Abstract
Relapsing-fever spirochetes achieve high cell densities (>108/ml) in their host's blood, while Lyme disease spirochetes do not (<105/ml). This striking contrast in pathogenicity of these two groups of bacteria suggests a fundamental difference in their ability to either exploit or survive in blood. Borrelia hermsii, a tick-borne relapsing-fever spirochete, contains orthologs to glpQ and glpT, genes that encode glycerophosphodiester phosphodiesterase (GlpQ) and glycerol-3-phosphate transporter (GlpT), respectively. In other bacteria, GlpQ hydrolyzes deacylated phospholipids to glycerol-3-phosphate (G3P) while GlpT transports G3P into the cytoplasm. Enzyme assays on 17 isolates of borreliae demonstrated GlpQ activity in relapsing-fever spirochetes but not in Lyme disease spirochetes. Southern blots demonstrated glpQ and glpT in all relapsing-fever spirochetes but not in the Lyme disease group. A Lyme disease spirochete, Borrelia burgdorferi, that was transformed with a shuttle vector containing glpTQ from B. hermsii produced active enzyme, which demonstrated the association of glpQ with the hydrolysis of phospholipids. Sequence analysis of B. hermsii identified glpF, glpK, and glpA, which encode the glycerol facilitator, glycerol kinase, and glycerol-3-phosphate dehydrogenase, respectively, all of which are present in B. burgdorferi. All spirochetes examined had gpsA, which encodes the enzyme that reduces dihydroxyacetone phosphate (DHAP) to G3P. Consequently, three pathways for the acquisition of G3P exist among borreliae: (i) hydrolysis of deacylated phospholipids, (ii) reduction of DHAP, and (iii) uptake and phosphorylation of glycerol. The unique ability of relapsing-fever spirochetes to hydrolyze phospholipids may contribute to their higher cell densities in blood than those of Lyme disease spirochetes.
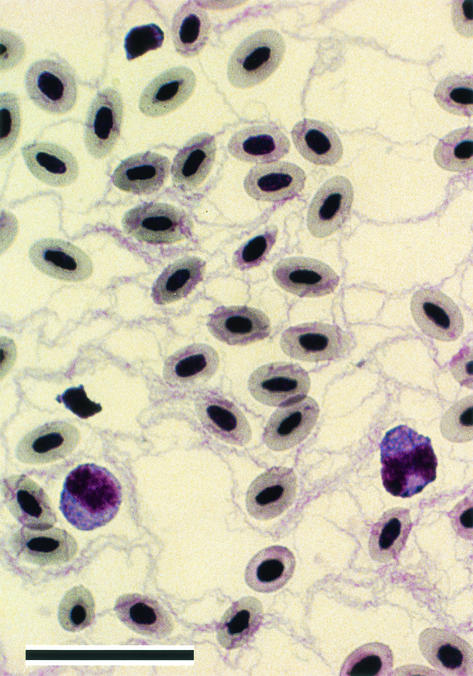
Pathogenic spirochetes belonging to the genus Borrelia are defined in part by their obligatory biological transmission by blood-feeding arthropods (6). These bacteria cause a variety of diseases in humans and other animals, are transmitted by numerous species of ticks or the human body louse, and are widely distributed around much of the world (12). Most of the species fall into one of two major groups related either to the relapsing-fever or Lyme disease spirochetes (28, 31). Relapsing-fever spirochetes cause a recurrent, febrile illness associated with pronounced bacteremias and are transmitted by fast-feeding argasid ticks of the genus Ornithodoros (1, 9). Lyme disease spirochetes cause a wide range of symptoms and are transmitted by the slow-feeding ixodid ticks of the genus Ixodes (19). A striking difference between the two groups of spirochetes is the cell density attained by the different groups in the peripheral blood. Relapsing-fever spirochetes and the related agent of fowl spirochetosis, Borrelia anserina, can achieve cell densities of 108 or more bacteria per ml of blood, making them easily detectable by light microscopy (Fig. 1). Lyme disease spirochetes also infect the peripheral blood and may be cultured from this tissue (3, 24, 43, 49); however, no reports demonstrate that these spirochetes attain cell densities high enough to allow their direct microscopic detection. This discrepancy in the levels of spirochetemia between relapsing-fever and Lyme disease spirochetes suggests a fundamental difference in the way these two groups of bacteria exploit or survive in the blood.
FIG. 1.
Spirochetemia of B. anserina with a very high cell density in the blood of a 12-day-old chicken 6 days after i.p. inoculation. The spirochetes far outnumber the nucleated red blood cells. Scale bar, 25 μm.
During the last 6 years, we have investigated a highly immunogenic protein in relapsing-fever spirochetes that stimulates a strong antibody response in patients having had tick-borne or louse-borne relapsing fever (27, 34). The bacterial gene encoding this protein, glycerophosphodiester phosphodiesterase (GlpQ), was first identified in Escherichia coli and was shown to hydrolyze deacylated phospholipids to an alcohol and glycerol-3-phosphate (G3P) (20). Our initial work also demonstrated that people who have had Lyme disease do not react serologically to this protein, and we were unable to find evidence by immunoblotting, PCR, or Southern blotting for its presence in the Lyme disease spirochete, Borrelia burgdorferi (34). The lack of glpQ in B. burgdorferi was confirmed when no ortholog to this gene was found in its genome (8, 13).
The presence or absence of a glpQ ortholog in spirochetes does not establish their ability to hydrolyze phospholipids. Therefore, in the present work we investigated numerous species of Borrelia for GlpQ activity and examined the glp locus at the DNA level. Our hypothesis is that GlpQ permits only the relapsing-fever group of spirochetes to acquire G3P from phospholipids. This metabolic advantage may contribute, in part, to their ability to achieve higher cell densities in the blood than do the Lyme disease spirochetes. The work we describe here is the first step toward testing this hypothesis.
MATERIALS AND METHODS
Borrelia strains and cultivation.
A total of 17 Borrelia isolates were studied, including 4 isolates of B. hermsii and single isolates of 13 other species (Table 1). These isolates originated primarily from ticks or humans from North America, Eurasia, and Africa. Borrelia crocidurae was provided by Sven Bergström, Umeå University, Umeå, Sweden. Borrelia miyamotoi was provided by Barbara Johnson, Centers for Disease Control and Prevention, Fort Collins, Colo. All spirochetes were cultured in modified Kelly's medium (complete BSK-H) (Sigma Chemical Co., Melville, N.Y.).
TABLE 1.
Borrelia species and isolates used in this study, their host source, and geographic origin
| Species and strain | Source
|
|
|---|---|---|
| Host | Location | |
| B. hermsii HS1 | Ornithodoros hermsi | Washington |
| B. hermsii DAH | Human | Washington |
| B. hermsii FRO | Human | Washington |
| B. hermsii YOR | Human | California |
| B. parkeri RML | Ornithodoros parkeri | Unknown |
| B. turicatae 91E135 | Ornithodoros turicata | Texas |
| B. crocidurae CR2A | Ornithodoros erraticus | Unknown |
| B. anserina BA2 | chicken | Unknown |
| B. coriaceae CO53 | Ornithodoros coriaceus | California |
| B. recurrentis 132 | Human | Sudan |
| B. miyamotoi FR 64b | Apodemus argenteus | Hokkaido, Japan |
| B. burgdorferi B31 | Ixodes scapularis | New York |
| B. garinii G2 | Human | Germany |
| B. afzelii VS 461 | Ixodes ricinis | Switzerland |
| B. bissettii DN127 | Ixodes pacificus | California |
| B. valaisiana VS116 | Ixodes ricinis | Switzerland |
| B. japonica HO14 | Ixodes ovatus | Hokkaido, Japan |
Glycerophosphodiester phosphodiesterase assay.
