Abstract
Cyanophycin (multi-l-arginyl-poly-l-aspartic acid) is a nitrogen storage polymer found in most cyanobacteria and some heterotrophic bacteria. The cyanobacterium Synechocystis sp. strain PCC 6803 accumulates cyanophycin following a transition from nitrogen-limited to nitrogen-excess conditions. Here we show that the accumulation of cyanophycin depends on the activation of the key enzyme of arginine biosynthesis, N-acetyl-l-glutamate kinase, by signal transduction protein PII.
Cyanophycin (multi-l-arginyl-poly-l-aspartic acid) is a nitrogen-rich reserve polymer present in most cyanobacteria (reviewed in references 4, 5, 34, and 43) as well as in some heterotrophic bacteria (27, 49). It consists of a poly-α-aspartic acid backbone, with arginine linked to the β-carboxyl group of almost every aspartyl residue via isopeptide bonds (44). Cyanophycin is synthesized by a single enzyme, cyanophycin synthetase, from aspartate and arginine in an ATP-dependent reaction using a still-unidentified primer (1, 2, 8, 17, 42, 48). The amount of cyanophycin in cyanobacteria varies considerably with growth conditions. Its content is usually less than 1% of dry weight in rapidly growing cultures but is high (up to 18%) in stationary-phase cultures and under conditions of unbalanced growth such as sulfate or phosphate limitation (6, 30, 40, 45). When nitrogen-starved cyanobacterial cultures were provided with combined nitrogen sources, a rapid but transient accumulation of cyanophycin occurred (3). The cyanophycin contents of Anabaena cylindrica and Synechocystis sp. strain PCC 6803 increased severalfold when translation was inhibited by chloramphenicol (6, 41), indicating that rapid synthesis of the polymer did not depend on de novo synthesis of cyanophycin synthetase and that consumption of amino acids by protein synthesis may compete with the accumulation of cyanophycin. Furthermore, no correlation was found between the extractable activity of cyanophycin synthetase and the rate of polymer accumulation (31). These and several similar studies could not, so far, elucidate the mechanism(s) by which cyanophycin accumulation is regulated. Recently, it was shown that the genes for cyanophycin metabolism are under nitrogen control in the diazotrophic strain Anabaena sp. strain PCC 7120 (35). Furthermore, an involvement of the signal transduction protein PII in the control of cyanophycin synthesis was suggested (19, 29) (see below).
The cyanobacterial PII protein is a member of the large family of PII signal transduction proteins, which play pervasive roles in nitrogen control in bacteria, plants, and some archaea (for recent reviews, see references 7 and 12). Similar to its Escherichia coli counterpart, PII from the cyanobacterium Synechococcus elongatus PCC 7942 binds ATP and 2-oxoglutarate in a synergistic manner (13, 24). In the presence of increased 2-oxoglutarate levels, corresponding to nitrogen-limited conditions, PII is phosphorylated at seryl residue 49 (14). Dephosphorylation of PII-P in Synechocystis sp. strain PCC 6803 is catalyzed by PphA, a phosphatase of the PP2C family (23), under conditions of low 2-oxoglutarate levels (39). Recently, the first molecular target of PII was identified in S. elongatus: N-acetyl-l-glutamate kinase (NAGK), which catalyzes the committed step in the cyclic arginine synthesis pathway (11). Its activity is strongly enhanced by complex formation with the nonphosphorylated form of PII, signaling nitrogen-excess conditions (19). Furthermore, the effector molecules 2-oxoglutarate, ATP, and ADP as well as Ca2+ modulate NAGK-PII complex formation (32). The NAGK-PII interaction seems to be universally conserved in oxygenic phototrophs, including higher plants (10, 46). Based on the key function of NAGK in arginine synthesis, we hypothesized that PII activation of arginine synthesis might play a role in the accumulation of cyanophycin under nitrogen-excess conditions (19). However, S. elongatus is one of a few cyanobacteria not able to synthesize cyanophycin, precluding investigation of this issue.
