Abstract
Biofilm formation is an increasing problem in medicine, due to the intrinsic resistance of microorganisms in the biofilm mode of growth against the host immune system and antimicrobial therapy. Adhesion is an important step in biofilm formation, influenced, among other factors, by the surface hydrophobicities and charges of both the substratum and the adhering microorganisms. Enterococcus faecalis strains generally display subpopulations with different surface charges, expressed as bimodal zeta potential distributions. Two-thirds of E. faecalis strains isolated from clogged biliary stents displayed such heterogeneity of surface charges in culture. In this study, the influence of this culture heterogeneity on initial adhesion and subsequent biofilm formation was investigated. Heterogeneous strains were retained in higher numbers on polystyrene than homogeneous strains. Also, biofilm formation was much more pronounced for heterogeneous strains than for homogeneous strains. In a population enriched to display only one subpopulation, fewer bacteria were retained than in its original heterogeneous culture. Also, the enriched subpopulation formed less biofilm than its original heterogeneous culture. The presence of ox bile during adhesion resulted in fewer retained bacteria, although heterogeneous strains were still retained in significantly higher numbers than were homogeneous strains, and, in general, the presence of ox bile reduced biofilm formation. The initial adhesion and biofilm formation were independent of the presence of the gene encoding the enterococcal surface protein (esp) or the expression of gelatinase (GelE). It is concluded that heterogeneity in cell surface charge represents an advantage for bacteria in the colonization of surfaces.
Many bacterial species are able to colonize the surfaces of biomedical devices and form biofilms. Biofilm growth protects the bacteria against host defenses and the action of antimicrobial agents, and therefore biofilms can be a source of persistent infections (3, 5). The human commensal Enterococcus faecalis is a major cause of nosocomial infections and is able to form biofilms on biomedical devices such as urinary catheters and central venous catheters (6). E. faecalis is also one of the most predominant strains involved in the formation of biofilms in biliary stents, used to palliate obstructions in the common bile duct (7). Clogging of biliary stents caused by microbial biofilms is a common complication, necessitating removal of the device, severely affecting the quality of life of the patient, and raising the cost of health care.
The majority of clinical isolates of E. faecalis have the ability to form a single-species biofilm in vitro (17, 23). Although the genetic basis of biofilm formation by E. faecalis is largely unknown, several factors that promote biofilm formation have been identified. The enterococcal surface protein (Esp) (17, 22, 23), the transcriptional regulator BopD (12), the quorum-sensing locus fsr (11, 18), and gelatinase (GelE) have all been reported to be involved in biofilm formation (11, 15).
Biofilm formation takes place in a complicated series of events that commences with the formation of a conditioning film on a surface and the subsequent adhesion of bacteria, followed by the formation of microcolonies and the production of a matrix of extracellular polymeric substances that define the biofilm.
Bacterial adhesion is governed by Lifshitz-van der Waals, electrostatic, and Lewis acid-base interactions between the bacteria and the substratum (26). These interactions originate both from the entire cell body and from more-specific, localized adhesion sites, such as proteins on the cell surface. Since nearly all surfaces occurring in nature carry a net negative charge under physiological conditions, electrostatic interactions in bacterial adhesion are mostly repulsive and have to be overcome by attractive Lifshitz-van der Waals, hydrophobic, and specific interaction forces.
In the determination of a bacterial property, such as cell surface charge or hydrophobicity, pure bacterial cultures are generally considered populations of identical organisms, although it is known that several strains display distinct subpopulations even in pure cultures. Subpopulations within one culture can differ in flagellation (21), natural competence (8), and cell surface charge (4). Surface charge is usually characterized by the zeta potential (24, 28), and in a heterogeneous culture, the zeta potentials measured may display either an extremely wide Gaussian distribution or more than one distinct Gaussian distribution (10, 21).
