Abstract
Neisseria gonorrhoeae has two porins, PIA and PIB, whose genes (porA and porB, respectively) are alleles of a single por locus. We recently demonstrated that penB mutations at positions 120 and 121 in PIB, which are presumed to reside in loop 3 that forms the pore constriction zone, confer intermediate-level resistance to penicillin and tetracycline (M. Olesky, M. Hobbs, and R. A. Nicholas, Antimicrob. Agents Chemother. 46:2811-2820, 2002). In the present study, we investigated the electrophysiological properties as well as solute and antibiotic permeation rates of recombinant PIB proteins containing penB mutations (G120K, G120D/A121D, G120P/A121P, and G120R/A121H). In planar lipid bilayers, the predominant conducting state of each porin variant was 30 to 40% of the wild type, even though the anion selectivity and maximum channel conductance of each PIB variant was similar to that of the wild type. Liposome-swelling experiments revealed no significant differences in the permeation of sugars or β-lactam antibiotics through the wild type or PIB variants. Although these results are seemingly contradictory with the ability of these variants to increase antibiotic resistance, they are consistent with MIC data showing that these porin mutations confer resistance only in strains containing an mtrR mutation, which increases expression of the MtrC-MtrD-MtrE efflux pump. Moreover, both the mtrR and penB mutations were required to decrease in vivo permeation rates below those observed in the parental strain containing either mtrR or porin mutations alone. Thus, these data demonstrate a novel mechanism of porin-mediated resistance in which mutations in PIB have no affect on antibiotic permeation alone but instead act synergistically with the MtrC-MtrD-MtrE efflux pump in the development of antibiotic resistance in gonococci.
In the 1970s and early 1980s, Neisseria gonorrhoeae, the etiological agent of the sexually transmitted infection gonorrhea, gradually became more resistant to penicillin and tetracycline until these antibiotics were discontinued as a first-line therapy. Resistance to these antibiotics can be either plasmid mediated or chromosomally mediated. Plasmid-mediated resistance to penicillin or tetracycline involves expression of a TEM-1-like β-lactamase (8, 30) or the TetM protein (25), respectively. In contrast, chromosomally mediated resistant N. gonorrhoeae (CMRNG) strains arise from cumulative mutations in endogenous genes or loci that gradually increase resistance until treatment failure occurs (3).
Four genes or loci (penA, mtrR, penB, and ponA) are known to be involved in high-level penicillin resistance (MICs, ≥2 μg/ml) in CMRNG strains, while the existence of a fifth resistance gene is inferred but not yet identified (3, 9, 32). penA (38, 39) and ponA (32, 33) encode alterations in the two essential penicillin-binding proteins (PBP 2 and PBP 1, respectively) that decrease their rates of acylation by β-lactam antibiotics. A single-base-pair deletion in the mtrR promoter abolishes transcription of the mtrR repressor and increases expression of the MtrC-MtrD-MtrE active efflux pump, which confers resistance to a diverse set of hydrophobic agents and detergents (14, 15, 29). penB, which encodes altered forms of porin IB (PIB), increases resistance to both penicillin and tetracycline (12, 28).
N. gonorrhoeae expresses one of two porins, PIA or PIB, whose genes (porA and porB, respectively) are alleles of a single por locus. In a previous study, we showed that mutations at positions 120 and 121 in PIB, which are predicted to reside within loop 3 that lines the pore of the channel, are critical in mediating resistance to penicillin and tetracycline (28). These mutations include a single G120K mutation and the double mutations G120D/A121D, G120R/A121H, and G120P/A121P. In addition, these data demonstrated that a charged amino acid at position 120 is highly preferred for mediating increased resistance to penicillin and tetracycline (28). Although we identified specific mutations that increase antibiotic resistance and their location within PIB, the mechanism(s) by which these alterations increase resistance is not clear. Mutations in loop 3 of other porins have been shown to have large effects on ion selectivity, pore size, and/or antibiotic permeation properties (1, 5, 23, 34), but the effects of the putative loop 3 mutations in PIB have not been elucidated.
To examine in more depth the mechanism(s) by which these mutations increase resistance, we determined whether these PIB mutations (i) alter the electrophysiological properties of the pore, including ion conductance and ion selectivity, (ii) constrict the size of the pore, and/or (iii) increase the hydrophilicity of the pore, thereby decreasing the permeability of relatively hydrophobic compounds such as penicillin and tetracycline. The latter phenomenon is observed in Escherichia coli porins, in which hydrophobic compounds permeate slower than hydrophilic compounds (27, 45). Recombinant PIB proteins were expressed in inclusion bodies in E. coli, renatured, and incorporated into planar lipid bilayers or liposomes. For each recombinant PIB, we determined the conductance and ion selectivity of the channel as well as the flux of a range of sugars or β-lactam antibiotics through the porin. Surprisingly, solute and antibiotic permeation rates of the four mutants were not significantly different from the permeation rates of wild-type porin. These paradoxical data are consistent with MIC experiments and in vivo permeation rates, as well as with earlier data (38) showing that overexpression of the MtrC-MtrD-MtrE efflux pump (via acquisition of the mtrR resistance determinant) is required for the penB porin mutants to confer increased resistance to penicillin and tetracycline.
MATERIALS AND METHODS
Chemicals.
All sugars and antibiotics were obtained from Sigma-Aldrich (St. Louis, MO), except for cephalexin (US Biochemical Corporation, Cleveland, OH) and cefotaxime (Hoechst-Roussel Pharmaceutical Inc., Somerville, NJ).
Bacterial strains, plasmids, and growth conditions.
N. gonorrhoeae strains used in this study are described in Table 1. Gonococcal transformations and MIC assays were performed as previously described (10, 28, 35). Plasmids encoding the porin variants used in the transformations also harbored an erm (porB mutants) or kan (porB) resistance cassette downstream of the coding sequence to aid in selection of recombinants. The D405N mutation in the mtrD gene, which also incorporated a new SalI site to help screen for transformants, was accomplished using QuickChange (Stratagene, La Jolla, CA). All mutations and resistance determinants introduced by transformation were verified by PCR amplification of genomic DNA and sequencing. E. coli BL21(DE3)* cells (Invitrogen, Carlsbad, CA) were transformed with por-containing plasmids, and colonies were grown on Luria-Bertani (LB) agar plates containing 50 μg/ml kanamycin.
TABLE 1.