A mutant of the PII phosphatase PphA homologue in the filamentous cyanobacterium Anabaena sp. strain PCC 7120 displays increased PII phosphorylation levels in heterocysts (28) and is impaired in formation of cyanophycin polar bodies. By contrast, cyanophycin accumulated in vegetative cells, implying that PII might be involved in controlling cyanophycin distribution along the filament. However, further studies of PII function in Anabaena are impeded by the lack of PII-null mutants in the Nostocales group (18). The present study was conducted to clarify the role of PII in cyanophycin accumulation. The strain Synechocystis PCC 6803 was used since it produces cyanophycin (1, 15) and mutants in the PII signaling system are available. In particular, we used a PII-null mutant (22) and a PphA-deficient mutant (23), which exhibits significantly delayed PII dephosphorylation upon ammonium addition (25), to study the correlation between PII phosphorylation status, NAGK activity, and cyanophycin accumulation following nitrogen-excess treatments.
The transformable wild-type Synechocystis sp. strain PCC 6803 (15) and the isogenic Synechocystis mutants MPphA (PphA deficient; pphA::kan [23]) and ΔPII (PII deficient; glnB::spc [22]) were routinely grown in liquid BG11 medium (38) supplemented with 5 mM NaHCO3. The MPphA strain was maintained with kanamycin (50 μg ml−1) selection and the ΔPII-strain with spectinomycin selection (35 μg ml−1). In the first set of experiments, wild-type cells of Synechocystis sp. strain PCC 6803 and the mutants ΔPII and MPphA were shifted from nitrogen-poor to nitrogen-excess conditions. Nitrogen-limited cultures were prepared by harvesting cells from 2 ml of nitrate-replete stock culture and resuspending them in 100 ml of modified BG11 medium (low-N BG11) containing 1 mM of nitrate. These cultures were grown in triple-baffled flasks with shaking at 30°C, under continuous illumination of 50 μmol photons m−2 s−1 from white fluorescent lamps. When an optical density at 750 nm of 0.8 to 1.0 was reached (after approximately 4 days for the wild-type and MPphA strains and 5 days for the ΔPII strain), cells started to get slightly bleached due to consumption of nitrate. After the time zero aliquots were taken ammonium chloride was added to a final concentration of 5 mM (nitrogen excess), and aliquots of the culture were harvested in the course of time. From these samples, the accumulation of cyanophycin, activity of NAGK, and phosphorylation status of PII were analyzed. Furthermore, at selected time points, the amount of cellular arginine was determined. Cyanophycin was extracted (40) from 10-ml samples and enzymatically hydrolyzed by recombinant cyanophycinase (37) and recombinant isoaspartyl dipeptidase from Synechocystis sp. strain PCC 6803 (20) to arginine and aspartic acid. The mass of the polymer was calculated from the liberated aspartic acid, quantified enzymatically (33). Values were reproducible within ±5%. For the determination of NAGK activity and the phosphorylation status of PII, cell extracts of the samples were prepared using a RiboLyser (Hybaid) as described previously (19) and protein concentration was estimated using the Bradford assay (9). One hundred micrograms of extract protein was used for a NAGK assay, and 5 μg of protein was used for PII phosphorylation state analysis. To measure the intracellular arginine level, cells from 4 ml of culture were harvested, suspended in 1 ml of 80% ethanol, and incubated for 3 h at 65°C. Following centrifugation, the supernatant was dried and the arginine content was determined by high-pressure liquid chromatography according to reference 16.