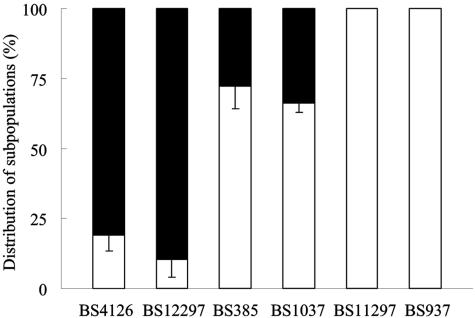
Culture heterogeneity in zeta potential is a common trait among E. faecalis strains (25), as 24 out of a collection of 46 strains displayed heterogeneous subpopulations. Four out of six E. faecalis strains isolated from biliary stents showed culture heterogeneity (Fig. 1 and Table 1), which was not influenced by growth medium, quorum sensing, the presence of surface proteins, such as Esp and Agg, or exposure to ox bile. However, growth in ox bile resulted in the disappearance of this heterogeneity. It was possible to enrich a heterogeneous culture for its most negatively charged subpopulation, although this enrichment was not permanent. Culture heterogeneity enhanced enterococcal adhesion to a glass surface (25).
FIG. 1.
Percentage distributions of subpopulations of the fresh clinical isolates E. faecalis BS4126, BS12297, BS385, BS1037, BS11297, and BS937. Distributions were determined with zeta potential measurements in 10 mM potassium phosphate (pH 7.0). In each case, the more-negative fraction is indicated in black and the less-negative fraction in white. The bars denote standard deviations for three experiments, with each experiment comprising 100 bacteria.
TABLE 1.
Zeta potentials of isolates measured in 10 mM potassium phosphate (pH 7.0)
| E. faecalis strain | Zeta potential (mV) for:
|
|
|---|---|---|
| Subpopulation 1 | Subpopulation 2 | |
| Heterogeneous | ||
| BS4126 | −27 ± 1 | −35 ± 1 |
| BS12297 | −24 ± 1 | −33 ± 2 |
| BS385 | −24 ± 2 | −35 ± 2 |
| BS1037 | −29 ± 4 | −40 ± 4 |
| Homogeneous | ||
| BS11297 | −30 ± 3 | |
| BS937 | −40 ± 4 | |
In this study, the effect of culture heterogeneity in zeta potential on retention and biofilm formation by E. faecalis strains isolated from clogged biliary stents was investigated.
MATERIALS AND METHODS
Strains and growth conditions.
E. faecalis strains BS4126, BS12297, BS385, BS1037, BS11297, and BS937 were freshly isolated from clogged biliary stents and characterized as described previously (25). Relevant, known characteristics of the isolates are summarized in Table 2. The zeta potential distributions of the isolates measured in 10 mM potassium phosphate (pH 7.0) are shown in Fig. 1. Isolates were routinely cultured from frozen stock on blood agar plates. Precultures were inoculated with a single colony from a plate in 3 ml tryptone soya broth (TSB) and grown overnight at 37°C. Cultures were grown from the precultures in 200 ml TSB overnight at 37°C.
TABLE 2.
Presence of esp and GelE in E. faecalis BS4126, BS12297, BS385, BS1037, BS11297, and BS937 and their cell surface hydrophobicities determined by water contact anglesa
| E. faecalis strain | Presencec of:
|
Contact angle (°)b | |
|---|---|---|---|
| esp | GelE | ||
| Heterogeneous | |||
| BS4126 | + | − | 29 |
| BS12297 | + | − | 27 |
| BS385 | − | − | 32 |
| BS1037 | + | − | 30 |
| Homogeneous | |||
| BS11297 | + | + | 28 |
| BS937 | + | − | 41 |
Data for esp and contact angles were taken from a report by van Merode et al. (25).
The water contact angle was measured for three separate cultures, yielding a standard deviation of less than 15% of the mean.
+, present; −, absent.
Gelatinase assay.
The gelatinase assay was performed as described previously (13). Briefly, 5 μl of an overnight culture and 5 μl of supernatant were incubated on a gelatin plate (0.8% gelatin and 5% agar). The gelatin plates were incubated for 48 h at 37°C. Hydrolysis of gelatin was determined by screening the plates for the appearance of a turbid halo around the colonies. The addition of trichloroacetic acid to the gelatin plate made the halo more vivid.
Retention assay.