Bacterial strains used in this study
| Strain | Descriptiona | Reference or source |
|---|---|---|
| FA1090 | Clinical isolate | 6 |
| FA6140 | Clinical isolate | 11 |
| FA19 | Clinical isolate | 35 |
| FA19 porBFA1090 | FA19 × pMO/porBFA1090 | This study |
| FA19 porB-G120K | FA19 × pMO/porB-G120K | This study |
| FA19 penA4 | FA19 × FA6140 | 28 |
| FA19 penA4 porBFA1090 | FA19 penA4 × pMO/porBFA1090 | This study |
| FA19 penA4 porB-G120K | FA19 penA4 × pMO/porB-G120K | This study |
| FA19 penA4 mtrR | FA19 penA4 × FA6140 | 28 |
| FA19 penA4 mtrR porBFA1090 | FA19 penA4 mtrR × pMO/porBFA1090 | 28 |
| FA19 penA4 mtrR porB-G120K | FA19 penA4 mtrR × pMO/porB-G120K | 28 |
| FA19 penA4 mtrR porB-G120D/A121D | FA19 penA4 mtrR × pMO/porB-G120D/A121D | 28 |
| FA19 penA4 mtrR porB-G120P/A121P | FA19 penA4 mtrR × pMO/porB-G120X/A121X | 28 |
| FA19 penA4 mtrR porB-G120R/A121H | FA19 penA4 mtrR × pMO/porB-G120X/A121X | 28 |
| FA19/Δrmp | FA19 lacking the Rmp protein | Chris Elkins, UNC-CHb |
| FA19/Δrmp porBFA1090 | FA19/Δrmp × pMO/porBFA1090 | This study |
| FA19 penA4 mtrR porB mtrD-D405N | FA19 penA4 mtrR porBFA1090 × pBS-mtrD-D405N | This study |
| FA19 penA4 mtrR porB-G120K mtrD-D405N | FA19 penA4 mtrR porB-G120K × pBS-mtrD-D405N | This study |
| L3481 | Penicillinase-producing porB strain (PPNG) | Marcia Hobbs, UNC-CH |
| L3481 porB-G120K | L3481 × FA19 porB-G120K | This study |
| L3481 mtrR | L3481 × FA19 penA mtrR porB | This study |
| L3481 mtrR porB-G120K | L3481 mtrR × FA19 porB-G120K | This study |
Plasmids used to create these strains have been described previously (28). pMO/porBFA1090 encodes the wild-type PIB from FA1090, pMO/porB-G120K encodes the PIB from FA1090 with the mutation Gly-120 to Lys, pMO/porB-G120D/A121D encodes the PIB from FA1090 with the mutations Gly-120 and Ala-121 to Asp, and pMO/porB-G120X/A121X refers to a library of porBFA1090 genes containing randomized codons at positions 120 and 121.
UNC-CH, University of North Carolina at Chapel Hill.
Purification of native PIB porin.
Native porin was isolated from strain FA19 Δrmp porBFA1090, which expresses PIB from strain FA1090 (wild type), following a Zwittergent-Ca2+-assisted purification procedure described by Wetzler et al. (44). An aliquot of the isolated porin in 50 mM Tris, pH 8.0, 10 mM EDTA, 5% Zwittergent 3,14 was submitted to chromatography on a Sephacryl-S300 gel filtration column (26 cm by 60 cm) and eluted with 100 mM Tris, pH 8.0, 200 mM NaCl, 10 mM EDTA, 0.05% Zwittergent 3,14. Fractions containing native PIB protein (determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) that eluted at the apparent molecular weight (MW) of trimers were pooled, concentrated, and dialyzed against 50 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.05% Zwittergent 3,14. Protein concentration was determined via Bradford assay.
Subcloning of N. gonorrhoeae porB and E. coli ompF genes.
The coding sequences for the mature forms of PIB (i.e., lacking the region encoding the leader sequence) were amplified from genomic DNA of the appropriate strains with Pfu polymerase. The 5′ primer (por-S3; 5′-CGCCATATGGATGTCACCCTGTACGGTGCCATCAAA-3′) was complementary to bases 58 to 84 of the porIB gene and contained an NdeI restriction site (in boldface) at its 5′ end. The NdeI site introduced an ATG start codon at the beginning of the coding sequence for the mature form of PIB and facilitated subcloning into the pT7-7K expression vector. The 3′ primer (por-U2; 5′-AAACTGCAGTATGGATAGATTCGTCATTCCCGC-3′) was complementary to a region ∼300 bp downstream of the porIB gene and contained a PstI site (in boldface) at its 5′ end. The mature coding sequence of ompF was amplified by PCR from the genomic DNA of E. coli strain MC1061. The 5′ primer (EC-OmpF-S1; 5-CGCCATATGGCAGAAATCTATAACAAAGATGG-3′) was complementary to bases 67 and 89 and contained an NdeI restriction site. The 3′ primer (EC-OmpF-U1; 5-AAACTGCAGTTAGAACTGGTAAACGATACCC-3′) contained a PstI site at its 5′ end and hybridized to bases 1069 and 1089, which are at the end of the coding sequence. The PCR products were subcloned into the NdeI and PstI sites of the expression vector, pT7-7K, which has a kanamycin resistance gene in place of the β-lactamase resistance gene (40). All constructs were verified by sequencing. E. coli BL21(DE3)* cells were transformed with pT7-7K/porB or pT7-7K/ompF plasmids, and transformants were selected on LB agar plates containing 50 μg/ml kanamycin.
Recombinant PIB protein expression and purification.
Three liters of BL21(DE3)* cells harboring one of the pT7-7K/porB or pT7-7K/ompF plasmids was grown in 2X YT, 50 μg/ml kanamycin at 37°C. Isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 0.5 mM, and cells were incubated for an additional 2 to 2.5 h. The cells were harvested by centrifugation at 3,600 × g for 20 min, resuspended in French press buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 2 mM EDTA, and 10% glycerol), and lysed by three passes through a French press at 16,000 lb/in2 immediately after the addition of 100 μl of freshly prepared phenylmethylsulfonyl fluoride (20 mg/ml in ethanol). Inclusion bodies were pelleted by centrifugation of the lysate at 10,000 × g for 10 min, and the large protein pellet was washed extensively in 10 mM Tris, pH 8.0, 1 mM EDTA with or without the addition of 1% Triton X-100.
The recombinant porins were refolded and purified following a modified procedure described by Qi et al. (31) for Neisseria porins. Briefly, the inclusion bodies were solubilized in TEN-urea buffer (10 mM Tris, pH 8.0, 10 mM EDTA, 100 mM NaCl, 8 M urea), adjusted to a concentration of ≤10 mg/ml, and mixed 1:1 with 10% Zwittergent 3,14 (Calbiochem, San Diego, CA). The porin proteins were slowly refolded by dialyzing the protein-detergent mixture in 200 ml of Buffer A (50 mM Tris, pH 8.0, 150 mM NaCl, 4 M urea, 0.05% Zwittergent 3,14), followed by a slow dilution of the urea by adding 1.8 liters of Buffer B (50 mM Tris, pH 8.0, 150 mM NaCl, 0.05% Zwittergent 3,14) at 1 ml/min for 30 h. The dialyzed and refolded samples were centrifuged at 10,000 × g to pellet any precipitant and loaded onto a Sephacryl-S300 gel filtration column equilibrated in 100 mM Tris, pH 8.0, 200 mM NaCl, 10 mM EDTA, and 0.05% Zwittergent 3,14, and refolded porin oligomers were eluted in the same buffer. Fractions containing trimeric porin proteins were pooled, concentrated, and dialyzed against 50 mM Tris, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.05% Zwittergent 3,14. Protein concentrations were determined via Bradford assay.