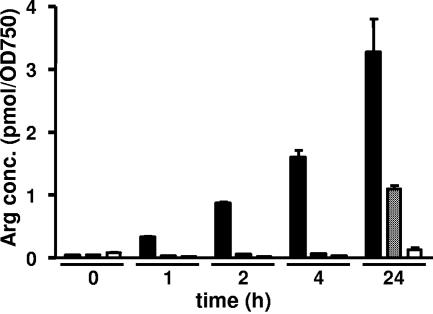
As shown in Fig. 1A, wild-type cells rapidly accumulated cyanophycin following ammonium treatment. By contrast, cyanophycin accumulation was completely absent in the PII-deficient mutant. The PphA-deficient strain showed an intermediate phenotype, having a delayed accumulation of cyanophycin compared to the wild type. Determination of NAGK activity revealed that, following ammonium upshift, wild-type cells rapidly increased the activity of this enzyme. By contrast, the PII-deficient mutant was unable to increase NAGK activity, the same result observed previously in PII-deficient cells of S. elongatus sp. strain PCC 7942 (19). The PphA-deficient cells showed a delayed increase of NAGK activity, compared to the wild type. Quantification of intracellular arginine following ammonium upshift revealed that the arginine level in wild-type cells increased immediately upon N upshift whereas it remained low in the PII-deficient mutant (Fig. 2). In the PphA mutant, increased arginine levels could be observed only in the last sample, which showed accumulated cyanophycin.
FIG. 1.
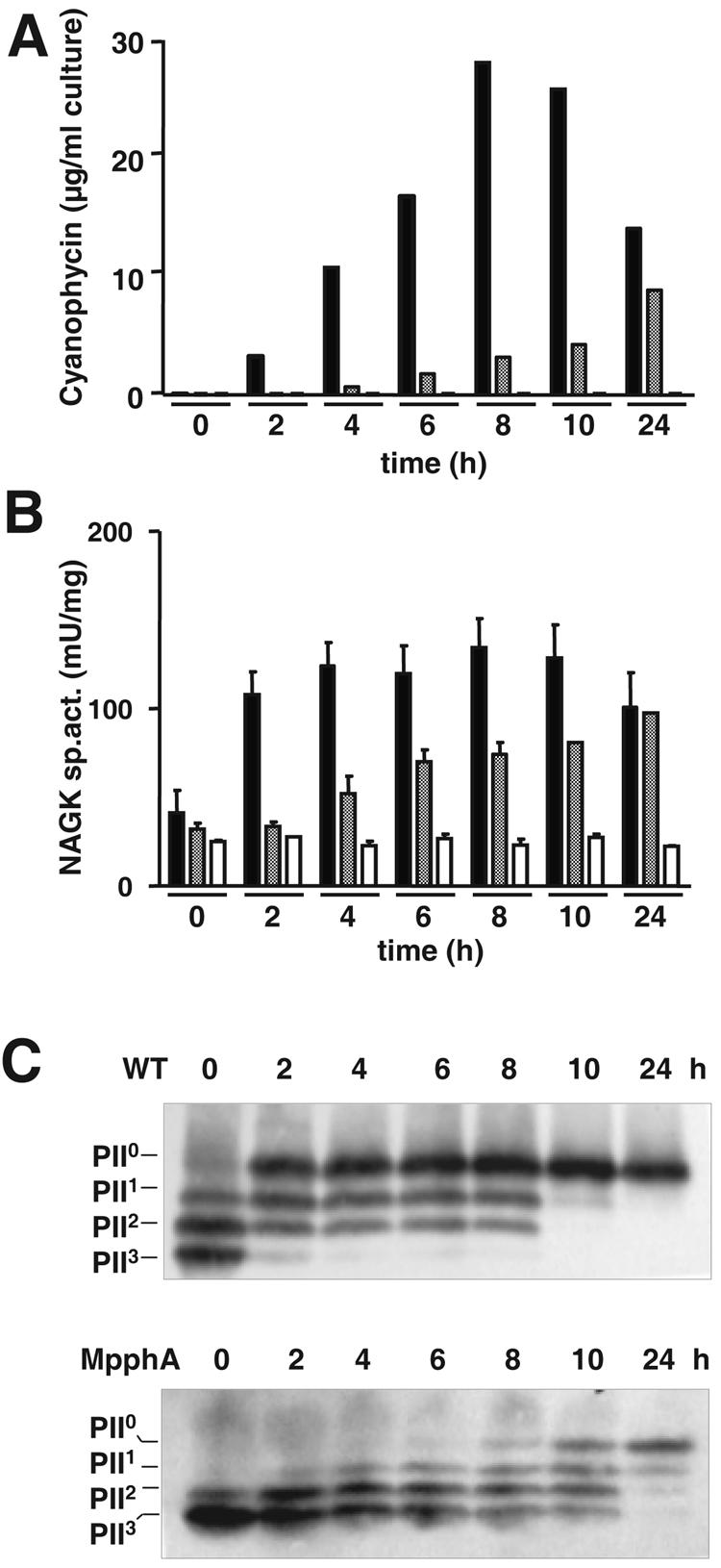
Analysis of cyanophycin accumulation (A) and NAGK activity (B) in cell extracts prepared from Synechocystis sp. strain PCC 6803 cells, which were grown under nitrate-limiting conditions and shifted to 5 mM ammonium chloride. Immediately before the shift (time zero) and after different time points, as indicated, aliquots were removed and extracts were prepared. The results from the Synechocystis wild-type strain (black bars), the MPphA strain (gray bars), and the ΔPII strain (open bars) are shown. Note that no cyanophycin was detectable in ΔPII cells. (C) Determination of the phosphorylation state of PII in the extracts of Synechocystis wild-type (top) and MPphA (bottom) cells that were analyzed as for panels A and B. The phosphorylation status of PII is shown on the left, with the superscripts indicating the numbers of phosphorylated subunits.