GlpQ activity in borrelia lysates was measured in a coupled spectrophotometric assay as described (4, 20, 37). Briefly, each Borrelia species was grown in 50 ml of medium to cell densities of 5.5 × 106 to 4.5 × 107 per ml as determined by microscopy and optical density at 600 nm (A600). The spirochetes were pelleted by centrifugation, suspended in phosphate-buffered saline (PBS), pelleted again, and suspended in cold assay buffer (1 M hydrazine hydrate with 1.5% glycine [pH 9.0]). The cells were lysed on ice by sonication with a Branson sonifier-cell disrupter 185 (VWR Scientific, San Francisco, Calif.). Each assay was done at 25°C in a 1-ml reaction mixture containing 10 mM CaCl2, 0.5 mM NAD, 0.5 mM glycerophosphorylcholine, 20 U of glycerol-3-phosphate dehydrogenase (G3PDH) (all from Sigma Chemical Co.), and various concentrations of borrelia lysate. Each borrelia lysate was tested three times, and each species was tested twice with a second 50-ml culture. Subcellular fractions of B. hermsii DAH were also prepared from duplicate cultures as described previously (7) and assayed for GlpQ activity. Positive control assays were run with glycerophosphorylcholine phosphodiesterase (Sigma Chemical Co.) in place of the borrelia lysate, and assays without G3PDH or substrate were done for negative controls. The reduction of NAD to NADH was measured by monitoring the increase in A340 with an Ultrospec 4000 spectrophotometer (Amersham Biosciences, Inc., Piscataway, N.J.). Absorbance was recorded for 10 to 15 min and the ΔA340 min−1 was determined. One unit of GlpQ activity is defined as the amount of enzyme that will hydrolyze 1 μmol of glycerophosphorylcholine min−1 as assessed by the reduction of NAD. Protein concentration was determined by the Bradford assay (Bio-Rad Laboratories, Hercules, Calif.)
DNA sequence analysis of the glp locus.
Our first report of GlpQ in B. hermsii included partial DNA sequence of the glp locus, which includes glpT and glpA flanking glpQ upstream and downstream, respectively (34). We used that same DNA library of B. hermsii DAH in E. coli from which clone pSPR75 containing the partial glp locus originated (34) and examined additional clones for DNA sequences that were part of this locus. DNA sequences were obtained (described below) from E. coli clones that contained overlapping segments of B. hermsii DNA and completed the contiguous glp locus.
The glp locus in B. burgdorferi B31 lacks glpT and glpQ upstream of glpA (13). In their place is a small open reading frame (ORF) of 276 bp, BB0242, that has no DNA sequence similarity to anything reported. We examined this region of the glp locus in the six species of B. burgdorferi sensu lato that we examined for GlpQ activity. We first used PCR to amplify the region from within BB0241 (glpK) through BB0242 and into BB0243 (glpA). The primers originating in glpK and glpA, respectively were BBK and BBD (Table 2) (Life Technologies, Baltimore, Md.). The predicted size of this fragment was 1,830 bp, based on the genomic sequence published for B. burgdorferi B31 (13). Total genomic DNA was purified from 500-ml cultures as described previously (38), quantified by UV spectrophotometry, and diluted to 0.05 μg for each 50-μl reaction mixture. Taq polymerase, deoxynucleoside triphosphates, and buffer were used as recommended by the manufacturer (Perkin-Elmer, Roche Molecular Systems, Inc., Branchburg, N.J.). PCR amplification began with heating at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 30 s, and extension at 72°C for 3 min. After the 35th cycle, an additional 7-min extension was done at 72°C.
TABLE 2.
Primers used for PCR and DNA sequencing
| Primer | Gene | Sequence (5′ to 3′) |
|---|---|---|
| BBK | BB0242 | AATGGTGGAGTTTATTTTGTGCCAGC |
| BBD | BB0242 | GGTTCTTCTTCTATCTCTTTTATTGGAATGTCAG |
| BBK + 1 | BB0242 | TCAATGCCAAAAAATCAAAAAG |
| BBK + 2 | BB0242 | AAAATTAAAACAAATGGTCTTTTCG |
| BBD + 1 | BB0242 | TGTTATTGCCAT(C/T)CTAGCATCATC |
| BBD + 2 | BB0242 | TACCGCAATGCCAAGACCTG |
| Bh GlpQ-1 | glpQ | AAGGTTAATAAATTATGT |
| Bh GlpQ-2 | glpQ | CTATGGTTTTATTTTTGT |
| GlpQ int 5′ | glpQ | TTATAGCTCACAGAGGT |
| GlpQ int 3′ | glpQ | ATTTGGGGTATCCAAGGT |
| GlpT int 5′ | glpT | ATAATTGCAGCAATATTA |
| GlpT int 3′ | glpT | CAAGATCAAGAGCATGAAGA |
| GPS1 | gpsA | TTACTTATATTGTTGGTCCA |
| GPS2 | gpsA | CTCCCTCTGGTAAATATCCA |
| GLPTQ-1 | glpTQ | TCTAGACCTTCAATGGAAAAAAGTAAAAGAGAAG |
| GLPTQ-2 | glpTQ | GGATCCGGCTATGGTTTTATTTTTGTGATGAAATTC |
PCR amplification products were visualized in agarose gels stained with ethidium bromide. Reaction mixtures that contained a single amplicon of the predicted size were processed with a PCR purification kit (Qiagen Inc., Valencia, Calif.). DNA sequencing reactions were performed with an ABI PRISM Dye Terminator cycle-sequencing ready reaction mix (Applied Biosystems, Inc., Foster City, Calif.) with slight modifications of the manufacturer's protocol to reduce the final volume to 15 μl. The mixtures were run for 45 cycles with a PTC-225 DNA Engine Tetrad thermal cycler (MJ Research, Inc., Waltham, Mass.). DNA sequences were determined with a model 3700 automated DNA sequencer (Applied Biosystems Inc.). Six primers were used to complete the double-stranded sequence of the approximately 1,830-bp amplification products (Table 2). Primers BBK, BBK+1, and BBK+2 generated the sequence for one strand, and primers BBD, BBD+1, and BBD+2 generated the sequence for the second strand. Nucleotide and deduced amino acid sequences were analyzed with Sequencher 4.1 (Gene Codes Corp., Ann Arbor, Mich.) and MacVector 6.0 software packages (Oxford Molecular, Beaverton, Oreg.). Alignments were constructed with the ClustalV program in the Lasergene (DNASTAR) software package and transferred into the PHYLIP-Phylogeny Inference Package (J. Felsenstein, PHYLIP-Phylogeny Inference Package, version 3.57c, Department of Genetics, University of Washington, Seattle, Wash.).
Southern blot analysis.
Genomic DNA samples from the 17 borreliae (Table 1) were examined for glpQ, glpT, and gpsA by Southern's method (41). DNA was digested with EcoRI at 37°C for 24 h, and the restriction fragments were separated by electrophoresis in a 0.75% agarose gel with TBE buffer (90 mM Tris, 90 mM boric acid, 20 mM EDTA). The DNA was stained in the gel with ethidium bromide and visualized by UV transillumination. The gel-bound DNA was depurinated in 0.25 N HCl for 10 min, denatured in 1.5 M NaCl-0.5 M NaOH for 40 min, and neutralized in 0.5 M Tris (pH 7.5)-1.5 M NaCl for 40 min. DNA was transferred overnight by capillary action onto MagnaGraph nylon membranes (Micron Separations Inc., Westborough, Mass.) with 20× SSC (1× SSC is 0.15 M NaCl and 15 mM sodium citrate [pH 7]) and cross-linked to the membrane with a UV Stratalinker 1800 (Stratagene, La Jolla, Calif.). The membranes were prehybridized at 65°C for 6 h in 60 ml of 5× SSC-0.1% (wt/vol) N-lauroylsarcosine sodium salt-0.02% sodium dodecyl sulfate (SDS)-6% blocking reagent (Roche Applied Science, Indianapolis, Ind.).