Planar lipid bilayer assays.
Bilayer experiments were carried out essentially as described previously (7, 20). Synthetic 1-palmitoyl-2-oleoyl-phosphatidylethanolamine and 1-palmitoyl-2-oleoyl-phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) in a 4:1 molar ratio were dissolved in n-decane to a final concentration of 50 mg/ml. Bilayers were formed by brushing lipids over a 400-μm-diameter hole in a polyvinyl difluoride partition separating two chambers (cis and trans), each containing an electrolyte solution of 200 mM NaCl and 10 mM HEPES, pH 7.4. To promote the incorporation of porins into the bilayer, 0.1% Triton X-100 (final) was added to the porin preparations immediately before addition to the cis chamber. Triton X-100 did not disrupt the stability of the bilayers or affect conductances. Porins were added at low concentrations (1 to 10 ng) to promote insertion of single channels and to delay multiple channel incorporations. Spontaneous channel incorporation occurred within 1 to 15 min. Membranes were voltage-clamped with a modified patch-clamp amplifier (PC501A; Warner Instruments, Hamden, CT). Voltages were defined as trans relative to cis, and current flow from trans to cis (equivalent to Cl− flow cis to trans) was recorded as positive current. Data were low-pass filtered at 200 Hz with an 8-pole Bessel filter and digitized at 1 kHz. Programs for data acquisition and analysis were written in Axobasic (Axon Instruments, Union City, CA).
Conductances for each porin were calculated from current-voltage plots. Current amplitudes at each voltage were determined from amplitude histograms. To determine ion selectivity, NaCl was added to the cis chamber to generate a gradient (0.4 M NaCl cis, 0.2 M NaCl trans). Current-voltage plots were constructed and the permeability ratios (Na+ versus Cl−) were calculated from the reversal potentials according to the Goldman-Hodgkin-Katz equation (16), where P is the permeability of the indicated ion, C is the concentration of the indicated ion, Erev is the reversal potential, e is the base of the natural logarithm, R is the gas constant, T is the absolute temperature, and F is the Faraday constant.
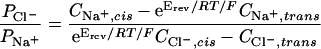 |
Permeation of sugars through porins reconstituted in liposomes.
Liposome-swelling assays with various sugars were performed precisely as described previously (26). Briefly, 2.4 μmol egg phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) and 0.2 μmol dicetylphosphate (Sigma-Aldrich, St. Louis, MO) in chloroform were combined and dried thoroughly in a Pyrex test tube. Lipids were suspended in either 0.2 ml water (for control liposomes) or 0.2 ml protein (1 μg) in water and sonicated (1 to 2 min) until translucent. The proteolipids were dried under vacuum and stored in a dark, evacuated desiccator overnight. Proteoliposomes were fully suspended in a solution of 5 mM Tris, pH 8.0, and 15% (wt/vol) Dextran T-40 (GE Healthcare, Piscataway, NJ) and left at room temperature for 1 h.
The isotonic concentrations of each sugar were determined empirically with control liposomes. Seventeen microliters of proteoliposomes was added to 0.6 ml of isotonic concentrations of each buffered sugar solution. Changes in optical density at 400 nm (OD400) were measured with a DU 650 Spectrophotometer (Beckman Instruments, Inc., Fullerton, CA) in kinetic mode at 1-s intervals for 90 to 180 s. The permeation of each sugar was determined in triplicate for separate liposome preparations, and the swelling rate was determined from the slopes of the linear portion of each OD curve (10 s) and plotted as the logarithmic percentage of arabinose permeation versus molecular weight. Linear regression was performed to determine the apparent molecular weight exclusion limit of each porin. The difference in the confidence intervals of the slopes of the best-fit lines was analyzed with GraphPad Prism (San Diego, CA) to compare sugar permeation rates between porins.
Permeation of β-lactam antibiotics through porins reconstituted in liposomes.
The determination of β-lactam antibiotic permeation through porins was essentially as described for sugars but with the following modifications (27). Multilamellar liposomes containing 120 μg of recombinant porins were prepared from 12.4 μmol of egg phosphatidylcholine and 0.4 μm dicetylphosphate exactly as described in the previous section. Proteoliposomes were suspended in a 0.8-ml solution of 12 mM stachyose, 4 mM sodium-NAD, and 1 mM imidazole-NAD, pH 6.0, and incubated at room temperature for 1 h. Stock solutions of each antibiotic were made fresh for each experiment and contained 25 mM of the sodium salt form of the β-lactam antibiotic, 1 mM sodium-NAD, and 1 mM imidazole-NAD (pH 6.0). The pH of each solution was carefully adjusted to 5.8 to 6.2 with NaOH. The permeation rate of arabinose in 1 mM sodium-NAD and 1 mM imidazole-NAD (pH 6.0) through the porins was also measured, and the rate of permeation of each antibiotic was plotted as a percentage of arabinose permeation. Differences in the swelling rates of each β-lactam antibiotic through the wild-type and G120K variant PIBs from five individual experiments were analyzed with Student t tests.
Nitrocefin hydrolysis assay.
β-Lactamase-producing strains of N. gonorrhoeae (L3481 and derivatives; Table 1) were grown overnight on GC medium base plates (Difco, Sparks, MD). The next morning, the cells were suspended in 50 mM Tris, 200 mM NaCl, 10 mM MgCl2 (TBS-M) plus 3% polyvinylpyrrolidone, washed once, and diluted to an OD600 of 0.5 and kept on ice. Nitrocefin (BD, Franklin Lakes, NJ) was dissolved in TBS-M at 25 μg/ml, and assays were initiated by adding 0.1 ml of cells to 0.4 ml nitrocefin solution, mixing, and monitoring the increase in absorbance at 480 nm. To control for the release of β-lactamase, cell suspensions were pelleted and the hydrolysis of nitrocefin by the supernatants was monitored as described above.
RESULTS
Native and recombinant wild-type PIB proteins have identical electrophysiological properties.