The ability of the E. faecalis strains to be retained by polystyrene surfaces was evaluated as follows. Strains were grown overnight in 200 ml TSB at 37°C, harvested by centrifugation (6,500 × g, 5 min, 10°C), and washed twice with 10 mM potassium phosphate (pH 7.0). The cells were resuspended in 10 mM potassium phosphate (pH 7.0) with and without 50 mg/ml ox bile (Merck) to an optical density at 600 nm (OD600) of 0.1, and 200 μl of this cell suspension was used to inoculate a well of a sterile 96-well polystyrene microtiter plate (Nunc F; Nalgene). After 2 h at 37°C with shaking (150 rpm), the wells were washed twice with 10 mM potassium phosphate (pH 7.0). To quantify the number of adhering bacteria able to withstand the washing, the wells were stained with 1% crystal violet. After being washed with 200 μl demineralized water, each well was air dried and the number of retained bacteria was quantified using a charge-coupled-device MXR camera mounted on a metallurgical microscope equipped with a 40× ultralong-working-distance objective. The mean count of retained bacteria was determined from three images from three replicate wells per experiment, and each experiment was repeated three times with separately cultured bacteria. The size of an image taken corresponds to 2.2 × 10−4 cm2.
Biofilm assay.
Bacteria adhering to polystyrene microtiter plates, after 2 h and washing as described above, were considered to be an initial biofilm and were further grown by the addition of 200 μl TSB containing 0.5% glucose with or without 50 mg/ml ox bile. After 24 h at 37°C with shaking (150 rpm), the wells were gently washed twice with 200 μl 10 mM potassium phosphate (pH 7.0) and stained with 200 μl 1% crystal violet for 30 min (17, 23). The wells were washed with 200 μl demineralized water to remove excess stain, and the crystal violet was solubilized in 200 μl of ethanol-acetone (80:20, vol/vol). The absorbance at 575 nm (A575) was determined using a microtiter plate reader (Fluostar Optima; BMG Labtech). All experiments included five replicate wells and a blank. All experiments were repeated three times with separately grown bacteria.
Biofilm formation was expressed as a percentage with respect to the A575 for E. faecalis BS4126 (A575, 7.86), which formed the most biofilm in this study and is used as a reference strain. For reference purposes, the A575 of strain OG1RF was 2.1.
Enrichment of a subpopulation of E. faecalis BS385.
To enrich E. faecalis BS385 into one subpopulation, this isolate was passed through an anion-exchange column as described previously (25). Briefly, the DEAE-Sephadex resin (Pharmacia) was preswollen in sterile 10 mM potassium phosphate (pH 7.0) and packed in a column (inside diameter, 5.5 by 1.5 cm). The column was equilibrated with sterile 10 mM potassium phosphate (pH 7.0) and checked for sterility by the addition of column material to fresh TSB. Since no growth appeared, the column material was considered to be sterile. A bacterial suspension in 30 ml 10 mM potassium phosphate (pH 7.0) with an OD600 of 1.0 was applied to the column, allowed to pass through by gravity, and collected in 5-ml fractions. The third fraction, which contained the lowest percentage in the less negatively charged subpopulation (25), was added to 200 ml TSB and grown overnight at 37°C. From this culture, the zeta potential distribution was determined and used for the retention assay. To grow biofilms, the OD600 of the third fraction was determined, and the original E. faecalis BS385 was suspended in 10 mM potassium phosphate (pH 7.0) to the same OD600. These cell suspensions were used to grow biofilms after the bacteria were allowed to adhere in microtiter plate wells, as described above.
Confocal laser scanning microscopy (CLSM).
Biofilms were grown in 12-well polystyrene tissue culture plates (Costar; Corning, Inc.) for 24 h in 2.5 ml medium, as described above. After the wells were gently washed twice with 2.5 ml 10 mM potassium phosphate (pH 7.0), the biofilms were stained with a Live/Dead BacLight viability kit (Molecular Probes) and incubated for 15 min in the dark. Confocal images were collected through the bottom of the plate using a Leica TCS NT microscope with a 40× long-working-distance objective.
Zeta potential distributions of biofilm bacteria.
Zeta potential distributions of biofilm organisms were determined by microelectrophoresis. Biofilm bacteria were grown in 12-well polystyrene tissue culture plates as described above. After the wells were gently washed twice with 2.5 ml 10 mM potassium phosphate (pH 7.0), biofilms were removed from the bottoms of the wells by sonication in 10 mM potassium phosphate (pH 7.0).
