of purified RpmE was mixed with the 15 A260 units of L31-depleted ribosomes. Therefore, 1.4 nmol of each of the two purified L31 proteins was used for this assay.
FIG. 4.
(A, B, and C) Competitive incorporation of RpmE and YtiA into L31-depleted ribosomes. Crude ribosomes prepared from RIK814 (rpmE::spc ytiA::erm) cells (A) and purified RpmE and YtiA proteins were used for the incorporation assay as described in the text. (B and C) RFHR 2D gels of the ribosome fraction before (B) or after (C) centrifugation. (D, E, and F) Incorporation of YtiA into RpmE-containing ribosomes. Crude ribosomes obtained from RIK802 (ytiA::erm) cells (D) and purified YtiA protein were mixed. (E and F) RFHR 2D gels of the ribosome fraction before (E) or after (F) centrifugation.
When these purified proteins were subjected to the sedimentation experiments without addition to the crude ribosomes, they were found in the supernatant fractions (data not shown), indicating that these purified proteins are soluble under this condition. Eighty-four percent of added YtiA, compared with the initial volume, is copelleted with ribosomes, but RpmE cannot be detected at all (Fig. 4B and C). It should be noted that these experiments were carried out with the addition of 1 mM ZnSO4. Taking these results together with the fact that similar results were obtained without addition of zinc (data not shown), it is evident that, independently of zinc, YtiA is incorporated into the ribosome more efficiently than RpmE.
To examine whether YtiA protein can liberate RpmE from ribosomes, we next performed the same experiments using purified YtiA protein and crude ribosomes obtained from the ytiA mutant (RIK802). Crude ribosomes originally contain RpmE, and the amount does not change after centrifugation in the presence or absence of 1 mM ZnSO4 (data not shown). Therefore, it is suggested that RpmE cannot be released from ribosomes in the absence of YtiA, even if cells encounter zinc-deficient conditions. As shown in Fig. 4E and F, the addition of purified YtiA (1.4 nmol) reduces the amount of RpmE copelleted with the ribosome, while 41% of the added YtiA is ribosome associated. We also found that the amount of RpmE copelleted with ribosomes depends on the concentration of YtiA added to the reaction. Indeed, RpmE could not be detected at all when 2.8 nmol of purified YtiA was added (data not shown). These results indicate that YtiA can be incorporated into at least a portion of the RpmE-containing ribosomes, and they further suggest that YtiA incorporation results in liberation of RpmE.
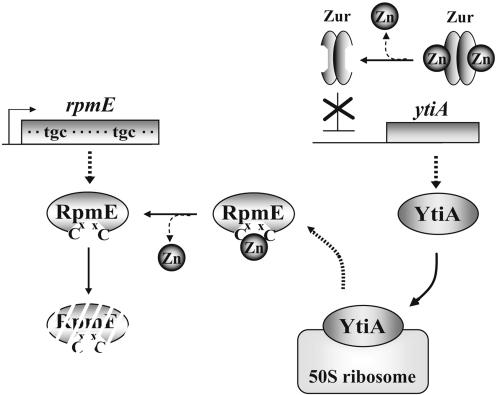
From these results, we propose a model for the alternation of RpmE and YtiA in the ribosome under changing zinc conditions (Fig. 5). In the absence of zinc, Zur is unable to bind to the “Zur box,” and derepression of ytiA occurs. Since the newly synthesized YtiA has a higher affinity for the ribosome than RpmE, YtiA can be efficiently incorporated into the ribosome and actively displaces bound RpmE. RpmE thus released is then degraded by an unknown protease(s). On the other hand, under these conditions newly synthesized RpmE would not be able to bind zinc and would thus be unstable. Since ribosomes are highly abundant in the cell, this alternation may be virtually able to increase the concentration of zinc ions which are available for other zinc-binding proteins in the cell. Therefore, this regulatory system would contribute to the zinc homeostasis in the cell under zinc-deficient conditions, as proposed by Panina et al. (16). However, the contribution of this alternation to zinc homeostasis may be partial, because the rpmE gene is dispensable for the cell growing under zinc-limiting conditions, though it grew slowly under these conditions (Fig. 1A).
FIG. 5.
Model of the replacement of RpmE by YtiA under zinc-deficient conditions. See the text for details.