Previous studies of porins from N. gonorrhoeae, N. meningitidis, and other bacterial species have demonstrated that recombinant porins expressed in inclusion bodies and refolded in vitro are structurally and functionally indistinguishable from native porins (21, 22, 31, 36). Thus, we purified and refolded gonococcal PIB proteins in a similar manner. However, to ensure that the refolded recombinant proteins retained endogenous properties, we purified native wild-type PIB (44) from N. gonorrhoeae strain FA19 Δrmp porBFA1090 (FA19 expressing PIB from FA1090 and containing a deletion of the Rmp protein that can copurify with porins [43]) and compared its conductance and ion selectivity with recombinant refolded wild-type PIB protein following incorporation into planar lipid bilayers. Both native and recombinant porins were primarily in the largest conducting state (i.e., each monomer of the trimer was open) and had slope conductances of 1.0 nS, with 0.2 M NaCl on both sides of the bilayer. In the presence of a twofold NaCl gradient (400 mM cis versus 200 mM trans), the average reversal potentials (Erev) for the native and recombinant channels were −6.3 ± 0.3 mV (n = 3) and −4.9 ± 1.2 mV (n = 4), respectively, demonstrating that both porins also had a similar preference for anions (Table 2). Taken together, these data demonstrate that native and recombinant wild-type porins have indistinguishable electrophysiological properties and that native function was preserved in the recombinant porin. This ensured that the properties of the recombinant porin variants could also be examined in a similar manner.
TABLE 2.
Electrophysiological properties of native and recombinant porin proteins in planar lipid bilayers
| Porin IB | Reversal potential | Ion selectivityb (PCl−/PNa+) |
|---|---|---|
| Native wild-type | −6.3 ± 0.3 | 2.2 ± 0.1 |
| Recombinant wild-type | −4.9 ± 1.2 | 1.8 ± 0.3 |
| G120K | −9.7 ± 0.9a | 3.7 ± 0.6 |
| G120D A121D | −6.4 ± 1.0 | 2.2 ± 0.3 |
| G120P A121P | −6.5 ± 0.6 | 2.3 ± 0.2 |
| G120R A121H | −7.5 ± 0.6 | 2.6 ± 0.3 |
Analysis of variance; P < 0.001.
Ion selectivity was derived from the mean reversal potential with the Goldman-Hodgkin-Katz equation (16) (see Materials and Methods).
Comparison of the electrophysiological properties of recombinant wild-type and variant PIB proteins.
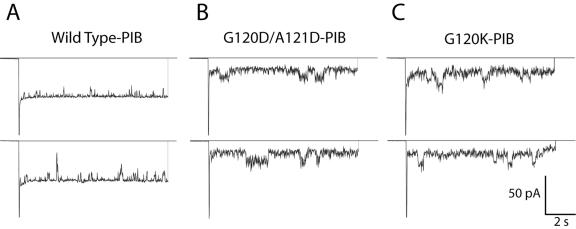
To ascertain whether recombinant wild-type and variant (G120K, G120D/A121D, G120P/A121P, and G120R/A121H) PIB proteins differed in their electrophysiological properties, we determined the conductance and ion selectivity of each PIB protein in planar lipid bilayers. Figure 1 shows representative traces of ion channel recordings from single insertions of wild-type, G120D/A121D, and G120K PIB proteins. Wild-type PIB channels were primarily in the largest conducting state (Fig. 1A). In contrast, the PIB variants were predominantly in subconductance states and displayed increased channel noise, which most likely reflected either fluctuations within the pores or rapid transitions between different conducting states (Fig. 1B and C). The highest conductance of the variants was similar to that of the wild type (∼1 nS), although the probability that the variants were in this state was low (data not shown).
FIG. 1.
Representative traces of current through PIB proteins in planar lipid bilayers. Single channels of recombinant PIB proteins were incorporated into artificial bilayers. Membranes were held at 0 mV to record the zero-current level and then were pulsed to −50 mV for 10 s. Bilayers were bathed in 200 mM NaCl, 10 mM HEPES, pH 7.4. Open transitions of the porin channels are downward.
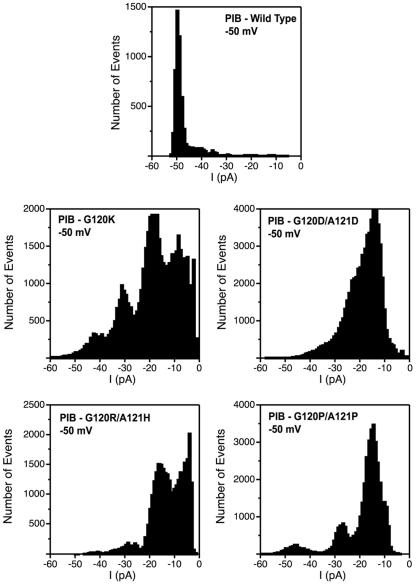
To characterize more clearly the differences in porin channel behavior, we constructed composite amplitude histograms from recordings at −50 mV of many separate single channel events. The wild-type PIB protein showed a dominant peak at −50 pA (conductance of 1.0 nS), whereas each of the PIB variants exhibited substantially smaller conductance states (Fig. 2). The most commonly observed conductance for each PIB variant was 30 to 40% of wild type. These data suggest that the pores of the PIB variants are similar to those of the wild type but exist in subconductance states much more often than wild-type PIB.
FIG. 2.
Composite all-point current histograms for wild-type and variant PIBs. Amplitude histograms were constructed by analyzing a series of current traces (n = 5 to 10) at −50 mV for single-porin channels from several separate experiments and plotting the number of events versus current amplitude (1-pA intervals). Bilayers were bathed in 200 mM NaCl, 10 mM HEPES, pH 7.4.
The ion selectivity of each PIB was determined in the presence of a twofold NaCl gradient (400 mM cis versus 200 mM trans) (Table 2). Whereas the G120D/A121D, G120P/A121P, and G120R/A121H PIB variants showed a similar preference for anions compared to the wild type, the G120K PIB variant showed a small but significant increase (∼2-fold) in its anion selectivity (Table 2). Therefore, each of the PIB variants showed little to no change in ion selectivity compared to wild-type PIB.
Sugar permeation through recombinant wild-type and variant PIB proteins.
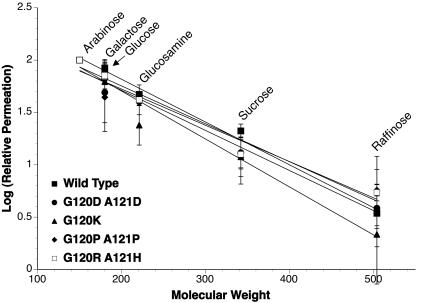
To test whether mutations in PIB decrease the size of the pore, we performed liposome-swelling experiments, which measured the permeation rates of uncharged sugars of increasing molecular weights (i.e., arabinose, galactose, glucose, N-acetyl-d-glucosamine, sucrose, raffinose, and stachyose; MWs of 150 to 666) through wild-type and variant PIBs. The permeation rates of arabinose (MW, 150) through each PIB were essentially identical (data not shown); therefore, the permeation rates of all other sugars were normalized to that of arabinose. Figure 3 demonstrates that an individual sugar permeated each PIB protein with similar rates, although the G120K PIB variant showed a slight decrease in the rate of permeation of N-acetyl-d-glucosamine (MW, 221) compared to the wild type or the other PIB variants. A plot of the log of relative permeation rates versus molecular weight revealed that the permeation rates of sugars through the wild-type and variant PIB proteins were not significantly different (95% confidence interval; P = 0.3). The approximate exclusion limit of each channel was defined as the molecular weight of a sugar that permeated at 10% of the rate of arabinose permeation. The apparent exclusion limits were between 360 and 400 Da for each of the PIBs, which were similar to the ∼400-Da solute exclusion limits observed for N. meningitidis PorB proteins (21, 22). Therefore, mutations in PIB proteins do not appear to decrease pore size.