Zeta potential distributions were measured in 10 mM potassium phosphate (pH 7.0) with a Lazer Zee model 501 meter (PenKem) equipped with image analysis options for zeta sizing. Briefly, the microelectrophoresis chamber was filled with a bacterial suspension with a density of 107 to 108 cells/ml, and a voltage difference of 150 V was applied over the chamber. The velocity of each individual bacterium was determined by image sequence analysis, and from this, its zeta potential was calculated, assuming that the Helmholtz-Smoluchowski equation holds. Zeta potential distributions were measured in triplicate for separately cultured bacteria.
Statistical analysis.
Differences in retention were analyzed for significance by Student's t test. Differences in optical densities of biofilms were analyzed for significance by the Mann-Whitney U test. Significance was defined as a P value of ≤0.05, and all evaluations were done with SPSS 12.0.2.
RESULTS
Retention of heterogeneous and homogeneous E. faecalis strains.
Heterogeneous and homogeneous E. faecalis strains were tested for the ability to adhere to a polystyrene surface. The number of enterococci able to withstand the washing are listed in Table 3, and the results show that significantly more bacteria of the heterogeneous strains (up to 10 times) were retained than bacteria of the homogeneous strains (P < 0.05, Student's t test). The presence of bile during adhesion led to a significant decrease in the number of retained bacteria, except in the case of BS11297. The difference in adhesion between heterogeneous and homogeneous strains remains clear: heterogeneous cultures are retained by polystyrene in significantly higher numbers than the homogeneous strains (P < 0.05, Student's t test).
TABLE 3.
Effect of ox bile on number of bacteria retained after 2 h in a polystyrene 96-well microtiter platea
| E. faecalis strain | No. of bacteria (106 cm−2) retained after 2 hb
|
|
|---|---|---|
| With ox bile | Without ox bile | |
| Heterogeneous | ||
| BS4126 | 11.5 ± 1.6 | 8.1 ± 1.4d |
| BS12297 | 7.8 ± 3.9 | 2.4 ± 0.9d |
| BS385 | 5.7 ± 0.8 | 2.5 ± 0.6d |
| BS1037 | 9.9 ± 0.9 | 2.0 ± 0.7d |
| Homogeneous | ||
| BS11297 | 1.0 ± 0.8c | 0.8 ± 0.2c |
| BS937 | 1.9 ± 1.4c | 0.4 ± 0.3c |
| Enriched subpopulation | ||
| BS385 | 4.1 ± 0.7e | ND |
Each plate contained 10 mM potassium phosphate with or without 50 mg/ml ox bile.
The results are the means ± standard deviations for three separate experiments performed in triplicate. ND, not determined.
P < 0.05, versus heterogeneous strains, Student's t test.
P < 0.05, versus without ox bile, Student's t test.
P < 0.05, versus BS385, Student's t test.
To confirm the role of culture heterogeneity in retention, the retention of the heterogeneous strain BS385 and that of its enriched subpopulation were compared. Whereas the more negative subpopulation of the original, planktonic culture constituted the minority (70% of the cells had zeta potentials of −24 ± 4 mV, and 30% of the cells had zeta potentials of −35 ± 2 mV), this subpopulation constituted a clear majority after anion-exchange chromatography (80% of the cells had zeta potentials of −34 ± 3 mV, and 20% of the cells had zeta potentials of −24 ± 2 mV). Bacteria of the original heterogeneous culture were retained on polystyrene surfaces significantly (P < 0.05, Student's t test) better than bacteria of the enriched subpopulation (Table 3).
Culture heterogeneity and biofilm formation.