Hybridization probes were produced with the PCR DIG probe synthesis kit (Roche Applied Science) as specified by the manufacturer. Primers for glpQ (GlpQ int 5′ and GlpQ int 3′), glpT (GlpT int 5′ and GlpT int 3′), and gpsA (GPS1 and GPS2) (Table 2) amplified DNA fragments of 706, 778, and 504 bp, respectively. Genomic DNA of B. hermsii DAH was used as the template to produce probes for glpQ and glpT, while DNA of B. burgdorferi B31 was used to produce the gpsA probe. PCR for all probes included an initial denaturation at 96°C for 3 min, 35 cycles of 94°C for 30 s, 55 to 56°C for 30 s, and 72°C for 2.5 min, and a final extension at 72°C for 7 min. The digoxigenin-labeled probes were denatured at 98°C for 10 min, added to 6 ml of fresh hybridization buffer with the membrane, and incubated at 55°C for 18 h. The membranes were washed with 2× SSC-0.1% SDS for 10 min at room temperature (RT) and 0.5× SSC-0.1% SDS for 30 min at 65°C, which lowered the stringency suggested by the manufacturer. The blots were incubated with anti-digoxigenin antibody conjugated to alkaline phosphatase and developed with the CDP-Star chemiluminescent substrate (both from Roche Applied Science). Hyperfilm ECL high-performance chemiluminescence film (Amersham Biosciences, Inc.) was exposed to membranes and developed to display the pattern of hybridization. Several films were exposed with increased time to each blot to confirm the results observed with the shorter exposures and to increase the sensitivity to detect less similar sequences.
Transformation of B. burgdorferi with glpTQ from B. hermsii.
High-passage, noninfectious B. burgdorferi B31-A was transformed with the shuttle vector pBSV2 (44), which contained glpT and glpQ from B. hermsii. Three attempts failed to transform low-passage, infectious spirochetes. The Expand PCR System (Roche, Indianapolis, Ind.) was used as specified by the manufacturer to amplify a 2,668-bp fragment from B. hermsii DAH that contained glpT and glpQ. Primers GLPTQ-1 and GLPTQ-2 (Table 2) for this amplification had restriction sites for XbaI and BamHI incorporated into them. The product contained 169 bp upstream of the translational start codon of glpT. The DNA fragment was cloned into pCR2.1-TOPO (Invitrogen, Carlsbad, Calif.) as specified by the manufacturer. The integrity of the construct was verified by restriction fragment length polymorphism, PCR, and DNA sequence analysis with primers GLPTQ-1, GLPTQ-2, GlpQ int 5′, GlpQ int 3′, GlpT int 5′, and GlpT int 3′ (Table 2). The plasmid was purified and transformed into B. burgdorferi B31-A as described previously (30, 44). Kanamycin-resistant colonies were screened by PCR for the presence of pBSV2 (44). Two transformants with glpTQ were randomly selected (transformants 21 and 25), and one transformant with only the shuttle vector was analyzed for the presence and activity of GlpQ. This work was approved by the Rocky Mountain Laboratories Biosafety Committee.
Gel electrophoresis and immunoblot analysis.
Whole-cell lysates of spirochetes were prepared as described previously (33). Proteins were separated by one-dimensional SDS-polyacrylamide gel electrophoresis (PAGE) with Laemmli buffer (18) and a vertical gel apparatus (Bethesda Research Laboratories-GIBCO, Gaithersburg, Md.). Proteins were blotted onto nitrocellulose membranes with Towbin buffer (46) and a Trans-Blot Cell (Bio-Rad Laboratories). The membranes were blocked overnight at RT with TSE-Tween (50 mM Tris [pH 7.4], 150 mM NaCl, 5 mM EDTA, 0.05% Tween 20) and incubated with rabbit anti-GlpQ antiserum diluted 1:500 (34). Bound antibodies were detected by 125I-labeled protein A autoradiography (33).
Synthesis and purification of recombinant GlpQ for immunization.
The glpQ gene of B. hermsii DAH was amplified by PCR using primers Bh GlpQ-1 and Bh GlpQ-2 (Table 2) and cloned into the pCRII vector as described previously (34). E. coli colonies were screened by PCR, and plasmid DNA from a positive clone was purified with a miniprep kit (Qiagen, Inc.) and digested with EcoRI. The restricted DNA fragment was purified with the QiaexII Gel Purification Kit (Qiagen, Inc.) and quantified by UV spectrophotometry. The pET-32a vector (Novagen Inc., Madison, Wis.) was digested with EcoRI and purified with the Edge BioSystems Quick Step cleanup kit (Edge BioSystems, Gaithersburg, Md.), quantified by UV spectrophotometry, treated with HK phosphatase (Epicentre, Madison, Wis.) for 1 h at 30°C, and heat inactivated at 65°C for 15 min. The B. hermsii glpQ PCR fragment was ligated into the pET-32a vector and transformed into E. coli XL1-Blue cells (Stratagene, La Jolla, Calif.) by electroporation and grown on Luria-Bertani broth plates with ampicillin (100 μg/ml). A single recombinant clone was collected and examined by PCR using primers Bh GlpQ-1 and Bh GlpQ-2. Vector DNA was purified from this recombinant clone with the miniprep kit, quantified, and transformed into chemically competent E. coli BLR(DE3) cells. A single recombinant was examined by PCR with the Bh GlpQ-1 and Bh GlpQ-2 primers and used for synthesis of the GlpQ fusion protein.
The His-GlpQ fusion protein was purified from E. coli cells following growth in Luria-Bertani broth with 100 μg of carbenicillin per ml, using the procedures described in the pET System Manual (Novagen). Cells were lysed by sonication, and the soluble protein fraction was passed through pre-charged Ni2+ Quick Columns provided in the HIS-Bind purification kit (Novagen), following as specified by the manufacturer, to separate the His-GlpQ fusion protein. The eluted sample was dialyzed with PBS at 4°C for 24 h in a Slide-A-Lyzer dialysis cassette (Pierce, Rockford, Ill.) to remove the salts and imidazole and examined by SDS-PAGE for purity, and the protein concentration was determined with the Bradford assay (Bio-Rad Laboratories). The enzymatic activity of the purified fusion protein was examined in triplicate as described above.
GlpQ immunization and challenge with B. hermsii.
Eight adult male BALB/c mice (Rocky Mountain Laboratories Animal Facility) were bled by cardiac puncture while anesthetized with isofluorane to obtain preimmunization serum samples. The next day, four mice were immunized intraperitoneally (i.p.) with 290 μg purified His-GlpQ protein in 0.5 ml of PBS-0.5 ml Ribi Adjuvant R-700 (Corixa, Hamilton, Mont.). Four mice were inoculated with only adjuvant. Twenty-four days later, the mice were boosted with His-GlpQ plus adjuvant or adjuvant only. Fourteen days later, blood was collected from each mouse and examined with the preimmunization samples by immunoblotting and enzyme-linked immunosorbent assay for reactivity with His-GlpQ. Only the serum samples from the GlpQ-immunized mice had reactivity with His-GlpQ. The enzyme-linked immunosorbent assay was performed as described previously (27), except that mouse rather than human serum samples were tested and the secondary antibody was goat anti-mouse immunoglobulin G (heavy and light chains) conjugated to horseradish peroxidase (Kirkegaard & Perry, Gaithersburg, Md.). Twenty-nine days after the boost, the mice were challenged with B. hermsii DAH by i.p. inoculation with infected mouse blood. Each mouse received approximately 2.5 × 106 spirochetes and was examined for spirochetemia daily for 14 days. For this, each mouse was anesthetized with isofluorane, the tip of the tail was nicked, and a drop of blood was expressed from the tail vein onto a glass microscope slide. A 2.5-μl volume of blood was transferred from the drop onto another glass slide lying over a circular template and spread with a toothpick to cover an area of 144 mm2. The blood was dried at RT, stained with Giemsa, and examined under a bright-field microscope (Nikon Eclipse 800) at ×600 magnification with a 60× oil immersion objective (area per field = 0.126 mm2). The spirochetes in 10 fields were counted, and the number of organisms per milliliter of mouse blood was estimated. The mice were bled 60 days after challenge, and their anti-B. hermsii titer was determined by an indirect fluorescent-antibody test with B. hermsii cells as antigen.