FIG. 3.
Sugar permeation through recombinant porin proteins reconstituted into liposomes. Proteoliposomes were made with 1 μg recombinant wild-type or mutant porins in the presence of T-40 Dextran. Aliquots (17 μl) were diluted into 0.6 ml of isotonic concentrations of a range of sugars, and the permeation rate of each sugar was determined from the initial change in OD400. The average permeation rate for each sugar was determined in triplicate for two separate proteoliposome preparations, and the log of the permeation rate (normalized to the permeation rate of arabinose) was plotted versus the molecular weight of the sugar (error bars represent the standard deviation). The lines were fitted via linear regression. Analyses of the 95% confidence intervals determined that the differences in slopes were not significant (P = 0.3).
β-Lactam antibiotic permeation through recombinant wild-type and variant PIB proteins.
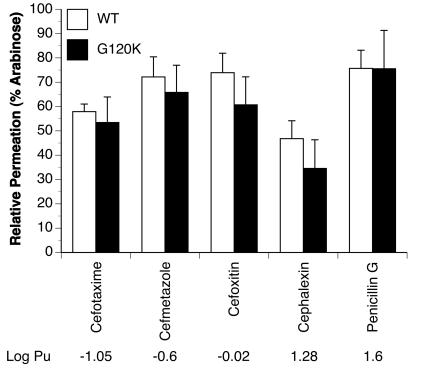
We also examined whether the mutations in PIB decrease the permeation of β-lactam antibiotics and whether the hydrophobicity of the β-lactam antibiotic had any influence on its permeation. Figure 4 shows the permeation rates of a set of β-lactam antibiotics with varying degrees of hydrophobic content through wild-type and G120K PIB proteins. Surprisingly, wild-type and G120K PIB proteins showed little to no difference in the permeability of each β-lactam antibiotic (Fig. 4). There was a tendency for the PIB-G120K to show slightly lower permeation rates than wild-type PIB, but the differences were not significant. Each of the other PIB variants showed a permeation profile for the antibiotics similar to that of the PIB-G120K variant (data not shown). Furthermore, in contrast to E. coli porins (45), the PIB proteins showed no relationship between the hydrophobicity of the β-lactam antibiotic and its rate of permeation. Therefore, mutations in PIB have minimal effects on the hydrophilic character of the pore and, importantly, on the permeation rates of a broad range of β-lactam antibiotics.
FIG. 4.
β-Lactam antibiotic permeation through recombinant porin proteins reconstituted into liposomes. Proteoliposomes were formed with 120 μg recombinant wild-type (WT) or G120K variant porins in the presence of stachyose and sodium-NAD. Aliquots (17 μl) were diluted into 0.6 ml of isotonic concentrations of β-lactam antibiotics of increasing hydrophobicity (from left to right on the plot), and the permeation rates of each antibiotic were determined from the initial change in OD400. The rates of antibiotic permeation in each experiment were normalized to the rate of arabinose permeation. The averages ± standard deviations of five separate experiments are shown. Differences in antibiotic permeation between wild-type and G120K PIBs for each antibiotic were determined via Student's t test (*, P < 0.05). The log partition coefficient (Pu; octanol-aqueous buffer) values are described in Yoshimura and Nikaido (45).
We also examined the permeation properties of a nongonococcal porin, OmpF, which is cation selective and would be expected to have a different permeation profile for β-lactam antibiotics than the anion-preferring gonococcal porins (2). OmpF was expressed in E. coli in inclusion bodies, renatured, and purified in exactly the same manner as described for gonococcal porins. The majority of the β-lactam antibiotics diffused substantially slower (35 to 65%) and showed a different rank order of permeation through E. coli OmpF proteins than through N. gonorrhoeae wild-type PIB (data not shown). Moreover, the rank order of permeation of the antibiotics was identical to that of previous reports (45) with the exception of cephalexin, which permeated relatively slower into OmpF-containing liposomes in our hands.
Antibiotic susceptibility profiles for strains containing wild-type or variant PIBs.
In order to correlate the permeation rates of the β-lactam antibiotics with their ability to inhibit gonococcal growth, we examined the antibiotic susceptibility profiles of the β-lactam antibiotics used in the permeation studies for a set of isogenic strains containing the wild-type PIB or variant (G120K, G120D/A121D, G120P/A121P, and G120R/A121H) PIBs (Table 3). These strains contained the first two resistance determinants, penA and mtrR, in addition to the various PIB proteins. Most β-lactam antibiotics had two- to threefold higher MICs for strains harboring the PIB variants compared to the strain containing the wild-type PIB. Because acquisition of penB also confers tetracycline resistance, the MICs of several tetracycline derivatives for the wild-type and variant PIB strains were also investigated (Table 3). Each PIB variant strain conferred approximately two- to threefold increases in resistance to each derivative compared to the wild-type PIB strain. As reported previously, tetracycline had a lower MIC in a penA mtrR strain harboring PIB-G120D/A121D than in similar strains harboring PIB proteins with the other mutations (28).
TABLE 3.
MICs of β-lactam and tetracycline antibiotics for strains containing wild-type (WT) or variant PIBs
| Antibiotic | MIC for the following PIBs in FA19 penA4 mtrRa
|
||||
|---|---|---|---|---|---|
| WT | G120D/ A121D | G120P/ A121P | G120R/ A121H | G120K | |
| Cefotaxime | 0.014b | 0.027 | 0.024 | 0.036 | 0.036 |
| Cefmetazole | 3.0 | 8.4 | 6.0 | 9.6 | 12.0 |
| Cefoxitin | 0.75 | 1.5 | 1.5 | 2.1 | 1.8 |
| Penicillin | 0.32 | 0.67 | 0.58 | 0.75 | 0.75 |
| Tetracycline | 0.45 | 1.0 | 1.5 | 1.5 | 1.5 |
| Oxytetracycline | 0.5 | 1.0 | 1.0 | 1.0 | 2.0 |
| Chlortetracycline | 1.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Doxycycline | 0.67 | 1.0 | 1.0 | 1.0 | 1.0 |
The identity of the porin in strain FA19 penA mtrR is shown. Strains were constructed by transformation with wild-type or altered porB DNA constructs as described in Materials and Methods.