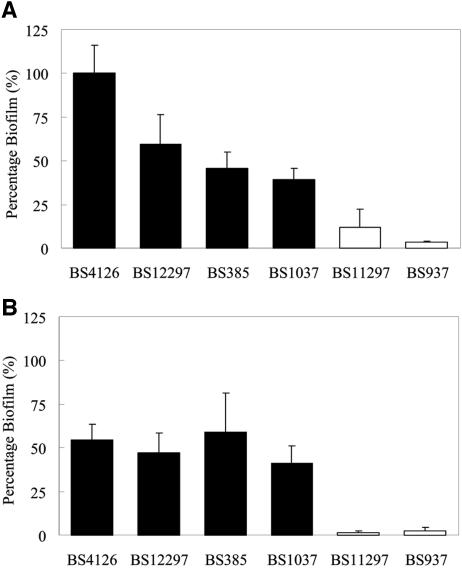
The abilities of heterogeneous and homogeneous strains to form biofilms were evaluated as well. All heterogeneous strains were categorized as strong biofilm-forming strains (A575 > 2), while the homogeneous strain BS11297 was a weak biofilm former (A575 greater than 0.5 but less than 1), and the homogeneous strain BS937 was categorized as a non-biofilm former (A575 ≤ 0.5) based on a report by Mohammed et al. (17). The heterogeneous strains formed significantly more biofilm than the homogeneous strains (P < 0.05, Mann-Whitney U test). All heterogeneous strains formed sizable biofilms, in contrast to homogeneous strains, which showed reduced abilities to form biofilms (less than 20% of reference strain BS4126) (Fig. 2A). The difference in ability to form biofilms between the heterogeneous and homogeneous strains increased when biofilms were grown in the presence of ox bile (Fig. 2B). In the presence of ox bile, all heterogeneous strains still formed sizable biofilms (>40%), while the two homogeneous strains formed considerably less biofilm (<5%). Interestingly, one heterogeneous strain, BS385, showed enhanced biofilm formation in the presence of ox bile.
FIG. 2.
Biofilm formation by E. faecalis BS4126, BS12297, BS385, BS1037, BS11297, and BS937 after 2 h of adhesion in the microtiter plate wells. The ability of the strains to form biofilms was expressed relative to the biofilm-forming ability of E. faecalis BS4126 in TSB containing 0.5% glucose. Biofilms were grown for 24 h in TSB containing 0.5% glucose in the absence (A) and presence (B) of 50 mg/ml ox bile. Heterogeneous strains are indicated in black and homogeneous strains in white. All data represent the means ± standard deviations for 15 determinations (three independent experiments, each performed five times).
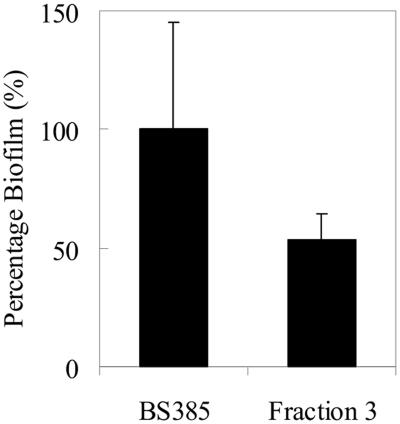
To further analyze the role of heterogeneity in biofilm formation, biofilm formation by the enriched subpopulation of E. faecalis BS385 was compared with biofilm formation by the original culture. The enriched subpopulation of E. faecalis BS385 had significantly less ability to form a biofilm than the original culture (P < 0.05, Mann-Whitney U test) (Fig. 3), indicating that culture heterogeneity is involved in biofilm formation. Note that the enriched culture is not completely homogeneous, which can account for the residual biofilm formation found.
FIG. 3.
Biofilm formation by heterogeneous E. faecalis BS385 and its subpopulation enriched in the more negatively charged bacteria. Note that in this case, biofilm formation was expressed relative to the biofilm-forming ability of E. faecalis BS385 (A575, 1.71). All data represent the means ± standard deviations for 15 determinations (three independent experiments, each performed five times).
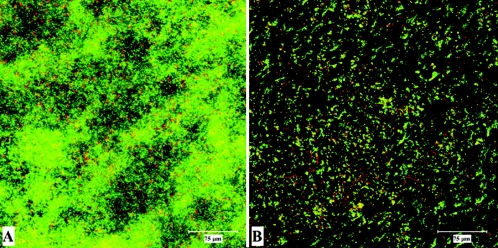
CLSM was used to examine biofilms formed by the heterogeneous and the homogeneous strains. Whereas the heterogeneous strains formed dense biofilms (Fig. 4), the biofilms formed by the homogeneous strains were not more than scattered microcolonies at the bottom of the 12-well plate.
FIG. 4.
Examples of CLSM images of biofilm growth of heterogeneous and homogeneous strains. The biofilm formed by the heterogeneous strain BS4126 (A) had a thickness of 42.9 μm, and the biofilm formed by the homogeneous strain BS11297 (B) had a thickness of 19.5 μm.
Zeta potentials of E. faecalis cells in a biofilm.