FIG. 2.
Quantification of arginine in ethanolic extracts from Synechocystis sp. strain PCC 6803 cells which had been grown under nitrate-limiting conditions and shifted to 5 mM ammonium chloride at time zero. The concentration of arginine is given as pmol Arg extracted per unit of optical density at 750 nm (OD750) of cells. The results from the Synechocystis wild-type strain (black bars), the MPphA strain (gray bars), and the ΔPII strain (open bars) are shown.
The activation state of NAGK, cyanophycin accumulation, and intracellular arginine concentration strongly correlated with the phosphorylation state of PII (Fig. 1C). Previously, we demonstrated that, in Synechococcus sp. strain PCC 7942, the nonphosphorylated form of PII strongly activates NAGK activity (19). Similarly, in the Synechocystis sp. strain PCC 6803 wild-type cells, dephosphorylation of PII correlates with an increase in NAGK activity. The increased NAGK activity is accompanied by increased intracellular arginine and cyanophycin concentrations. In contrast, the delay in PII dephosphorylation in the MPphA strain correlated with delayed NAGK activation and cyanophycin and arginine accumulation. Immunoblot analysis using NAGK-specific antibodies revealed that the amount of NAGK protein did not significantly change during the time course of the experiment (data not shown).
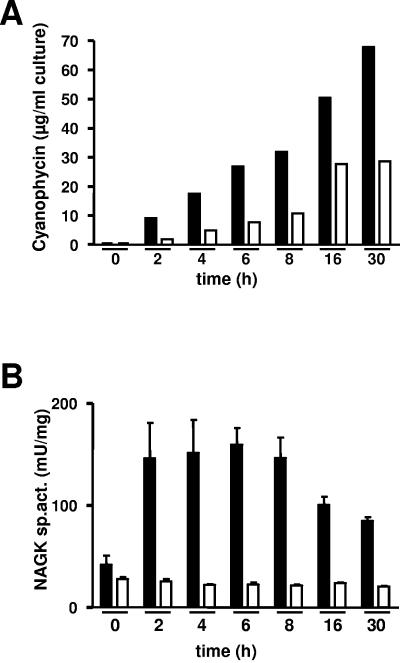
The above results strongly suggested that PII-mediated NAGK activation is responsible for increased arginine synthesis, which then leads to cyanophycin accumulation. To verify independently that impaired cyanophycin synthesis in PII-deficient cells is indeed due to limiting arginine levels and not caused by impaired cyanophycin synthetase activity (26), ammonium upshift experiments in the presence of 5 mM arginine were carried out with wild-type and PII-deficient cells (Fig. 3). Synechocystis has a highly active arginine transport system (28), resulting in a rapid uptake of externally added arginine. As shown in Fig. 3A, the PII-deficient mutant, despite low NAGK activity, was now able to accumulate cyanophycin, although to a lesser extent than the wild type (Fig. 3B). The difference between wild type and mutant may be due to the lack of internally synthesized arginine in the ΔPII strain or may indicate an additional requirement for PII in cyanophycin synthesis. The recently discovered PII receptor PamA in Synechocystis may be considered in this context (47). In any case, cyanophycin accumulation can be restored in the PII-deficient mutant by bypassing the impaired NAGK activity through external addition of arginine, implying that cyanophycin synthesis in the ΔPII strain was limited by the availability of arginine.