Electron microscopy.
B. hermsii whole cells were prepared for negative staining as described previously (10) and examined with a Philips CM10 transmission electron microscope (FEI, Hillsboro, Oreg.). Thin sections of B. hermsii cells were prepared for labeling as follows. Spirochete cell pellets were washed either with 0.1 M Tris buffer to permeabilize the outer membrane or with Hanks' balanced salt solution to keep the outer membrane intact. The cells were lightly fixed with 4% paraformaldehyde in 0.1 M sodium cacodylate buffer for 20 min and incubated overnight at 4°C with either mouse anti-GlpQ or mouse anti-Vsp33 antiserum. The samples were washed with 0.1 M sodium cacodylate buffer and incubated for 1 h with secondary Nanogold-conjugated antibody reagents (Nanoprobes, Inc., Stony Brook, N.Y.). Cells were washed with distilled H2O and further fixed with 2.5% glutaraldehyde-4% paraformaldehyde in 0.1 M sodium cacodylate buffer for 1 h. Samples were washed three times for 5 min each, with distilled H2O, and the Nanogold was enhanced with silver for 4 min with HQ silver reagents (Nanoprobes, Inc.). Samples were fixed further with 1.0% osmium tetroxide-0.8% potassium ferrocyanide in 0.1 M sodium cacodylate, dehydrated with a graded ethanol series, embedded in Spurr's resin, sectioned with an RMC MT-7000 ultramicrotome (Boeckeler, Tucson, Ariz.), and stained with 1% uranyl acetate and Reynold's lead citrate. The samples were viewed as described above for negatively stained spirochetes.
Inoculations of other species of Borrelia into mice and chickens.
B. burgdorferi and B. miyamotoi were inoculated into mice to determine if these bacteria could produce spirochetemias of sufficient density to be detectable by microscopy. Cultures of B. burgdorferi B31 (passage 5 after isolation from laboratory-infected Ixodes scapularis) and B. miyamotoi FR64b (low passage, exact history unknown) were each inoculated i.p. into two adult male BALB/c mice (RML). Blood from these mice was examined for 10 days postinoculation as described above for mice infected with B. hermsii. Infection of mice with B. burgdorferi was confirmed 23 days postinoculation by xenodiagnosis and isolation of spirochetes from the urinary bladder (32). B. anserina LSP, (BSK culture, passage 13) was inoculated i.p. into three 6-day-old Red Broiler chickens (Phinney Hatchery, Walla Walla, Wash.), and blood was collected daily from the wing vein of each bird to monitor spirochetemia. Thin smears of blood were made on glass microscope slides, dried at RT, fixed with methanol, immersed in Wright's stain for 30 min, rinsed with distilled H2O, dried, and examined under a bright-field microscope as described above.
Nucleotide sequence accession numbers.
DNA sequences for B. hermsii have the following GenBank accession numbers: glpF, AF506979; glpK, AF506980; glpT, AF506981; glpA, AF506982; gpsA, AF506983. The DNA sequence for glpQ was deposited previously (U40762) (34). DNA sequences for BB0242 have the following accession numbers: B. burgdorferi, AF509485; B. afzelii, AF509486; B. valaisiana, AF509487, B. bissettii, AF509488, B. garinii, AF509489; B. japonica, AF509490.
RESULTS
Glycerophosphodiester phosphodiesterase activity.
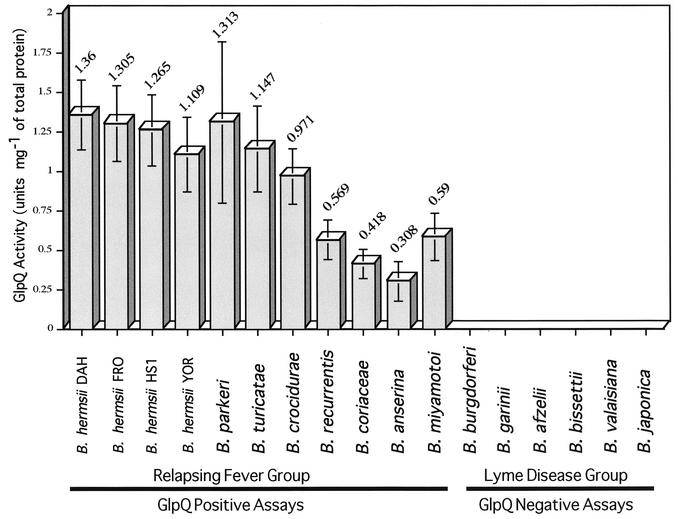
Whole-cell extracts of four strains of B. hermsii and single strains of 13 other species of Borrelia were assayed for GlpQ enzyme activity. Duplicate cultures and three different volumes of cell extracts of each culture were tested, which resulted in six assays for each strain (Fig. 2). The eight isolates of relapsing-fever spirochetes, B. anserina, B. coriaceae, and B. miyamotoi, had enzyme activity; however, no activity was observed with the six species of B. burgdorferi sensu lato.
FIG. 2.
GlpQ-specific activity in Borrelia species. Each assay was performed six times, and the average activity is shown above each bar. Vertical lines represent one standard deviation above and below the mean activity. Only spirochetes in the relapsing-fever group had specific activity.
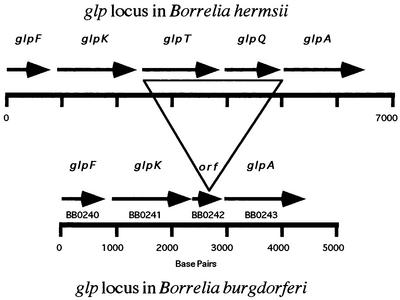
DNA sequence analysis of the glp locus.
DNA sequence analysis of the glp locus in B. hermsii identified five contiguous ORFs homologous to genes in E. coli and other bacteria that are involved with glycerol and G3P transport and metabolism: glpF (glycerol facilitator), glpK (glycerol kinase), glpT (G3P transporter), glpQ, and glpA (G3PDH) (Fig. 3) (20, 21). An additional clone contained the complete gene for G3P synthase (gpsA). The glp locus in B. burgdorferi B31 contains glpF (BB0240), glpK (BB0241), and glpA (BB0243) in the same relative positions but lacks glpT and glpQ (Fig. 3) (13). In their place between glpK and glpA is a small ORF, BB0242, that has no sequence similarity to anything deposited in GenBank (13). We examined this part of the glp locus in B. burgdorferi B31 and five other species in the B. burgdorferi sensu lato complex. glpK and glpA were present in all six species, but the region between these genes varied from 254 to 406 bp. All species contained an ORF between glpK and glpA that was homologous to BB0242 but whose product varied in size from 41 to 92 amino acids, the latter for B. burgdorferi B31, which was identical to that published previously for this strain (13). In the other species, this ORF was smaller and in some cases contained major deletions. In B. garinii and B. japonica, the coding capacity of BB0242 was only 42 and 41 amino acids, respectively. None of the six species contained DNA sequences in this region of the glp locus that were similar to glpT or glpQ of B. hermsii.
FIG. 3.