MICs (in micrograms/milliliter) were determined as described previously (28). MICs are the averages of three independent experiments.
Influence of the MtrC-MtrD-MtrE efflux pump on porin-mediated antibiotic resistance and permeation.
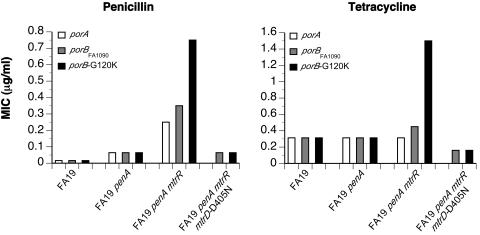
The data described above demonstrate that neither the size of the pore nor the permeation rate of a broad range of β-lactam and tetracycline antibiotics are significantly altered in PIB with mutations at residues 120 and 121, yet these mutant porins are capable of conferring intermediate resistance to a variety of antibiotics. To further investigate this paradox, we constructed isogenic strains of FA19, FA19 penA4, and FA19 penA4 mtrR containing PIA, PIB, or PIB-G120K and determined the MICs of penicillin and tetracycline for each of these strains (Fig. 5). In the absence of the mtrR resistance determinant, isogenic strains containing any of the porins, including PIB-G120K, displayed identical levels of resistance to both antibiotics. Following acquisition of the mtrR determinant, modest increases in the MICs of both antibiotics were observed for the FA19 penA4 mtrR porBFA1090 strain compared to the PIA-containing isogenic strain, FA19 penA4 mtrR. Penicillin and tetracycline resistance was then further increased by two- to threefold in FA19 penA4 mtrR porB-G120K. These data demonstrate that the mtrR mutation must also be present for the variant porins to increase resistance.
FIG. 5.
MICs of isogenic strains with varying resistance genes and porin proteins. penA4 and mtrR resistance determinants from FA6140 genomic DNA were transformed into FA19, which contains PIA, in a stepwise manner. Strains containing wild-type or variant PIBs were created via transformation with plasmid constructs containing either wild-type or mutant porB DNA, respectively (as described in Olesky et al. [28]; see Table 1). MICs are the averages of three experiments.
Because the mtrR mutation increases expression of the MtrC-MtrD-MtrE efflux pump, the requirement for the mtrR mutation to increase the MIC of penicillin and tetracycline in penB strains could be due to either (i) increased efflux pump activity as a result of MtrC-MtrD-MtrE overexpression or (ii) increased efflux pump protein, which could bind to the porin and influence antibiotic influx directly. To distinguish between these two possibilities, we constructed strains of FA19 penA4 mtrR porBFA1090 and FA19 penA4 mtrR porB-G120K also containing a D405N mutation in the mtrD gene. The analogous mutation in the mexB gene encoding the MexA-MexB efflux pump from Pseudomonas aeruginosa (47% sequence identity to MtrD) was shown to completely disrupt efflux activity without affecting protein expression (13). Both strains containing the mtrD-D405N mutation showed large decreases in the MICs of penicillin and tetracycline (Fig. 5) to levels observed in CMRNG strains containing a deletion in mtrD. Moreover, the MICs for both strains were identical, again highlighting the inability of the mutant porin to alter resistance in the absence of an active MtrC-MtrD-MtrE efflux pump.
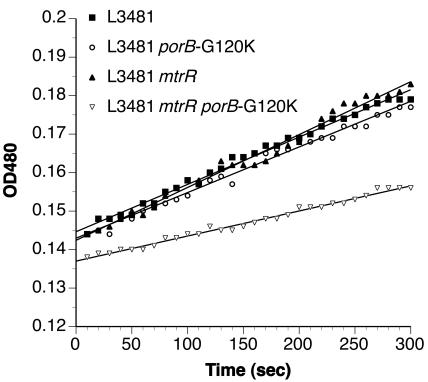
To examine the permeability of porins in vivo, we measured the hydrolysis of the chromogenic β-lactam antibiotic nitrocefin in cells expressing periplasmic β-lactamase. In this assay, the diffusion of the antibiotics across the outer membrane is the rate-limiting step in their hydrolysis (41). L3481, a β-lactamase-producing PIB strain, was used as a recipient for the mtrR and porB-G120K resistance determinants. As shown in Fig. 6, the rates of hydrolysis of nitrocefin were essentially identical in L3481, L3481 porB-G120K, and L3481 mtrR. In contrast, L3481 mtrR porB-G120K showed a reduced rate of hydrolysis, consistent with a decrease in outer membrane permeability.
FIG. 6.
Nitrocefin hydrolysis assays in β-lactamase-producing gonococcal strains also harboring mtrR and porIB-G120K mutations. L3481 cells (a β-lactamase-producing PIB strain) harboring the indicated resistance determinants (Table 1) were used to assess the permeation of antibiotics in vivo as described in Materials and Methods. Permeation across the outer membrane is the rate-limiting step in the hydrolysis of nitrocefin (41). This experiment was repeated twice with similar results.
DISCUSSION
In a previous study, we identified a set of PIB mutations (G120K, G120D/A121D, G120P/A121P, and G120R/A121H) that confer resistance to penicillin and tetracycline (28). These mutations were hypothesized to reside in loop 3, the extracellular loop that folds into the barrel and forms the constriction zone. Because mutations in loop 3 of other porins are extremely effective in altering pore size and ion and antibiotic permeation properties (23, 34, 36), we expected that the PIB mutations studied here would have similar effects. Although our data demonstrated that each of the PIB variants had substantially lower predominant conducting states and increased channel noise compared to wild-type PIB, the variants in fact showed no significant differences in pore size, ion permeation, and antibiotic permeation rates. Moreover, our results confirmed and extended earlier studies showing that overexpression of the MtrC-MtrD-MtrE efflux pump in N. gonorrhoeae is necessary to observe increased resistance to penicillin and tetracycline conferred by penB mutations (38, 42).
In concordance with other porin studies (31), the structural and functional properties of recombinant wild-type PIB porin, which was overexpressed in inclusion bodies in E. coli and refolded in vitro, were indistinguishable from native PIB purified from gonococci. Furthermore, wild-type PIB, either native or recombinant, exhibited ion conductance and ion selectivity values in planar lipid bilayers that were very similar to those previously reported for Neisseria porins (19, 20, 37). The wild-type channels were primarily in an open state, but occasionally they were closed in distinct levels reflecting the trimeric porin structure. We infrequently observed a small closed-channel current as described by Mauro et al. (20). Gonococcal porins have been reported to exhibit voltage-dependent closings at ±80 mV in planar lipid bilayers; however, our experimental design did not address the voltage-dependent gating behaviors of the PIB channels at these potentials (19, 37).