FIG. 3.
Analysis of cyanophycin accumulation (A) and NAGK activity (B) in cell extracts from Synechocystis sp. strain PCC 6803 cells, which had been grown under nitrate-limiting conditions and shifted to 5 mM ammonium chloride with 5 mM arginine. Immediately before the shift (time zero) and after different time points, as indicated, aliquots were removed and extracts were prepared. The results from the Synechocystis wild-type strain are shown by the black bars and those from the ΔPII strain by open bars.
Arginine has a dual role in cyanobacteria, first as an amino acid for protein synthesis and second as a nitrogen buffer, storing excess nitrogen in the form of cyanophycin and making it easily available through efficient arginine metabolism (21, 36). PII controls the committed step in arginine synthesis as it activates NAGK activity by complex formation. In addition to increasing NAGK catalytic activity, complex formation with PII also causes a dramatic reduction in arginine feedback inhibition. Whereas free NAGK was almost completely inhibited by arginine concentrations above 50 μM, the PII-complexed NAGK was barely inhibited (32). Therefore, under physiological conditions of PII-NAGK complex formation, efficient arginine synthesis occurs in the presence of appreciably higher levels of arginine compared to conditions favoring PII-NAGK complex dissociation. Complex formation occurs with nonphosphorylated PII at low levels of 2-oxoglutarate, corresponding to nitrogen-rich conditions. Under nitrogen-poor conditions, however, complex formation is impaired, since PII is phosphorylated and the 2-oxoglutarate concentrations are high (19, 32). Cyanophycin synthetase in Synechocystis sp. strain PCC 6803 has a Km for arginine of 49 μM (2), a concentration which is already inhibitory for free NAGK but not for NAGK in complex with PII. Therefore, under nitrogen-poor conditions, cyanophycin cannot be formed. Instead, arginine levels should be just sufficient to meet the requirement for protein synthesis, since the Km values of aminoacyl-tRNA synthetases for their cognate amino acids are usually in the micromolar range. Under conditions of nitrogen excess, however, the NAGK-PII complex is formed and arginine synthesis is stimulated, allowing cyanophycin synthesis to occur. The other substrates of cyanophycin synthetase, aspartate and ATP (exhibiting Km values for Asp of 0.45 mM and for ATP of 0.2 mM [2]), do not seem to limit the reaction in the PII-deficient mutant, since arginine addition alone was sufficient to restore cyanophycin synthesis. The regulation of NAGK activity by PII in response to the nitrogen status thus provides the mechanistic basis for the dual role of arginine: in the nonactivated state, NAGK activity is sufficient to provide arginine for the purpose of protein synthesis; in the PII-activated state, excess nitrogen can be stored in the form of cyanophycin.
Acknowledgments
We thank S. Bedu (Marseille) for the ΔPII strain used in this study.
This work was supported by grants from the DFG (Fo195/4 and Lo286/6-2). M.M. was supported by the Graduiertenkolleg 370 at the University of Giessen.