In order to gain more information on the stage of biofilm formation at which culture heterogeneity plays a role, the zeta potential of cells within a biofilm was determined (Table 4). After 24 h of biofilm growth, most strains did not show culture heterogeneity anymore, except for E. faecalis BS4126 and BS385. Interestingly, BS4126 showed culture heterogeneity in the absence of ox bile, while BS385 showed culture heterogeneity in the presence of ox bile. The zeta potentials of the E. faecalis strains within a biofilm were, in general, less negative than the zeta potentials of the planktonically grown E. faecalis strains.
TABLE 4.
Zeta potential distributions of subpopulations of E. faecalis BS4126, BS12297, BS385, BS1037, BS11297, and BS937 in biofilm growth in culture with and without ox bilea
| E. faecalis strain | Without ox bile
|
With ox bile
|
||||
|---|---|---|---|---|---|---|
| Subpopulation 1
|
Subpopulation 2 zeta potential (mV) | Subpopulation 1
|
Subpopulation 2 zeta potential (mV) | |||
| Zeta potential (mV) | % of culture | Zeta potential (mV) | % of culture | |||
| Heterogeneous | ||||||
| BS4126 | −32 ± 4 | 76 | −22 ± 0 | −27 ± 3 | 100 | Absent |
| BS12297 | −33 ± 3 | 100 | Absent | −35 ± 3 | 100 | Absent |
| BS385 | −25 ± 3 | 100 | Absent | −35 ± 2 | 50 | −29 ± 3 |
| BS1037 | −26 ± 2 | 100 | Absent | −28 ± 2 | 100 | Absent |
| Homogeneous | ||||||
| BS11297 | −26 ± 4 | 100 | Absent | CD | ||
| BS937 | CD | CD | ||||
Zeta potential distributions were determined in 10 mM potassium phosphate (pH 7.0). The results are the means ± standard deviations for three separate experiments performed in triplicate. CD, could not be determined.
DISCUSSION
The results show that culture heterogeneity plays an important role in enterococcal adhesion to the surface and as such is pivotal to biofilm formation, but once biofilm formation progresses, culture heterogeneity disappears.
Clinical E. faecalis strains with heterogeneous zeta potential distributions adhered significantly better than homogeneous strains. Bacterial adhesion is determined by an interplay between hydrophobic and electrostatic interactions (2). The cell surface hydrophobicities of the bacteria as determined by measuring water contact angles were similar for all strains studied (Table 2), indicating that differences in hydrophobicity are likely not to be involved in the observed differences in adhesion and retention. This method is not sensitive to culture heterogeneity, and differences in hydrophobicity among subpopulations thus remain undetected. When the bacteria approach the polystyrene surface, they experience an electrostatic repulsion since both the bacteria and the polystyrene surface are negatively charged (14). This electrostatic repulsion is stronger for the bacteria with a more-negative zeta potential. Heterogeneous cultures consist of two subpopulations that have differential negative charges and zeta potentials. When one of these subpopulations is enriched, adhesion is reduced, indicating that heterogeneity in zeta potential is responsible for the observed increase in adhesion rather than the presence of other strain-specific traits.
During biofilm formation in biliary stents, it may be expected that bile is present, and therefore it is clinically relevant to study bacterial adhesion in the presence of bile. Although the presence of bile during adhesion decreases the adhesion and retention of the E. faecalis strains, the heterogeneous strains still adhered significantly better than the homogeneous strains. Previously, it was shown that the presence of bile during growth, and not the mere exposure to bile, influences culture heterogeneity (25). The reduction in adhesion in the presence of bile that was found for the heterogeneous cultures cannot be explained by changes in zeta potential distribution or by changes in hydrophobicity. A possible explanation for the observed effect of bile might be found in the changes in cell metabolism due to the bactericidal nature of bile (9). Alternatively, the main constituents of bile are bile acids, which are amphipathic molecules that lower the surface tension of the medium (16). A change in surface tension between the medium and surface could interfere with the adhesion of bacteria, and a lower medium-surface tension theoretically results in decreased bacterial adhesion (1).
