Abstract
Streptomyces coelicolor differentiates on solid agar media by forming aerial hyphae that septate into spores. We here show that differentiation also occurs in standing liquid minimal media. After a period of submerged growth, hyphae migrate to the air interface, where they become fixed by a rigid reflecting film. Colonies that result from these hyphae form sporulating aerial hyphae. In addition, submerged hyphae in the liquid minimal medium may attach to the surface. Liquid standing cultures easily become anoxic only 1 to 2 mm below the surface. Yet, biomass increases, implying the existence of metabolic pathways supporting anaerobic growth.
Streptomyces coelicolor is a filamentous gram-positive soil bacterium that undergoes a complex life cycle of morphological differentiation on solid agar medium. After a submerged feeding mycelium has been formed, hyphae escape the aqueous environment to grow into the air. This process is mediated by a surface-active peptide, SapB, that lowers the water surface tension (15). As a result, colonies are covered with a white fluffy layer of aerial hyphae. These aerial hyphae eventually septate into chains of grey-pigmented spores that are spread by wind or animals. These spores may give rise to a new mycelium. Aerial hyphae and spores possess several surface layers that make them hydrophobic. The rodlet layer is one of these layers. Its formation is mediated by the homologous rodlin proteins RdlA and RdlB (4) that are produced by hyphae in contact with air or a hydrophobic solid.
S. coelicolor mutants have been isolated that are impaired in formation of aerial hyphae (bld mutants) or in formation of spores (whi mutants). bld mutants grown on complex medium are affected in the production of the surface-active peptide SapB (16) and thus cannot escape into the air by their inability to lower the water surface tension (15). whi mutants are not impaired in SapB production. The aerial hyphae of these mutants, however, are affected in septation, cell wall thickening, and/or spore pigmentation. The phenotypes of a number of bld mutants are known to be suppressed by growth on minimal medium supplemented with mannitol, arabinose, or other sugars (8, 16).
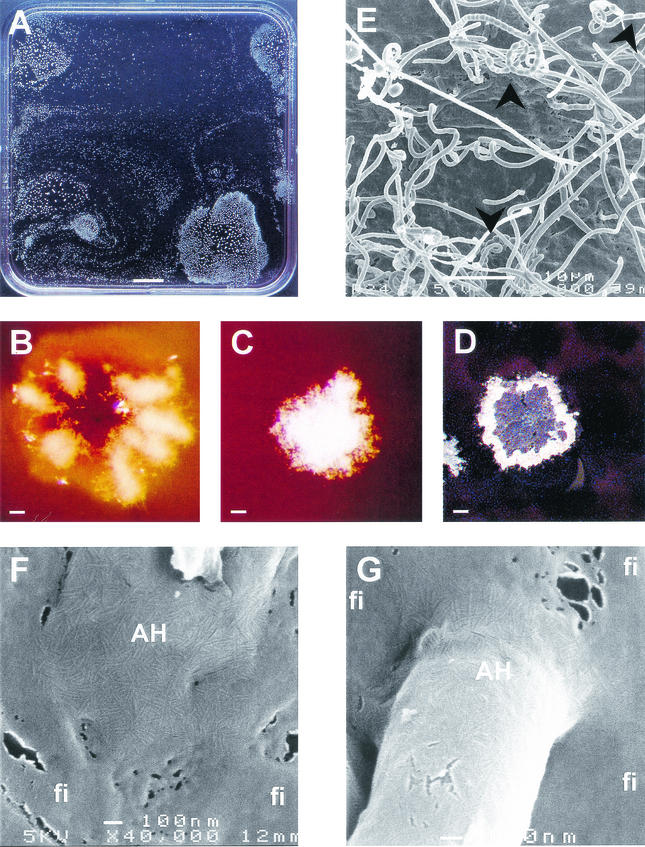
In contrast to agar media, differentiation does not occur in liquid shaken cultures. So far, it has not been reported whether formation of aerial hyphae and spores occurs in standing liquid cultures of S. coelicolor. To investigate this, S. coelicolor M145 (9) was grown at 30°C in polystyrene microtiter plates (Costar 3370; Corning Inc.) or in petri dishes. Minimal NMMP medium (9) was used in the absence of polyethylene glycol 6000 and supplemented with 25 mM glucose and 0.25% Casamino Acids (gNMMP) or 25 mM mannitol (mNMMP) as the carbon source. Alternatively, S. coelicolor was grown in the complete medium tryptic soy broth, R2YE, or YEME (9). Spores or mycelium from a liquid shaken culture were used as inoculum. A submerged mycelium was produced in all media. In contrast, aerial hyphae were only formed in minimal medium (gNMMP and mNMMP). After 3 days of submerged growth in these media, colonies formed at the water-air interface from which aerial hyphae developed (Fig. 1A). These colonies were fixed at this interface by a rigid light-reflecting film that surrounded the colony. Scanning electron microscopy revealed that the film had no clear ultrastructure and was therefore not an extension of the rodlet layer that coats the aerial hyphae (Fig. 1F and G). The rigid film was rich in protein but did not contain SapB or rodlins as determined by immunodetections with antibodies raised against these polypeptides (data not shown). The film could function as a fungal hydrophobin membrane by lowering the water surface tension (18). Colonies grown on gNMMP formed few aerial hyphae often restricted to radial zones or rings (Fig. 1B). In contrast, colonies grown on mNMMP formed a confluent layer of aerial hyphae (Fig. 1C). The aerial hyphae formed on both media metamorphosed into grey-pigmented spores (Fig. 1DE). Viability of these spores was similar to that of spores isolated from agar cultures before and after freeze drying (results not shown). bld and whi mutants (bldJ261, bldD53 [17], bldH109 [3], bldA39 [12], whiA [J2401], whiB [J2402], whiG [J2400] [7], whiH [J2403 [K. Flärdh, John Innes Center, Norwich, United Kingdom], whiI [J2450] [1], whiJ [J2452] [J. A. Aínsa, John Innes Center]) did not form floating air-interface colonies on gNMMP and mNMMP, and consequently aerial hyphae could not be formed. However, submerged growth was unaffected, as was shown by total protein determinations using the DC protein determination kit (Bio-Rad).
FIG.1.
Differentiating colonies of S. coelicolor at the air interface of standing liquid cultures. (A) Top view of a liquid standing culture grown on mNMMP. (B) Young interface colonies grown on glucose form aerial hyphae confined to rings or radial zones. (C) In contrast, those grown on mannitol form a confluent layer. (D) Aerial hyphae of cultures grown on mNMMP or gNMMP further differentiate to form grey-pigmented spores, as could be observed in more detail by scanning electron microscopy (E). The colonies are fixed at the interface by a rigid film (fi). This film has a different ultrastructural appearance than that of the rodlet layer covering aerial hyphae (AH) (F and G). Arrowheads indicate the position of spores. Bars represent, respectively, 1 cm (A), 100 μm (B to D), 10 μm (E), and 100 nm (F and G).
The standing cultures studied may resemble flooded soil, a condition S. coelicolor may escape from by forming floating sporulating colonies. How the hyphae move from the lower zone in the liquid to the air interface to form a sporulating colony is intriguing since S. coelicolor has no means of motility by, e.g., flagella. Within the genome sequence of S. coelicolor two gene clusters, SCO0649-SCO0658 and SCO6499-SCO6508, are present with homology to gas vesicle gene clusters from halophilic archaea and cyanobacteria (2). It is therefore not unlikely that S. coelicolor forms gas vesicles to become buoyant to reach the air interface.