REFERENCES
- 1.Aboulmagd, E., F. B. Oppermann-Sanio, and A. Steinbüchel. 2000. Molecular characterization of the cyanophycin synthetase from Synechocystis sp. strain PCC 6803. Arch. Microbiol. 174:297-306. [DOI] [PubMed] [Google Scholar]
- 2.Aboulmagd, E., F. B. Oppermann-Sanio, and A. Steinbüchel. 2001. Purification of Synechocystis sp. strain PCC 6308 cyanophycin synthetase and its characterization with respect to substrate and primer specificity. Appl. Environ. Microbiol. 67:2176-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, M. M., and F. Hutchinson. 1980. Nitrogen limitation in the cyanobacterium Aphanocapsa 6308. Arch. Microbiol. 128:1-7. [Google Scholar]
- 4.Allen, M. M. 1984. Cyanobacterial cell inclusions. Annu. Rev. Microbiol. 38:1-25. [DOI] [PubMed] [Google Scholar]
- 5.Allen, M. M. 1988. Inclusions: cyanophycin. Methods Enzymol. 167:207-213. [Google Scholar]
- 6.Allen, M. M., F. Hutchinson, and P. M. Weathers. 1980. Cyanophycin granule polypeptide formation and degradation in the cyanobacterium Aphanocapsa 6308. J. Bacteriol. 141:687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arcondeguy, T., R. Jack, and M. Merrick. 2001. PII signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol. Mol. Biol. Rev. 65:80-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg, H., K. Ziegler, K. Piotukh, K. Baier, W. Lockau, and R. Volkmer-Engert. 2000. Biosynthesis of the cyanobacterial reserve polymer multi-L-arginyl-poly-L-aspartic acid (cyanophycin): mechanism of the cyanophycin synthetase reaction studied with synthetic primers. Eur. J. Biochem. 267:5561-5570. [DOI] [PubMed] [Google Scholar]
- 9.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 10.Burillo, S., I. Luque, I. Fuentes, and A. Contreras. 2004. Interaction between the nitrogen signal transduction protein PII and N-acetyl glutamate kinase in organisms that perform oxygenic photosynthesis. J. Bacteriol. 186:3346-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caldovich, L., and M. Tuchman. 2003. N-acetylglutamate and its changing role through evolution. Biochem. J. 372:279-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forchhammer, K. 2004. Global carbon/nitrogen control by PII signal transduction in cyanobacteria: from signals to targets. FEMS Microbiol. Rev. 28:319-333. [DOI] [PubMed] [Google Scholar]
- 13.Forchhammer, K., and A. Hedler. 1997. Phosphoprotein PII from cyanobacteria. Eur. J. Biochem. 244:869-875. [DOI] [PubMed] [Google Scholar]
- 14.Forchhammer, K., and N. Tandeau de Marsac. 1995. Phosphorylation of the PII protein (glnB gene product) in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 177:5812-5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grigorieva, G., and S. Shestakov. 1982. Transformation in the cyanobacterium Synechocystis sp. PCC 6803. FEMS Microbiol. Lett. 13:367-370. [Google Scholar]
- 16.Hagemann, M., J. Vinnemeier, I. Oberpichler, R. Boldt, and H. Bauwe. 2005. The glycine decarboxylase complex is not essential for the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Biol. 7:15-22. [DOI] [PubMed] [Google Scholar]
- 17.Hai, T., F. B. Oppermann-Sanio, and A. Steinbüchel. 1999. Purification and characterization of cynophycin and cyanphycin synthetase from the thermophilic Synechococcus sp. MA19. FEMS Microbiol. Lett. 181:229-236. [DOI] [PubMed] [Google Scholar]
- 18.Hanson, T. E., K. Forchhammer, N. Tandeau de Marsac, and J. C. Meeks. 1998. Characterization of the glnB gene product of Nostoc punctiforme strain ATCC 29133: glnB or the PII protein may be essential. Microbiology 144:1537-1547. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich, A., M. Maheswaran, U. Ruppert, and K. Forchhammer. 2004. The Synechococcus elongatus PII signal transduction protein controls arginine synthesis by complex formation with N-acetyl-L-glutamate kinase. Mol. Microbiol. 52:1303-1314. [DOI] [PubMed] [Google Scholar]