The heterogeneous strains formed biofilms, in contrast to the homogeneous strains, which lacked this ability. This difference in biofilm growth was not caused by strain-specific traits (esp and GelE), since an enriched subpopulation of a heterogeneous strain was reduced in its biofilm-forming ability. Because of the observed low adhesive potential of homogeneous strains, it is possible that homogeneous strains might be unable to anchor themselves to the surface, thus not providing a strong basis for a biofilm. In addition, once a strain grows into a biofilm, its heterogeneity generally disappears, except in the case of BS4126, and the strains display homogeneous traits, indicating that heterogeneity plays a role in adhesion and retention in the initial stages of biofilm formation. Interestingly, it was determined that planktonic bacteria in the well with biofilms formed by heterogeneous strains displayed heterogeneous zeta potential distributions. Planktonic cells above biofilms formed by homogeneous strains remained homogeneous. Less biofilm was formed in the presence of bile than in the absence of bile, regardless of the zeta potential distribution of the population. Interestingly, E. faecalis BS385, the best biofilm former in the presence of ox bile, displays two different subpopulations with different surface charges even in its biofilm mode of growth.
Several factors are reported to be involved in adhesion and biofilm formation by E. faecalis. Esp was found to be enriched in infection-derived enterococcal strains (20) and has been associated with enhanced adhesion to abiotic surfaces and biofilm formation by E. faecalis, especially in the presence of 0.5% (wt/vol) glucose (22, 23). The expression of Esp promotes a strong interaction between substratum and bacteria, leading to a higher number of adherent bacteria (27). Of the six E. faecalis strains studied here, five were esp positive (Table 2). The only esp-negative strain, BS385, is heterogeneous in zeta potential, and, of all heterogeneous strains, it shows the lowest retention to polystyrene (Table 3), confirming the role of esp in retention. In contrast, the two homogeneous strains are esp positive and hardly adhere to the polystyrene. Therefore, the presence of esp alone does not necessarily lead to a high number of adhering bacteria. In addition, the data presented here indicated no association between biofilm formation and the presence of esp, which is in accordance with previous reports (15, 19); an esp-negative strain was fully able to form a biofilm, and two esp-positive strains did not form biofilms, though 0.5% (wt/vol) glucose was present during biofilm formation.
Previous reports also state that gelatinase (GelE) is involved in biofilm formation by E. faecalis (11, 15). GelE is able to hydrolyze small, biologically active peptides such as gelatin or collagen. Hancock and Perego (11) showed that the E. faecalis fsr quorum-sensing system controls biofilm formation via the production of GelE. In the present study, however, only a non-biofilm-forming isolate expressed GelE, while the four biofilm-forming strains did not express GelE either when planktonically grown or when grown as biofilms (Table 2).
In summary, whereas only a partial involvement in biofilm formation has been established for esp and GelE, culture heterogeneity in surface charge in axenic cultures of E. faecalis represents an absolute advantage for the bacteria in colonizing surfaces and forming biofilms.
REFERENCES
- 1.Absolom, D. R., F. V. Lamberti, Z. Policova, W. Zingg, C. J. van Oss, and A. W. Neumann. 1983. Surface thermodynamics of bacterial adhesion. Appl. Environ. Microbiol. 46:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, Y. H., and R. J. Friedman. 1998. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 43:338-348. [DOI] [PubMed] [Google Scholar]
- 3.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 4.Cowan, M. M., H. C. van der Mei, I. Stokroos, and H. J. Busscher. 1992. Heterogeneity of surfaces of subgingival bacteria as detected by zeta potential measurements. J. Dent. Res. 71:1803-1806. [DOI] [PubMed] [Google Scholar]
- 5.Davies, D. G. 2003. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2:114-122. [DOI] [PubMed] [Google Scholar]
- 6.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowidar, N., H. J. Kolmos, H. Lyon, and P. Matzen. 1991. Clogging of biliary endoprostheses. A morphologic and bacteriologic study. Scand. J. Gastroenterol. 26:1137-1144. [DOI] [PubMed] [Google Scholar]