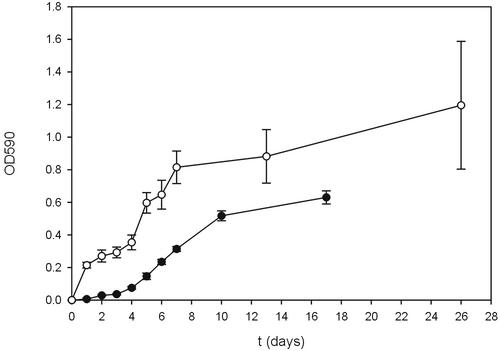
In minimal medium, not only were aerial hyphae formed but submerged hyphae also attached to the hydrophobic surface of the microtiter plates. In contrast, hyphae did not adhere in the nutrient-rich media YEME, tryptic soy broth, and R2YE. Attachment of hyphae grown in gNMMP and mNMMP was quantified using a staining assay with crystal violet, which was measured at an optical density at 590 nm (11, 13). The number of attaching hyphae increased with culture age (Fig. 2) and correlated in a linear way with the increase in total biomass as measured by total protein determination (data not shown). Cultures grown on glucose formed more biomass than those grown on mannitol, and more hyphae attached. The stationary phase was reached after 7 and 10 days of growth in gNMMP and mNMMP, respectively, after which the number of attaching hyphae no longer increased. Recently, it was shown that hyphae in contact with air or a hydrophobic solid produce rodlins. These proteins are involved in formation of the rodlet layer of aerial hyphae and mediate attachment to a hydrophobic surface (4). The latter may be instrumental in the establishment of symbiotic or pathogenic interactions. For instance, streptomycetes have been shown to associate in a beneficial way to the cuticle of leaf-cutting ants, which grow fungi as their main source of food (5, 6).
FIG. 2.
Attachment of hyphae in standing liquid cultures of S. coelicolor grown on glucose (○) or mannitol (•) minimal medium. OD590 (optical density at 590 nm) represents the amount of crystal violet solubilized from crystal violet-stained adhering hyphae.
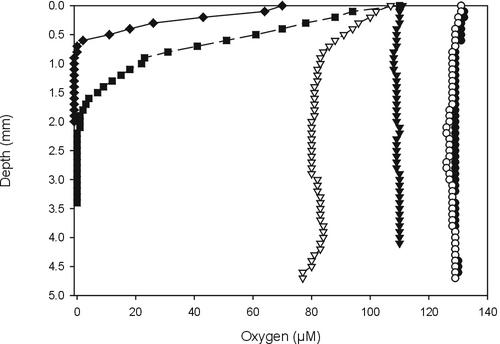
Growing standing liquid cultures are expected to develop oxygen gradients. Indeed, using a sensitive Clark-type oxygen microsensor (10) it was shown that 7-day-old YEME and gNMMP cultures were anoxic at a depth of 0.7 and 2 mm, respectively (Fig. 3). Yet, biomass still increased as measured by total protein determinations (data not shown), indicating an active metabolism. Standing mNMMP cultures also developed oxygen gradients, but they were less steep and cultures did not become anoxic (Fig. 3). Apparently, diffusion matches the requirement for oxygen, as can be explained by the reduced growth rate in this medium (Fig. 2). Because steep oxygen gradients are present in soil (14), S. coelicolor should be able to overcome or even grow under conditions with no or low amounts of oxygen. The genome sequence of S. coelicolor revealed gene clusters, e.g., genes SCO0216-SCO0219, SCO4947-SCO4950, and SCO6532-SCO6535, that putatively encode a typical four-subunit respiratory nitrate reductase (2). This protein is involved in anaerobic metabolism, indicating that S. coelicolor possesses enzymes to accommodate metabolism under anoxic or microaerobic conditions. Because S. coelicolor did not form colonies at the air interface in anoxic nutrient-rich standing cultures, oxygen availability may not be the signal for their formation.
FIG. 3.
Oxygen profiles of 7-day-old standing cultures of S. coelicolor grown on YEME (♦), gNMMP (▪), or mNMMP (▵). Abiotic oxygen profiles of YEME (▾), gNMMP (○), and mNMMP (•) served as controls.
In conclusion, standing cultures of S. coelicolor are an attractive model system to study a wide range of phenomena not studied thus far, such as attachment of streptomycetes to solids, differentiation at a liquid interface, and anaerobic metabolism.
Acknowledgments
We thank Ietse Stokroos for performing the scanning electron microscopy, Joanne Willey for supplying the bld strains, and Keith Chater (JIC, the Biotechnology and Biological Sciences Research Council [BBSRC]) for supplying the whi strains.