- 20.Hejazi, M., K. Piotukh, J. Mattow, R. Deutzmann, and W. Lockau. 2002. Isoaspartyl dipeptidase activity of plant-type asparaginases. Biochem. J. 364:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrero, A. 2004. New targets of the PII signal transduction protein identified in cyanobacteria. Mol. Microbiol. 52:1225-1228. [DOI] [PubMed] [Google Scholar]
- 22.Hisbergues, M., R. Jeanjean, F. Joset, N. Tandeau de Marsac, and S. Bedu. 1999. Protein PII regulates both inorganic carbon and nitrate uptake and is modified by a redox signal in Synechocystis PCC 6803. FEBS Lett. 463:216-220. [DOI] [PubMed] [Google Scholar]
- 23.Irmler, A., and K. Forchhammer. 2001. A PP2C-type phosphatase dephosphorylates the PII signalling protein in the cyanobacterium Synechocystis PCC 6803. Proc. Natl. Acad. Sci. USA 98:12978-12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamberov, E. S., M. A. Atkinson, and A. J. Ninfa. 1995. The Escherichia coli signal transduction protein is activated upon binding 2-ketoglutarate and ATP. J. Biol. Chem. 270:17797-17807. [DOI] [PubMed] [Google Scholar]
- 25.Kloft, N., G. Rasch, and K. Forchhammer. 2005. Protein phosphatase PphA from Synechocystis sp. PCC 6803: the physiological framework of PII-P dephosphorylation. Microbiology 151:1275-1283. [DOI] [PubMed] [Google Scholar]
- 26.Krehenbrink, M., and A. Steinbüchel. 2004. Partial purification and characterization of a non-cyanobacterial cyanophycin synthetase from Acinetobacter calcoaceticus strain ADP1 with regard to substrate specificity, substrate affinity and binding to cyanophycin. Microbiology 150:2599-2608. [DOI] [PubMed] [Google Scholar]
- 27.Krehenbrink, M., F. B. Oppermann-Sanio, and A. Steinbüchel. 2002. Evaluation of non-cyanobacterial genome sequences for occurrence of genes encoding protein homologous to cyanophycin synthetase and cloning of an active cyanophycin synthetase from Acinetobacter sp. strain DSM 587. Arch. Microbiol. 177:371-380. [DOI] [PubMed] [Google Scholar]
- 28.Labarre, J., P. Thuriaux, and F. Chauvat. 1987. Genetic analysis of amino acid transport in the facultatively heterotrophic cyanobacterium Synechocystis sp. strain 6803. J. Bacteriol. 169:4668-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laurent, S., K. Forchhammer, L. Gonzalez, T. Heulin, C. C. Zhang, and S. Bedu. 2004. Cell-type specific modification of PII is involved in the regulation of nitrogen metabolism in the cyanobacterium Anabaena PCC 7120. FEBS Lett. 576:261-265. [DOI] [PubMed] [Google Scholar]
- 30.Lawry, N. H., and R. D. Simon. 1982. The normal and induced occurrence of cyanophycin inclusion bodies in several blue-green algae. J. Phycol. 18:391-399. [Google Scholar]
- 31.Mackerras, A. H., N. M. Dechazal, and G. D. Smith. 1990. Transient accumulations of cyanophycin in Anabaena cylindrica and Synechocystis 6308. J. Gen. Microbiol. 136:2057-2065. [Google Scholar]
- 32.Maheswaran, M., C. Urbanke, and K. Forchhammer. 2004. Complex formation and catalytic activation by the PII signaling protein of N-acetyl-L-glutamate kinase from Synechococcus elongatus strain PCC 7942. J. Biol. Chem. 279:55202-55210. [DOI] [PubMed] [Google Scholar]
- 33.Möllering, H. 1985. Laspartate and L-arginine, p. 350-357. In H. U. Bergmeyer, J. Bergmeyer, and M. Grassl (ed.), Methods of enzymatic analysis, 3rd ed., vol. VIII. VCH Verlagsgesellschaft, Weinheim, Germany. [Google Scholar]
- 34.Oppermann-Sanio, F. B., and A. Steinbüchel. 2002. Occurrence, functions and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften 89:11-22. [DOI] [PubMed] [Google Scholar]