- 8.Dubnau, D. 1991. Genetic competence in Bacillus subtilis. Microbiol. Rev. 55:395-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flahaut, S., J. Frere, P. Boutibonnes, and Y. Auffray. 1996. Comparison of the bile salts and sodium dodecyl sulfate stress responses in Enterococcus faecalis. Appl. Environ. Microbiol. 62:2416-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glynn, J. R., Jr., B. M. Belongia, R. G. Arnold, K. L. Ogden, and J. C. Baygents. 1998. Capillary electrophoresis measurements of electrophoretic mobility for colloidal particles of biological interest. Appl. Environ. Microbiol. 64:2572-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hancock, L. E., and M. Perego. 2004. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 186:5629-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hufnagel, M., S. Koch, R. Creti, L. Baldassarri, and J. Huebner. 2004. A putative sugar-binding transcriptional regulator in a novel gene locus in Enterococcus faecalis contributes to production of biofilm and prolonged bacteremia in mice. J. Infect. Dis. 189:420-430. [DOI] [PubMed] [Google Scholar]
- 13.Kanemitsu, K., T. Nishino, H. Kunishima, N. Okamura, H. Takemura, H. Yamamoto, and M. Kaku. 2001. Quantitative determination of gelatinase activity among enterococci. J. Microbiol. Methods 47:11-16. [DOI] [PubMed] [Google Scholar]
- 14.Kirby, B. J., and E. E. F. Hasselbrink. 2004. Zeta potential of microfluidic substrates: 2. Data for polymers. Electrophoresis 25:203-213. [DOI] [PubMed] [Google Scholar]
- 15.Kristich, C. J., Y.-H. Li, D. G. Cvitkovitch, and G. M. Dunny. 2004. Esp-independent biofilm formation by Enterococcus faecalis. J. Bacteriol. 186:154-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luner, P. E. 2000. Wetting properties of bile salt solutions and dissolution media. J. Pharm. Sci. 89:382-395. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed, J. A., W. Huang, S. R. Nallapareddy, F. Teng, and B. E. Murray. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillai, S. K., G. Sakoulas, G. Eliopoulus, R. C. Moellering, B. E. Murray, and R. T. Inouye. 2004. Effects of glucose on fsr-mediated biofilm formation in Enterococcus faecalis. J. Infect. Dis. 190:967-970. [DOI] [PubMed] [Google Scholar]
- 19.Sandoe, J. A. T., I. R. Witherden, J. H. Cove, J. Heritage, and M. H. Wilcox. 2003. Correlation between enterococcal biofilm formation in vitro and medical-device-related infection potential in vivo. J. Med. Microbiol. 52:547-550. [DOI] [PubMed] [Google Scholar]
- 20.Shankar, V., A. S. Baghdayan, M. M. Huycke, G. Lindahl, and M. S. Gilmore. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 67:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streger, S. H., S. Vainberg, H. Dong, and P. B. Hatzinger. 2002. Enhancing transport of Hydrogenophaga flava ENV735 for bioaugmentation of aquifers contaminated with methyl tert-butyl ether. Appl. Environ. Microbiol. 68:5571-5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tendolkar, P. M., A. S. Baghdayan, M. S. Gilmore, and N. Shankar. 2004. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 72:6032-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toledo-Arana, A., J. Valle, C. Solano, M. J. Arrizubieta, C. Cucarella, M. Lamata, B. Amorena, J. Leiva, J. R. Penadés, and I. Lasa. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67:4538-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Mei, H. C., and H. J. Busscher. 2001. Electrophoretic mobility distributions of single-strain microbial populations. Appl. Environ. Microbiol. 67:491-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Merode, A. E. J., H. C. van der Mei, H. J. Busscher, K. Waar, and B. P. Krom. 2006. Enterococcus faecalis strains show culture heterogeneity in cell surface charge. Microbiology 152:807-814. [DOI] [PubMed] [Google Scholar]
- 26.Van Oss, C. J. 2003. Long-range and short-range mechanisms of hydrophobic attraction and hydrophilic repulsion in specific and aspecific interactions. J. Mol. Recognit. 16:177-190. [DOI] [PubMed] [Google Scholar]
- 27.Waar, K., H. C. van der Mei, H. J. M. Harmsen, J. E. Degener, and H. J. Busscher. 2002. Enterococcus faecalis surface proteins determine its adhesion mechanism to bile drain materials. Microbiology 148:1863-1870. [DOI] [PubMed] [Google Scholar]
- 28.Wilson, W. W., M. M. Wade, S. C. Holman, and F. R. Champlin. 2001. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 43:153-164. [DOI] [PubMed] [Google Scholar]