G. van Keulen and D. Claessen are supported by a grant of the Dutch Program EET (Economy, Ecology and Technology), a joint initiative of the Ministries of Economic Affairs, Education, Culture, and Sciences and of Housing, Spatial Planning, and the Environment (EETK98020).
REFERENCES
- 1.Aínsa, J. A., H. D. Parry, and K. F. Chater. 1999. A response regulator-like protein that functions at an intermediate stage of sporulation in Streptomyces coelicolor A3(2). Mol. Microbiol. 34:607-619. [DOI] [PubMed] [Google Scholar]
- 2.Bentley, S. D., K. F. Chater, A.-M. Cerdeño-Tárraga, G. L. Challis, N. R. Thompson, et al. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. [DOI] [PubMed] [Google Scholar]
- 3.Champness, W. C. 1988. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J. Bacteriol. 170:1168-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claessen, D., H. A. B. Wösten, G. Van Keulen, O. G. Faber, A. M. C. R. Alves, W. G. Meijer, and L. Dijkhuizen. 2002. Two novel homologous proteins of Streptomyces coelicolor and Streptomyces lividans are involved in the formation of the rodlet layer and mediate attachment to a hydrophobic surface. Mol. Microbiol. 44:1483-1492. [DOI] [PubMed] [Google Scholar]
- 5.Currie, C. R. 2001. A community of ants, fungi, and bacteria: a multilateral approach to studying symbiosis. Annu. Rev. Microbiol. 55:357-380. [DOI] [PubMed] [Google Scholar]
- 6.Currie, C. R., J. A. Scott, R. C. Summerbell, and D. Malloch. 1999. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398:701-704. [Google Scholar]
- 7.Flärdh, K., K. C. Finlay, and K. F. Chater. 1999. Association of early sporulation genes with suggested developmental decision points in Streptomyces coelicolor A3(2). Microbiology 145:2229-2243. [DOI] [PubMed] [Google Scholar]
- 8.Hopwood, D. A. 1988. Towards an understanding of gene switching in Streptomyces, the basis of sporulation and antibiotic production. Proc. R. Soc. Lond. B 235:121-138. [DOI] [PubMed] [Google Scholar]
- 9.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom.
- 10.Kühl, M., and N. P. Revsbech. 1998. Microsensors for the study of interfacial biogeochemical processes, p. 180-210. In B. P. Boudreau and B. B. Jorgensen (ed.), The benthic boundary layer. Oxford University Press, Oxford, United Kingdom.
- 11.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 12.Piret, J. M., and K. F. Chater. 1985. Phage-mediated cloning of bldA, a region involved in Streptomyces coelicolor morphological development, and its analysis by genetic complementation. J. Bacteriol. 163:965-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds, T. B., and G. R. Fink. 2001. Bakers' yeast, a model for fungal biofilm formation. Science 291:878-881. [DOI] [PubMed] [Google Scholar]
- 14.Sextone, A. J., N. P. Revsbech, T. B. Parkin, and J. M. Tiedje. 1985. Direct measurement of oxygen profiles and denitrification rates in soil aggregates. Soil Sci. Soc. Am. J. 49:645-651. [Google Scholar]
- 15.Tillotson, R. D., H. A. B. Wösten, M. Richter, and J. M. Willey. 1998. A surface active protein involved in aerial hyphae formation in the filamentous fungus Schizophyllum commune restores the capacity of a bald mutant of the filamentous bacterium Streptomyces coelicolor to erect aerial structures. Mol. Microbiol. 30:595-602. [DOI] [PubMed] [Google Scholar]
- 16.Willey, J., R. Santamaria, J. Guijarro, M. Geistlich, and R. Losick. 1991. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by S. coelicolor. Cell 65:641-650. [DOI] [PubMed] [Google Scholar]
- 17.Willey, J. M., J. Schwedock, and R. Losick. 1993. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 7:895-903. [DOI] [PubMed] [Google Scholar]
- 18.Wösten, H. A. B., M.-A. van Wetter, L. G. Lugones, H. J. Busscher, and J. G. H. Wessels. 1999. How a fungus escapes the water to grow into the air. Curr. Biol. 9:85-88. [DOI] [PubMed] [Google Scholar]