- 35.Picossi, S., A. Valladares, E. Flores, and A. Herrero. 2004. Nitrogen-regulated genes for the metabolism of cyanophycin, a bacterial nitrogen reserve polymer. J. Biol. Chem. 279:11582-11592. [DOI] [PubMed] [Google Scholar]
- 36.Quintero, M. J., A. M. Muro-Pastor, A. Herrero, and E. Flores. 2000. Arginine catabolism in the cyanobacterium Synechocystis sp. strain PCC 6803 involves the urea cycle and arginase pathway. J. Bacteriol. 182:1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richter, R., M. Hejazi, R. Kraft, K. Ziegler, and W. Lockau. 1999. Cyanophycinase, a peptidase degrading the cyanobacterial reserve material multi-L-arginyl-poly-L-aspartic acid (cyanophycin): molecular cloning of the gene of Synechocystis sp. PCC 6803, expression in Escherichia coli, and biochemical characterization of the purified enzyme. Eur. J. Biochem. 263:163-169. [DOI] [PubMed] [Google Scholar]
- 38.Rippka, R. 1988. Isolation and purification of cyanobacteria. Methods Enzymol. 167:3-27. [DOI] [PubMed] [Google Scholar]
- 39.Ruppert, U., A. Irmler, N. Kloft, and K. Forchhammer. 2002. The novel protein phosphatase PphA from Synechocystis PCC 6803 controls dephosphorylation of the signalling protein PII. Mol. Microbiol. 44:855-864. [DOI] [PubMed] [Google Scholar]
- 40.Simon, R. D. 1973. Measurement of the cyanophycin granule polypeptide contained in the blue-green alga Anabaena cylindrica. J. Bacteriol. 114:1213-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon, R. D. 1973. The effect of chloramphenicol on the production of cyanophycin granule polypeptide in the blue green alga Anabaena cylindrica. Arch. Microbiol. 92:115-122. [DOI] [PubMed] [Google Scholar]
- 42.Simon, R. D. 1976. The biosynthesis of multi-L-arginyl-poly(L-aspartic acid) in the filamentous cyanobacterium Anabaena cylindrica. Biochim. Biophys. Acta 422:407-418. [DOI] [PubMed] [Google Scholar]
- 43.Simon, R. D. 1987. Inclusion bodies in the cyanobacteria: cyanophycin, polyphosphate, polyhedral bodies, p. 199-225. In P. Fay and C. van Baalen (ed.), The cyanobacteria. Elsevier, Amsterdam, The Netherlands.
- 44.Simon, R. D., and P. Weathers. 1976. Determination of the structure of the novel polypeptide containing aspartic acid and arginine which is found in cyanobacteria. Biochim. Biophys. Acta 420:165-176. [DOI] [PubMed] [Google Scholar]
- 45.Stephan, D. P., H. G. Ruppel, and E. K. Pistorius. 2000. Interrelation between cyanophycin synthesis, L-arginine catabolism and photosynthesis in the cyanobacterium Synechocystis sp. strain PCC 6803. Z. Naturforsch. 55c:927-942. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama, K., T. Hayakawa, T. Kudo, T. Ito, and T. Yamaya. 2004. Interaction of N-acetylglutamate kinase with a PII-like protein in rice. Plant Cell Physiol. 45:1768-1778. [DOI] [PubMed] [Google Scholar]
- 47.Takashi, O., S. Sato, S. Tabata, and K. Tanaka. 2005. Identification of PamA as a PII-binding membrane protein important in nitrogen-related and sugar-catabolic gene expression in Synechocystis sp. PCC 6803. J. Biol. Chem. 280:34684-34690. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler, K., A. Diener, C. Herpin, R. Richter, R. Deutzmann, and W. Lockau. 1998. Molecular characterization of cyanophycin synthetase, the enzyme catalyzing the biosynthesis of the cyanobacterial reserve material multi-L-arginyl-poly-L-aspartate (cyanophycin). Eur. J. Biochem. 254:154-159. [DOI] [PubMed] [Google Scholar]
- 49.Ziegler, K., R. Deutzmann, and W. Lockau. 2002. Cyanophycin synthetase-like enzymes of non-cyanobacterial eubacteria: characterization of the polymer produced by a recombinant synthetase of Desulfitobacterium hafniense. Z. Naturforsch. 57c:522-529. [DOI] [PubMed] [Google Scholar]