The largest differences between the wild-type and variant PIBs were in their ion-conducting properties, including lower conductance states and increased channel noise. The conductance properties of the PIB variants suggest that either the internal regions of the pore are altered or that the individual subunits of the trimers are open less often and for much shorter times than those of the wild type. Even though the ion conductances of the porin variants were markedly altered, liposome-swelling assays showed that mutations in PIB do not alter the size of the pore or the permeation rates of neutral sugars and β-lactam antibiotics. Because the pore sizes were unchanged in the PIB variants, our data are more consistent with changes in the gating properties of the PIB variants and not with alterations within the pore itself.
The increased channel noise and increased time spent in subconductance states observed in the variant PIBs are reminiscent of the changes in the channel properties of the OmpC porin with mutations designed to disrupt the interaction of loop 3 with the outer barrel (17, 18). The electrophysiological properties of these mutant porins were examined by patch clamp of liposome blisters containing multiple porin channels. Some of the mutant porins displayed a dramatic increase in the closing rate and decrease in open probability relative to wild-type OmpC (17, 18). These changes were interpreted to be the result of changes in the flexibility of loop 3 within the barrel, caused by the loss of hydrogen bonds and/or salt bridges between loop 3 and the outer barrel. It is tempting to speculate that mutations in loop 3 of PIB also may increase the flexibility of loop 3. Unfortunately, the effects of the OmpC mutations on antibiotic permeation are unknown, so a correlation of changes in electrophysiological properties to changes in permeation of larger compounds cannot be made. The data presented here with gonococcal porins suggest that changes in the electrophysiological properties of PIB do not correlate with changes in solute and antibiotic permeation rates.
Gill et al. (12) speculated that aspartic acid residues at positions 120 and 121 in loop 3 of PIB decrease antibiotic diffusion by anion repulsion. However, this mechanism is not supported by our data. No differences in the permeation rates of monoanionic β-lactam antibiotics were observed through wild-type and variant PIBs, and both the wild-type and PIB variants showed similar preferences for anions, even with the variants containing either an acidic (Asp) or basic (Lys and Arg) amino acid at position 120. The lack of an effect on ion selectivity by charged amino acid substitutions seems to argue against the prediction that residues 120 and 121 line the channel of the pore. These residues may instead face towards the outer wall or may even be located outside of the pore. A definitive description of the changes induced by these mutations must await the three-dimensional structure of PIB.
Recently, an antibiotic-resistant strain of Enterobacter aerogenes was identified containing a mutation in the major porin that conferred resistance to a variety of cephalosporin antibiotics. The porin contained a mutation (G112D) in loop 3, in a position similar to that of the PIB mutations, and was shown to have a significantly reduced single-channel ion conductance and reduced pore size (5). The authors concluded that the smaller pore size of the mutant mediated increased antibiotic resistance, but liposome-swelling assays with β-lactam antibiotics were not performed to determine whether antibiotic fluxes through the mutant porin were also altered. The loop 3 mutation identified in the De et al. study, G112D, was similar to the G120D/A121D variant described in this study (5). However, the effects of the G112D mutation in the E. aerogenes porin were very different than the G120D/A121D mutations in PIB, as we saw no significant differences in the pore sizes of wild-type and mutant PIBs.
It is unclear at the present time how the penB mutations increase antibiotic resistance, especially given that the mutations had no effect on the permeation rates of sugars and antibiotics and the absolute requirement for the presence of the mtrR determinant to increase antibiotic resistance (and decrease permeation) in strains containing the PIB variants. Some reports have shown that expression of nonspecific porins can be negatively regulated by modulators of the multidrug resistance (Mdr) system, which is homologous to the Mtr system, resulting in decreased permeability of the outer membrane to antibiotics (4, 24). However, we showed previously that porin expression is not decreased in strains with the PIB variants (28).
So why do the PIB mutants increase antibiotic resistance only when the MtrC-MtrD-MtrE efflux pump is overexpressed? One possibility is that only a slight decrease in antibiotic permeation through porins is required to confer resistance when expression of the efflux pump is deregulated. This scenario would be consistent with the small (but not significant) decreases observed in the antibiotic permeation rates through PIB-G120K compared to wild-type PIB. An alternative hypothesis is that the PIB variants may interact directly with the Mtr efflux pump and work cooperatively to decrease the periplasmic concentrations of antibiotic. In this scenario, mutant PIB proteins may form a complex with the MtrC-MtrD-MtrE efflux pump, and the close proximity of the efflux pump may allow for small differences in antibiotic permeation to be magnified.
Acknowledgments
This work was supported by grant AI-036901 from the National Institutes of Health.
We gratefully acknowledge the invaluable advice of Janne Cannon. We also thank Lisa Lyford, Tom Kawula, and Lee Graves for helpful and constructive discussions and Mei Hu and Jamie Alan for technical support and suggestions.
REFERENCES
- 1.Bauer, K., M. Struyve, D. Bosch, R. Benz, and J. Tommassen. 1989. One single lysine residue is responsible for the special interaction between polyphosphate and the outer membrane porin PhoE of Escherichia coli. J. Biol. Chem. 264:16393-16398. [PubMed] [Google Scholar]
- 2.Benz, R., A. Schmid, and R. E. Hancock. 1985. Ion selectivity of gram-negative bacterial porins. J. Bacteriol. 162:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon, J. G., and P. F. Sparling. 1984. The genetics of the gonococcus. Annu. Rev. Genet. 38:111-133. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, S. P., L. M. McMurry, and S. B. Levy. 1988. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J. Bacteriol. 170:5416-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De, E., A. Basle, M. Jaquinod, N. Saint, M. Mallea, G. Molle, and J. M. Pages. 2001. A new mechanism of antibiotic resistance in Enterobacteriaceae induced by a structural modification of the major porin. Mol. Microbiol. 41:189-198. [DOI] [PubMed] [Google Scholar]