On the other hand, the function of L31 proteins in the ribosome remains unclear at present. In fact, sucrose density gradient ultracentrifugation analyses suggested that L31 mutations almost did not affect 70S ribosome formation and did not change the compositions of ribosomal proteins in the crude ribosomes (data not shown). Therefore, we cannot fully confirm that this alternation would be required not only for zinc homeostasis but also for another physiological role at present. Further investigation will be needed to clarify the function of L31 and the physiological role of the alternation between RpmE and YtiA.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (C) and Creative Scientific Research (16GS0304), from the Ministry of Education, Culture, Sports, Science and Technology of Japan. H.N. was supported by a Grant-in-Aid for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Eistetter, A. J., P. D. Butler, R. R. Traut, and T. G. Fanning. 1999. Characterization of Escherichia coli 50S ribosomal protein L31. FEMS Microbiol. Lett. 180:345-349. [DOI] [PubMed] [Google Scholar]
- 2.Gaballa, A., and J. D. Helmann. 1998. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J. Bacteriol. 180:5815-5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaballa, A., T. Wang, R. W. Ye, and J. D. Helmann. 2002. Functional analysis of the Bacillus subtilis Zur regulon. J. Bacteriol. 184:6508-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy, S. J. S., C. G. Kurland, P. Voynow, and G. Mora. 1969. The ribosomal proteins of Eschericha coli. I. Purification of the 30S ribosomal proteins. Biochemistry 8:2897-2905. [DOI] [PubMed] [Google Scholar]
- 5.Henne, A., H. Bruggemann, C. Raasch, A. Wiezer, T. Hartsch, H. Liesegang, A. Johann, T. Lienard, O. Gohl, R. Martinez-Arias, C. Jacobi, V. Starkuviene, S. Schlenczeck, S. Dencker, R. Huber, H. P. Klenk, W. Kramer, R. Merk, G. Gottschalk, and H. J. Fritz. 2004. The genome sequence of the extreme thermophile Thermus thermophilus. Nat. Biotechnol. 22:547-553. [DOI] [PubMed] [Google Scholar]
- 6.Herold, M., and K. H. Nierhaus. 1987. Incorporation of six additional proteins to complete the assembly map of the 50S subunit from Escherichia coli ribosomes. J. Biol. Chem. 262:8826-8833. [PubMed] [Google Scholar]
- 7.Imamura, D., K. Kobayashi, J. Sekiguchi, N. Ogasawara, M. Takeuchi, and T. Sato. 2004. spoIVH (ykvV), a requisite cortex formation gene, is expressed in both sporulating compartments of Bacillus subtilis. J. Bacteriol. 186:5450-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joseph, P., J. R. Fantino, M. L. Herbaud, and F. Denizot. 2001. Rapid orientated cloning in a shuttle vector allowing modulated gene expression in Bacillus subtilis. FEMS Microbiol. Lett. 205:91-97. [DOI] [PubMed] [Google Scholar]
- 9.Kunst, F., et al. 1997. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 10.Makarova, K. S., V. A. Ponomarev, and E. V. Koonin. 2001. Two C or not two C: recurrent disruption of Zn-ribbons, gene duplication, lineage-specific gene loss, and horizontal gene transfer in evolution of bacterial ribosomal proteins. Genome Biol. 2:0033.1-0033.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murayama, R., G. Akanuma, Y. Makino, H. Nanamiya, and F. Kawamura. 2004. Spontaneous transformation and its use for genetic mapping in Bacillus subtilis. Biosci. Biotechnol. Biochem. 68:1672-1680. [DOI] [PubMed] [Google Scholar]
- 12.Nanamiya, H., G. Akanuma, Y. Natori, R. Murayama, S. Kosono, T. Kudo, K. Kobayashi, N. Ogasawara, S.-M. Park, K. Ochi, and F. Kawamura. 2004. Zinc is a key factor in controlling alternation of two types of L31 protein in the Bacillus subtilis ribosome. Mol. Microbiol. 52:273-283. [DOI] [PubMed] [Google Scholar]
- 13.Nanamiya, H., Y. Ohashi, K. Asai, S. Moriya, N. Ogasawara, M. Fujita, Y. Sadaie, and F. Kawamura. 1998. ClpC regulates the fate of a sporulation initiation sigma factor, σH protein, in Bacillus subtilis at elevated temperatures. Mol. Microbiol. 29:505-513. [DOI] [PubMed] [Google Scholar]
- 14.Nierhaus, K. H. 2004. Assembly of the prokaryotic ribosome, p. 85-105. In K. H. Nierhaus and D. N. Wilson (ed.), Protein synthesis and ribosome structure. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- 15.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488-2492. [DOI] [PubMed] [Google Scholar]
- 16.Panina, E. M., A. A. Mironov, and M. S. Gelfand. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. USA 100:9912-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Schagger, H., and G. T. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 19.Toda, T., T. Tanaka, and M. Itaya. 1996. A method to invert DNA segments of the Bacillus subtilis 168 genome by recombination between two homologous sequences. Biosci. Biotechnol. Biochem. 60:773-778. [DOI] [PubMed] [Google Scholar]
- 20.Yusupov, M. M., G. Z. Yusupova, K. Lieberman, T. N. Earnest, J. H. D. Cate, and H. F. Noller. 2001. Crystal structure of the ribosome at 5.5 Å resolution. Science 292:883-896. [DOI] [PubMed] [Google Scholar]