Comparison of the glp (glycerol transport and metabolism) locus in B. hermsii and B. burgdorferi. Both species contain glpF (glycerol facilitator), glpK (glycerol kinase), and glpA (G3PDH). B. hermsii has glpQ (glycerophosphodiester phosphodiesterase) and glpT (G3P transporter). These genes are replaced in B. burgdorferi with a small ORF (BB0242) that matches nothing in the database. These results suggest that B. hermsii, but not B. burgdorferi, has the potential to acquire G3P through the catabolism of deacylated phospholipids and to use this G3P in glycolysis and the synthesis of new phospholipids.
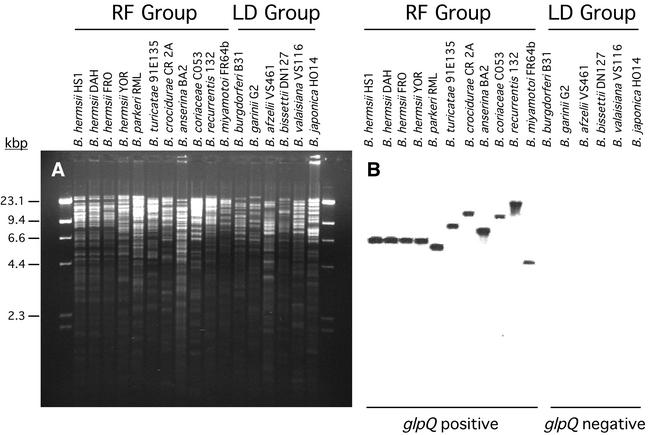
Southern blot analysis.
DNA samples from 17 borreliae were analyzed for glpQ, glpT, and gpsA. Spirochetes that had GlpQ enzymatic activity bound probes to glpQ (Fig. 4) and glpT (data not shown). Both of these probes bound to the same restriction fragment, which was anticipated from the sequence data for B. hermsii. None of the DNA from the six isolates of B. burgdorferi sensu lato hybridized to these probes, which supported the notion that glpQ and glpT were absent from their entire genome and not only from the glp locus examined above. All the borreliae hybridized with the gpsA probe (data not shown), which supported the universal presence of this gene and the potential for all these borreliae to produce G3P from dihydroxyacetone phosphate (DHAP).
FIG. 4.
Distribution of glpQ among Borrelia species demonstrated by Southern blot analysis. (A) Agarose gel with genomic DNA digested with EcoRI and stained with ethidium bromide. (B) Hybridization pattern with the glpQ probe, which was identical to the hybridization pattern with the glpT probe (data not shown). Molecular size standards in kilobase pairs are on the left. The relapsing-fever (RF) spirochetes were positive with both probes, while the Lyme disease (LD) group of spirochetes were negative. All samples hybridized with the gpsA probe (data not shown).
Heterologous expression of glpQ in B. burgdorferi.
We demonstrated by DNA sequence analysis, Southern blotting, and enzyme assays that the presence of glpQ in the relapsing-fever group of spirochetes correlated with our biochemical data. However, these analyses do not prove that glpQ is responsible for the hydrolysis of phospholipids. To definitively establish that glpQ of the relapsing-fever spirochetes encodes this function, we cloned the gene into B. burgdorferi, one of the species that lacks GlpQ activity, and assayed again for activity. We analyzed two clones of B. burgdorferi that were transformed with the shuttle vector pBSV2 containing glpTQ from B. hermsii. Double-stranded DNA sequence analysis of PCR products confirmed the presence and integrity of the genes in the transformants (data not shown). SDS-PAGE and immunoblot analysis demonstrated the synthesis of GlpQ by both transformants but not by B. burgdorferi that contained the shuttle vector without the glpTQ insert (Fig. 5). We could not confirm the synthesis of GlpT in the transformants because we were unable to produce specific antiserum to this protein. We failed three times to produce GlpT as a purified His fusion protein in E. coli that we could use to immunize rabbits to produce a specific antiserum. Whole-cell lysates of the two B. burgdorferi transformants with glpTQ had GlpQ activity (2.81 and 2.62 U/mg), whereas B. burgdorferi with only the shuttle vector did not. The GlpQ activity of the transformed B. burgdorferi was higher than the activities measured for B. hermsii and other species, possibly because of a higher copy number of plasmid-encoded glpTQ in the transformants. These results demonstrated that although wild-type B. burgdorferi normally lacks GlpQ and corresponding activity, this species is capable of synthesizing enzymatically active protein when transformed with glpTQ from B. hermsii. These results, in association with enzyme activity of the purified recombinant protein (see below), also verified that glpQ from B. hermsii was responsible for the hydrolysis of phospholipids.
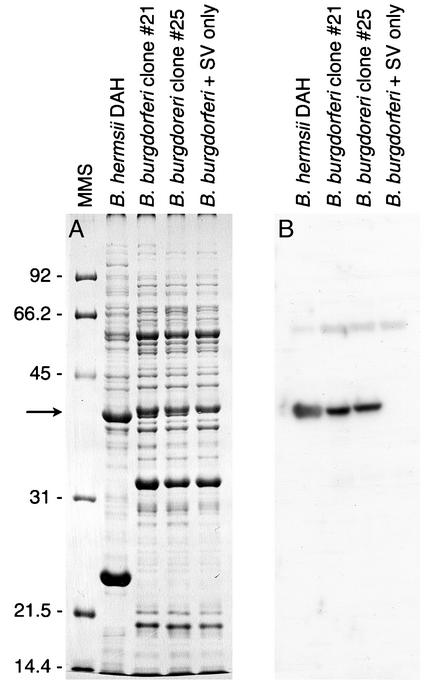
FIG. 5.
Immunoblot analysis of B. burgdorferi transformed with the shuttle vector containing glpTQ from B. hermsii. (A) SDS-PAGE analysis of whole-cell lysates of B. hermsii, two transformed clones (clones 21 and 25) of B. burgdorferi (glpTQ+), and B. burgdorferi with only the shuttle vector (SV) (glpTQ−). Proteins were stained with Coomassie blue, and molecular mass standards (MMS) are shown at the left in kilodaltons. (B) Immunoblot analysis with rabbit anti-GlpQ antibody. B. hermsii and the glpTQ-transformed clones of B. burgdorferi produced GlpQ (arrow), while B. burgdorferi with only the shuttle vector did not.
Cellular localization of GlpQ in B. hermsii.
Immunogold labeling and negative staining of intact spirochetes detected Vsp33, but not GlpQ, on the outer surface of B. hermsii (Fig. 6A and B). Additionally, GlpQ was not detected when the outer membrane of the cell was kept intact prior to labeling and analysis of thin sections (Fig. 6C). In contrast, when the outer membrane was disrupted from the protoplasmic cylinder, GlpQ was labeled intensely (Fig. 6D). The disrupted outer membrane was also labeled with anti-Vsp33 antibody (data not shown). Different cellular fractions of B. hermsii DAH were also assayed for enzyme activity (data not shown). Both the cell lysate and total membrane fractions had threefold greater activity than did the soluble fraction. Together, these results demonstrate that GlpQ is anchored to either the inner or outer membrane but not to the outer surface of the spirochete.
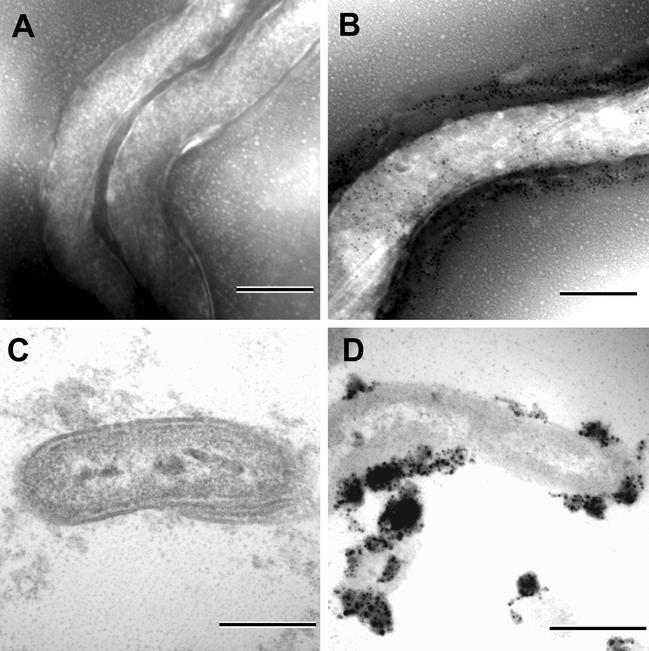
FIG. 6.