- 6.Dempsey, J. A., W. Litaker, A. Madhure, T. L. Snodgrass, and J. G. Cannon. 1991. Physical map of the chromosome of Neisseria gonorrhoeae FA1090 with locations of genetic markers, including opa and pil genes. J. Bacteriol. 173:5476-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai, S. A., and R. L. Rosenberg. 1997. Pore size of the malaria parasite's nutrient channel. Proc. Natl. Acad. Sci. USA 94:2045-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon, J. A., and K. H. Yeung. 1989. Beta-lactamase plasmids and chromosomally mediated antibiotic resistance in pathogenic Neisseria species. Clin. Microbiol. Rev. 2(Suppl.):S125-S133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dougherty, T. J. 1986. Genetic analysis and penicillin-binding protein alterations in Neisseria gonorrhoeae with chromosomally mediated resistance. Antimicrob. Agents Chemother. 30:649-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dougherty, T. J., A. E. Koller, and A. Tomasz. 1980. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 18:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faruki, H., and P. F. Sparling. 1986. Genetics of resistance in a non-β-lactamase-producing gonococcus with relatively high-level penicillin resistance. Antimicrob. Agents Chemother. 30:856-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill, M. J., S. Simjee, K. Al-Hattawi, B. D. Robertson, C. S. Easmon, and C. A. Ison. 1998. Gonococcal resistance to beta-lactams and tetracycline involves mutation in loop 3 of the porin encoded at the penB locus. Antimicrob. Agents Chemother. 42:2799-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan, L., and T. Nakae. 2001. Identification of essential charged residues in transmembrane segments of the multidrug transporter MexB of Pseudomonas aeruginosa. J. Bacteriol. 183:1734-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagman, K. E., W. Pan, B. G. Spratt, J. T. Balthazar, R. C. Judd, and W. M. Shafer. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611-622. [DOI] [PubMed] [Google Scholar]
- 15.Hagman, K. E., and W. M. Shafer. 1995. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J. Bacteriol. 177:4162-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hodgkin, A. L., and B. Katz. 1949. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 108:37-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, N., and A. H. Delcour. 1998. The spontaneous gating activity of OmpC porin is affected by mutations of a putative hydrogen bond network or of a salt bridge between the L3 loop and the barrel. Protein Eng. 11:797-802. [DOI] [PubMed] [Google Scholar]
- 18.Liu, N., H. Samartzidou, K. W. Lee, J. M. Briggs, and A. H. Delcour. 2000. Effects of pore mutations and permeant ion concentration on the spontaneous gating activity of OmpC porin. Protein Eng. 13:491-500. [DOI] [PubMed] [Google Scholar]
- 19.Lynch, E. C., M. S. Blake, E. C. Gotschlich, and A. Mauro. 1984. Studies of porins spontaneously transferred from whole cells and reconstituted from purified proteins of Neisseria gonorrhoeae and Neisseria meningitidis. Biophys. J. 45:104-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauro, A., M. Blake, and P. Labarca. 1988. Voltage gating of conductance in lipid bilayers induced by porin from outer membrane of Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. USA 85:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minetti, C. A., M. S. Blake, and D. P. Remeta. 1998. Characterization of the structure, function, and conformational stability of PorB class 3 protein from Neisseria meningitidis. A porin with unusual physicochemical properties. J. Biol. Chem. 273:25329-25338. [DOI] [PubMed] [Google Scholar]
- 22.Minetti, C. A., J. Y. Tai, M. S. Blake, J. K. Pullen, S. M. Liang, and D. P. Remeta. 1997. Structural and functional characterization of a recombinant PorB class 2 protein from Neisseria meningitidis. Conformational stability and porin activity. J. Biol. Chem. 272:10710-10720. [DOI] [PubMed] [Google Scholar]
- 23.Misra, R., and S. A. Benson. 1988. Isolation and characterization of OmpC porin mutants with altered pore properties. J. Bacteriol. 170:528-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Misra, R., and P. R. Reeves. 1987. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J. Bacteriol. 169:4722-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morse, S. A., S. R. Johnson, J. W. Biddle, and M. C. Roberts. 1986. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob. Agents Chemother. 30:664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikaido, H., K. Nikaido, and S. Harayama. 1991. Identification and characterization of porins in Pseudomonas aeruginosa. J. Biol. Chem. 266:770-779. [PubMed] [Google Scholar]
- 27.Nikaido, H., and E. Y. Rosenberg. 1983. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J. Bacteriol. 153:241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olesky, M., M. Hobbs, and R. A. Nicholas. 2002. Identification and analysis of amino acid mutations in porin IB that mediate intermediate-level resistance to penicillin and tetracycline in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:2811-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan, W., and B. G. Spratt. 1994. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol. Microbiol. 11:769-775. [DOI] [PubMed] [Google Scholar]
- 30.Phillips, I. 1976. β-Lactamase producing penicillin-resistant gonococcus. Lancet ii:656-657. [DOI] [PubMed] [Google Scholar]
- 31.Qi, H. L., J. Y. Tai, and M. S. Blake. 1994. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect. Immun. 62:2432-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ropp, P. A., M. Hu, M. Olesky, and R. A. Nicholas. 2002. Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 46:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ropp, P. A., and R. A. Nicholas. 1997. Cloning and characterization of the ponA gene encoding penicillin-binding protein 1 from Neisseria gonorrhoeae and Neisseria meningitidis. J. Bacteriol. 179:2783-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saint, N., K. L. Lou, C. Widmer, M. Luckey, T. Schirmer, and J. P. Rosenbusch. 1996. Structural and functional characterization of OmpF porin mutants selected for larger pore size. II. Functional characterization. J. Biol. Chem. 271:20676-20680. [PubMed] [Google Scholar]
- 35.Sarubbi, F. A. J., E. Blackman, and P. F. Sparling. 1974. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J. Bacteriol. 120:1284-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmid, B., L. Maveyraud, M. Kromer, and G. E. Schulz. 1998. Porin mutants with new channel properties. Protein Sci. 7:1603-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song, J., C. A. Minetti, M. S. Blake, and M. Colombini. 1998. Successful recovery of the normal electrophysiological properties of PorB (class 3) porin from Neisseria meningitidis after expression in Escherichia coli and renaturation. Biochim. Biophys. Acta 1370:289-298. [DOI] [PubMed] [Google Scholar]
- 38.Sparling, P. F., F. A. J. Sarubbi, and E. Blackman. 1975. Inheritance of low-level resistance to penicillin, tetracycline, and chloramphenicol in Neisseria gonorrhoeae. J. Bacteriol. 124:740-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spratt, B. G. 1988. Hybrid penicillin-binding proteins in penicillin-resistant strains of Neisseria gonorrhoeae. Nature 332:173-176. [DOI] [PubMed] [Google Scholar]
- 40.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Gelder, P., N. Saint, R. van Boxtel, J. P. Rosenbusch, and J. Tommassen. 1997. Pore functioning of outer membrane protein PhoE of Escherichia coli: mutagenesis of the constriction loop L3. Protein Eng. 10:699-706. [DOI] [PubMed] [Google Scholar]
- 42.Veal, W. L., R. A. Nicholas, and W. M. Shafer. 2002. Overexpression of the MtrC-MtrD-MtrE efflux pump due to an mtrR mutation is required for chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. J. Bacteriol. 184:5619-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wetzler, L. M., M. S. Blake, K. Barry, and E. C. Gotschlich. 1992. Gonococcal porin vaccine evaluation: comparison of Por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. J. Infect. Dis. 166:551-555. [DOI] [PubMed] [Google Scholar]
- 44.Wetzler, L. M., M. S. Blake, and E. C. Gotschlich. 1988. Characterization and specificity of antibodies to protein I of Neisseria gonorrhoeae produced by injection with various protein I-adjuvant preparations. J. Exp. Med. 168:1883-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimura, F., and H. Nikaido. 1985. Diffusion of β-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob. Agents Chemother. 27:84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]