Immunogold labeling for GlpQ and Vsp33 in B. hermsii. (A) Negative stain with no GlpQ detected on the outer surface with anti-GlpQ antibody and secondary antibody conjugated to gold particles. (B) Negative stain with Vsp33 detected on the outer surface with anti-Vsp33 antibody. (C) No GlpQ detected in thin sections with the outer membrane intact. (D) GlpQ detected in thin sections when the membranes were disrupted from the protoplasmic cylinder. Scale bars, 0.25 μm.
GlpQ immunization and challenge with B. hermsii in mice.
Four mice immunized with His-GlpQ seroconverted to this fusion protein with ELISA titers of ≥1:102,400. The eight preimmunization serum samples and control samples from four mice receiving only adjuvant were not reactive at a 1:100 dilution. Immunoblot analysis with the immune serum samples (1:100) and whole-cell lysates of B. hermsii showed reactivity and specificity to a single protein consistent with the size of GlpQ (data not shown). These results demonstrated the presence of anti-GlpQ antibody and that the ELISA reactivity with the fusion His-GlpQ protein was not due solely to anti-His antibody in the immune serum samples. The purified fusion protein also had extremely high GlpQ enzymatic activity (60 ± 13 U/mg). Mice were challenged with B. hermsii by needle inoculation and examined daily for 2 weeks. All four mice receiving only adjuvant and three of four mice immunized with His-GlpQ developed detectable spirochetemias after challenge (peaks of 8.1 × 107 to 6.4 × 108 spirochetes per ml of blood), followed by one or two relapses. All eight mice seroconverted with high immunofluorescent-antibody titers (1:512 to 1:4,096) to the entire spirochete after challenge, which does not occur after immunization only with GlpQ (27). These results demonstrated that immunization with His-GlpQ failed to provide protection in mice when they were challenged with needle-inoculated spirochetes.
Microscopic detection of spirochetes in the blood of mice and chickens.
Few if any attempts have been made to visualize spirochetes in the blood other than for species that cause relapsing fever. Therefore, mice were inoculated with cultures of B. burgdorferi or B. miyamotoi and blood from their tail vein was examined for 10 days for the presence of spirochetes. Neither of two mice inoculated with B. burgdorferi had detectable spirochetes, although infection was confirmed by transmission to I. scapularis larvae (xenodiagnosis) and isolation of spirochetes from the urinary bladder. Both mice inoculated with B. miyamotoi had detectable spirochetemias that peaked 6 days postinoculation, with 1.6 × 106 and 7.8 × 106 spirochetes per ml of blood. Three chickens injected with B. anserina were spirochetemic on days 1 through 6 postinoculation, with the densities on the last day becoming too high to count accurately (Fig. 1). The ability of B. miyamotoi and B. anserina to achieve densities in the blood that are detectable by microscopy, and the ability of these organisms to hydrolyze deacylated phospholipids support our hypothesis that these phenomena may be related.
DISCUSSION
Our results demonstrate a fundamental difference among Borrelia species in their pathways to provide G3P, an important intermediate for metabolism and phospholipid synthesis (21, 22) (Fig. 7). Our conclusion is based on enzyme assays, DNA sequence, Southern blot analyses, and heterologous expression data presented here, additional sequence and immunoblot analyses we reported previously (27, 34), and two pregenomic studies on lipid metabolism (23, 26). Therefore, Borrelia species can be separated into two groups based on the presence of GlpQ and GlpT. This division corresponds to the two primary phylogenetic groups based on DNA sequences of the flagellin and 16S rRNA genes (14, 28). We may broadly define the groups as either relapsing-fever spirochetes or Lyme disease spirochetes, although there are members of each group that are not yet known to be pathogenic. Species of the relapsing-fever group have glpQ and glpT, both of which are absent from the Lyme disease species group. Thus, species in the relapsing-fever group can acquire G3P by the hydrolysis of deacylated phospholipids via GlpQ and the transport of G3P across the cytoplasmic membrane via GlpT (Fig. 7, pathway 3), whereas Lyme disease spirochetes lack the genes for both functions.
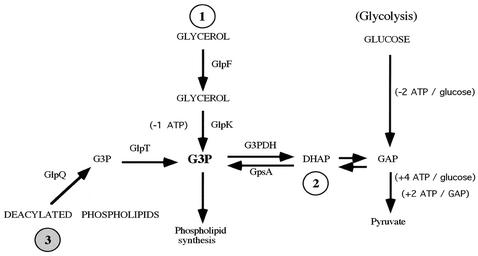
FIG. 7.
The three pathways for the acquisition of G3P in relapsing-fever and Lyme disease spirochetes. Evidence for pathways 1 and 2 is present in both groups of spirochetes, while pathway 3 is restricted to the relapsing-fever spirochetes.
Two other pathways for the acquisition of G3P exist in bacteria (22) (Fig. 7). Glycerol may cross the cytoplasmic membrane, facilitated by GlpF, and be phosphorylated by glycerol kinase, GlpK (Fig. 7, pathway 1). Also, DHAP, an intermediate of glycolysis, may be reduced by glycerol-3-phosphate synthase (GpsA) (Fig. 7, pathway 2). This reaction was supported by Livermore et al., who demonstrated that B. hermsii incorporated [14C]glucose into phosphatidylcholine (23). The genomic sequence of B. burgdorferi B31 identified homologs of glpF, glpK, glpA, and gpsA (13). Our analysis of part of the glp locus demonstrated glpK and glpA in B. burgdorferi and five additional species of the B. burgdorferi sensu lato complex and demonstrated glpF and glpK in B. hermsii. By Southern blot analysis, we also identified homologs of gpsA in all the borreliae examined. Therefore, all species of Borrelia probably have the genetic potential to produce G3P via the phosphorylation of glycerol and by the reduction of DHAP. However, we demonstrate that relapsing-fever spirochetes can acquire G3P through a third pathway that involves the catabolism of deacylated phospholipids.
Our efforts to identify the cellular location of GlpQ have focused on the DAH isolate of B. hermsii. In our earlier analysis, proteinase K digestion of intact spirochetes did not reduce the amount of this protein detectable by immunoblotting (34). This suggested that GlpQ was not exposed on the outer surface. Following our report (34), Shang et al. also described the presence of GlpQ (Gpd) in B. hermsii and identified the protein in preparations of outer membrane vesicles, although they concluded that the exact location of the protein was uncertain (36). The DNA sequences of glpQ from B. hermsii and other species we examined are consistent with a signal peptide and signal peptidase II cleavage site (27, 34, 36). While this is not proven, the sequence data suggest that GlpQ is a lipoprotein (50), possibly anchored to the cytoplasmic or outer membrane. In the present study, we obtained additional evidence regarding the localization of GlpQ in B. hermsii. First, significant enzyme activity was found with the membrane fraction of the spirochete. Second, immunogold labeling and electron microscopic analysis detected GlpQ associated with the membranes of disrupted cells but not on the outer surface of intact spirochetes. Third, immunization with purified recombinant GlpQ failed to provide protection in mice when they were challenged by needle inoculation with B. hermsii. The immunization and challenge experiment was done because of the conflicting reports concerning the ability of GlpQ from Treponema pallidum to produce partial immunity in rabbits that were challenged intradermally with syphilis spirochetes (5, 37). These studies also differed in their conclusion regarding the location of GlpQ in T. pallidum, one being the outer membrane (5) and the other being the periplasm associated with the cytoplasmic membrane (37). In E. coli, GlpQ is nonlipidated and soluble in the periplasm (20). In Haemophilus influenzae, it is a lipoprotein exposed on the outer surface of the bacterium, stimulates a protective immune response (17), and assists in the acquisition of choline from the membrane of eukaryotic host cells (11). The evidence for B. hermsii demonstrates that GlpQ is most probably bound to the periplasmic side of the inner or outer membrane.
Our analysis of the glp locus found a small ORF, BB0242, in six species of B. burgdorferi sensu lato. This ORF was first identified in the genomic sequence of B. burgdorferi B31 (13), and its location was the same in the species we examined, between glpK and glpA, where glpT and glpQ are in B. hermsii (Fig. 3). However, no two sequences of BB0242 were the same, and they varied considerably in size and completeness. The variation in these sequences among the six species examined is most easily explained by multiple independent deletions in this locus. The DNA sequences of the six BB0242 ORFs matched nothing in the databases, and we found no sequences with any similarity to them in the genomic sequence of B. hermsii currently being compiled in our laboratory. We suspect that BB0242 represents a nonfunctional ORF, at least in some of the isolates we examined, that replaced glpT and glpQ in the Lyme disease group of spirochetes. Therefore, we think that part of the diversification of the genus Borrelia into two main groups involved the loss of GlpT and GlpQ from the ancestral relapsing-fever spirochetes to give rise to the Lyme disease spirochetes.
Our transformation of noninfectious B. burgdorferi with glpTQ from B. hermsii demonstrated that high-passage Lyme disease spirochetes survived and produced enzymatically active GlpQ; it also verified the association of glpQ with enzyme activity. This experiment also demonstrated that the B. hermsii promoters for these genes were functional in B. burgdorferi. The ability to combine comparative genomics with the heterologous expression of genes between closely related species of spirochetes has great potential. B. burgdorferi and B. hermsii are closely related (16) but differ significantly in their pathogenicity, the diseases induced, and the arthropod vectors required for transmission (31). When a complete genomic comparison is available for these two species of spirochetes, the heterologous expression of genes in one species that are unique to the other can be expanded beyond our effort presented here. Such manipulations should result in altered phenotypes across species that elucidate gene product function and mechanisms of pathogenicity.
The two groups of Borrelia species differ in their growth in vertebrate blood. Relapsing-fever spirochetes may attain levels of greater than 108 bacteria per ml of blood and are easily detectable by microscopy (31). Lyme disease spirochetes also produce bacteremias and may be cultured from the blood of patients (3, 24, 43, 49), but the spirochetes do not attain densities high enough in the blood of humans or mice for direct microscopic detection. In our present work, B. miyamotoi was shown to have the glpQ gene and GlpQ activity and to produce detectable spirochetemias in mice. This species is more closely related to the relapsing-fever spirochetes than to the Lyme disease spirochetes (15), but it forms a distinct cluster with Borrelia lonestari, Borrelia theileri, and a novel species of Borrelia not yet named (2, 29, 35). In contrast to the true relapsing-fever spirochetes, these four species infect ixodid rather than argasid ticks (2, 15, 35, 40, 47). Like B. miyamotoi, B. theileri also produces a spirochetemia detectable by microscopy (39, 40). B. lonestari and the novel Borrelia species have not yet been observed microscopically in blood, but we predict that this may be possible. B. anserina is also more closely related to the relapsing-fever spirochetes than to the Lyme disease spirochetes (14, 15, 28). This species causes a nonrelapsing avian spirochetosis, contains active GlpQ, and produces extremely high spirochetemias in birds (Fig. 1).
Might the presence of GlpQ and GlpT in the relapsing-fever spirochetes, and hence a third pathway for acquisition of G3P, contribute to the ability of these bacteria to achieve higher densities in the host's peripheral blood than do the Lyme disease spirochetes? The relapsing-fever and related spirochetes have the potential to utilize deacylated phospholipids as a carbon source for glycolysis and for the synthesis of new phospholipids (Fig. 7), whereas the Lyme disease spirochetes do not. The acquisition of G3P from phospholipids would conserve ATPs in the cell, in contrast to its synthesis from the other two pathways (Fig. 7). No ATP is required for the hydrolysis of a deacylated phospholipid, whereas one ATP is required to phosphorylate glycerol and two ATPs are required to convert glucose to fructose-1,6-biphosphate in the first stage of glycolysis (48). Additionally, diverting one 3-carbon intermediate from glycolysis to produce G3P would eliminate two ATPs gained from this molecule further down the glycolytic pathway. Hence, the acquisition of G3P from phospholipids costs the cell no ATP, conserves the ATP produced by glycolysis, and can contribute to the net gain in ATP when catabolized in the second stage of glycolysis. The presence of GlpQ may provide the relapsing-fever group of spirochetes an energetic advantage over the Lyme disease group of spirochetes.
Phosphatidylcholine (lecithin) is the most abundant phospholipid in mammalian erythrocytes and serum (25) and is also the major phospholipid in B. hermsii cells (23). This phospholipid is also taken up and released by mature erythrocytes (45); therefore, the outer surface of the red blood cells may have higher concentrations of phosphatidylcholine than does the serum. Brachyspira (Serpulina) hyodysenteriae (formerly Treponema hyodysenteriae), the spirochetal agent of swine dysentery, does not grow without lipids but grows well when cholesterol and phosphatidylcholine are added to the medium (42). Washed erythrocyte cell membranes also support good growth of this spirochete in medium free of other lipids, demonstrating that red blood cells can provide a phospholipid, most probably phosphatidylcholine, required for growth (42). Future efforts will be directed toward testing the hypothesis that relapsing-fever spirochetes forage phospholipids in the serum and from the surface of red blood cells and toward determining the role that this plays in affecting the density of spirochetes in blood.
Acknowledgments
We thank Jim Musser, Frank Gherardini, and Joe Hinnebusch for reviewing the manuscript and Gary Hettrick for providing graphic art work.
REFERENCES
- 1.Barbour, A. G., and S. F. Hayes. 1986. Biology of Borrelia species. Microbiol. Rev. 50:381-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour, A. G., G. O. Maupin, G. J. Teltow, C. J. Carter, and J. Piesman. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 173:403-409. [DOI] [PubMed] [Google Scholar]
- 3.Benach, J. L., E. M. Bosler, J. P. Hanrahan, J. L. Coleman, G. S. Habicht, T. F. Bast, D. J. Cameron, J. L. Ziegler, A. G. Barbour, W. Burgdorfer, R. Edelman, and R. A. Kaslow. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 308:740-742. [DOI] [PubMed] [Google Scholar]
- 4.Bublitz, C., and O. Wieland. 1962. Glycerokinase. Methods Enzymol. 5:354-361. [Google Scholar]
- 5.Cameron, C. E., C. Castro, S. A. Lukehart, and W. C. Van Voorhis. 1998. Function and protective capacity of Treponema pallidum subp. pallidum glycerophosphodiester phosphodiesterase. Infect. Immun. 66:5763-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canale-Parola, E. 1984. Order 1. Spirochaetales Buchanan 1917, 163AL, p. 38-39. In N. R. Krieg (ed.), Bergey's manual of sytematic bacteriology, vol. 1. The Williams & Williams Co., Baltimore, Md.
- 7.Carroll, J. A., and F. C. Gherardini. 1996. Membrane protein variations associated with in vitro passage of Borrelia burgdorferi. Infect. Immun. 64:393-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin, M. S., D. E. Anderson, Jr., T. G. Schwan, P. C. Shoemaker, S. N. Banerjee, B. O. Kassen, and W. Burgdorfer. 1998. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin. Infect. Dis. 26:122-131. [DOI] [PubMed] [Google Scholar]
- 10.El-Hage, N., K. Babb, J. A. Carroll, N. Lindstrom, E. R. Fischer, J. C. Miller, R. D. Gilmore Jr., L. M. Mbow, and B. Stevenson. 2002. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp lipoproteins. Microbiology 147:821-830. [DOI] [PubMed] [Google Scholar]
- 11.Fan, X., H. Goldfine, E. Lysenko, and J. N. Weiser. 2001. The transfer of choline from the host to the bacterial cell surface requires glpQ in Haemophilus influenzae. Mol. Microbiol. 41:1029-1036. [DOI] [PubMed] [Google Scholar]
- 12.Felsenfeld, O. 1971. Borrelia. Strains, vectors, human and animal borreliosis. Warren H. Green, Inc., St. Louis, Mo.
- 13.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. V. Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga, M., and Y. Koreki. 1995. The flagellin gene of Borrelia miyamotoi sp. nov. and its phylogenetic relationship among Borrelia species. FEMS Microbiol. Lett. 134:255-258. [DOI] [PubMed] [Google Scholar]
- 15.Fukunaga, M., Y. Takahashi, Y. Tsuruta, O. Matsushita, D. Ralph, M. McClelland, and M. Nakao. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 45:804-810. [DOI] [PubMed] [Google Scholar]
- 16.Hyde, F. W., and R. C. Johnson. 1984. Genetic relationship of Lyme disease spirochetes to Borrelia, Treponema, and Leptospira spp. J. Clin. Microbiol. 20:151-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janson, H., L. O. Heden, and A. Forsgren. 1992. Protein D, the immunoglobulin D-binding protein of Haemophilus influenzae, is a lipoprotein. Infect. Immun. 60:1336-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London). 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lane, R. S., J. Piesman, and W. Burgdorfer. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587-609. [DOI] [PubMed] [Google Scholar]
- 20.Larson, T. J., M. Ehrmann, and W. Boos. 1983. Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J. Biol. Chem. 258:5428-5432. [PubMed] [Google Scholar]
- 21.Lin, E. C. C. 1987. Dissimilatory pathways for sugars, polyols, and carboxylates, p. 244-284. In F. C. Neidhardt, J. L. Ingraham, B. Magasanik, K. B. Low, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, D.C.
- 22.Lin, E. C. C. 1976. Glycerol dissimilation and its regulation in bacteria. Annu. Rev. Microbiol. 30:535-578. [DOI] [PubMed] [Google Scholar]
- 23.Livermore, B. P., R. F. Bey, and R. C. Johnson. 1978. Lipid metabolism of Borrelia hermsi. Infect. Immun. 20:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maraspin, V., E. Ruzic-Sabljic, J. Cimperman, S. Lotric-Furlan, T. Jurca, R. N. Picken, and F. Strle. 2001. Isolation of Borrelia burgdorferi sensu lato from blood of patients with erythema migrans. Infection 29:65-70. [DOI] [PubMed] [Google Scholar]
- 25.Nelson, G. J. 1967. Lipid composition of erythrocytes in various mammalian species. Biochim. Biophys. Acta 144:221-232. [DOI] [PubMed] [Google Scholar]
- 26.Pickett, J., and R. Kelly. 1974. Lipid catabolism of relapsing fever borreliae. Infect. Immun. 9:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porcella, S. F., S. J. Raffel, M. E. Schrumpf, M. E. Schriefer, D. T. Dennis, and T. G. Schwan. 2000. Serodiagnosis of louse-borne relapsing fever with glycerophosphodiester phosphodiesterase (GlpQ) from Borrelia recurrentis. J. Clin. Microbiol. 38:3561-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ras, N. M., B. Lascola, D. Postic, S. J. Cutler, F. Rodhain, G. Baranton, and D. Raoult. 1996. Phylogenesis of relapsing fever Borrelia spp. Int. J. Syst. Bacteriol. 46:859-865. [DOI] [PubMed] [Google Scholar]
- 29.Rich, S. M., P. M. Armstrong, R. D. Smith, and S. R. Telford III. 2001. Lone star tick-infecting borreliae are most closely related to the agent of bovine borreliosis. J. Clin. Microbiol. 39:494-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuels, D. S. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol. Biol. 47:253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwan, T. G., W. Burgdorfer, and P. A. Rosa. 1999. Borrelia, p. 746-758. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 32.Schwan, T. G., W. Burgdorfer, M. E. Schrumpf, and R. H. Karstens. 1988. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J. Clin. Microbiol. 26:893-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwan, T. G., K. K. Kime, M. E. Schrumpf, J. E. Coe, and W. J. Simpson. 1989. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect. Immun. 57:3445-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwan, T. G., M. E. Schrumpf, B. J. Hinnebusch, D. E. Anderson, and M. E. Konkel. 1996. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J. Clin. Microbiol. 34:2483-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scoles, G. A., M. Papero, L. Beati, and D. Fish. 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoon. Dis. 1:21-34. [DOI] [PubMed] [Google Scholar]
- 36.Shang, E. S., J. T. Skare, H. Erdjument-Bromage, D. R. Blanco, P. Tempst, J. N. Miller, and M. A. Lovett. 1997. Sequence analysis and characterization of a 40-kilodalton Borrelia hermsii glycerophosphodiester phosphodiesterase homolog. J. Bacteriol. 179:2238-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shevchenko, D. V., T. J. Sellati, D. L. Cox, O. V. Shevchenko, E. J. Robinson, and J. D. Radolf. 1999. Membrane topology and cellular location of the Treponema pallidum glycerophosphodiester phosphodiesterase (GlpQ) ortholog. Infect. Immun. 67:2266-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson, W. J., C. F. Garon, and T. G. Schwan. 1990. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb. Pathog. 8:109-118. [DOI] [PubMed] [Google Scholar]
- 39.Smith, R. D., J. Brener, M. Osorno, and M. Ristic. 1978. Pathobiology of Borrelia theileri in the tropical cattle tick, Boophilus microplus. J. Invertebr. Pathol. 32:182-190. [DOI] [PubMed] [Google Scholar]
- 40.Smith, R. D., G. S. Miranpuri, J. H. Adams, and E. H. Ahrens. 1985. Borrelia theileri: isolation from ticks (Boophilus microplus) and tick-borne transmission between splenectomized calves. Am. J. Vet. Res. 46:1396-1398. [PubMed] [Google Scholar]
- 41.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 42.Stanton, T. B., and C. P. Cornell. 1987. Erythrocytes as a source of essential lipids for Treponema hyodysenteriae. Infect. Immun. 55:304-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, P. E., R. Thalken, J. L. Bono, and P. Rosa. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714-721. [DOI] [PubMed] [Google Scholar]
- 45.Telen, M. J., and R. E. Kaufman. 1998. The mature erythrocyte, p. 193-227. In G. R. Lee, J. Foerster, J. Lukens, F. Paraskevas, J. P. Greer, and G. M. Rodgers (ed.), Wintrobe's clinical hematology, 10th ed, vol. 1. The Williams & Williams Co., Baltimore, Md.
- 46.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trees, A. J. 1978. The transmission of Borrelia theileri by Boophilus annulatus (Say, 1821). Trop. Anim. Health Prod. 10:93-94. [DOI] [PubMed] [Google Scholar]
- 48.White, D. 1995. The physiology and biochemistry of prokaryotes. Oxford University Press, New York, N.Y.
- 49.Wormser, G. P., S. Bittker, D. Cooper, J. Nowakowski, R. B. Nadelman, and C. Pavia. 2000. Comparison of the yields of blood cultures using serum or plasma from patients with early Lyme disease. J. Clin. Microbiol. 38:1648-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, H. C., and M. Tokunaga. 1986. Biogenesis of lipoproteins in bacteria. Curr. Top. Microbiol. Immunol. 125:127-157. [DOI] [PubMed] [Google Scholar]