Abstract
A search of the pvd pyoverdine biosynthesis locus of Pseudomonas aeruginosa identified an open reading frame, PA2387, whose product exhibited a sequence similar to those of a number of so-called extracytoplasmic- function sigma factors responsible for siderophore-dependent expression of iron-siderophore receptors in Escherichia coli and Pseudomonas putida. Deletion of this gene, dubbed fpvI, compromised pyoverdine-dependent FpvA ferric pyoverdine receptor production and fpvA gene expression, while the cloned gene stimulated fpvA expression. A Fur-binding site was identified immediately upstream of fpvI, consistent with the observed iron-regulated expression of fpvI and fpvA.
With few exceptions, almost all bacteria require iron for growth and survival (27). Iron acquisition in nature is complicated, however, due to the low solubility of iron under aerobic conditions at neutral pH (27). Pathogenic organisms face similar restrictions in human hosts, since iron is generally sequestered intracellularly in heme-containing compounds, or in fluids, by iron-binding proteins such as lactoferrin and transferrin (39). Many bacteria overcome this problem by synthesizing high-affinity iron chelators called siderophores (29). Together with siderophore-specific outer membrane receptors, these facilitate the uptake of iron required to sustain growth and pathogenesis (28).
Pseudomonas aeruginosa, an opportunistic human pathogen (10), produces two known siderophores, pyoverdine (6) and pyochelin (5), in response to iron limitation. Pyoverdine is the superior chelator, at least at neutral pH (23), and is required for in vivo growth and virulence (24, 34, 48). Genes involved in the biosynthesis of pyoverdine localize in two gene clusters, the pvc operon (45, 46) and the pvd locus (22, 26, 36, 50), which are implicated in the synthesis of the chromophore and peptide moieties, respectively. The fpvA gene encoding the ferric pyoverdine receptor is also localized in the pvd cluster (22, 35, 36).
Although it is an essential nutrient for growth and pathogenesis, iron, in excess, is toxic to cells (12). Thus, uptake genes are tightly regulated by intracellular iron levels, mediated by the Fur repressor (8, 13, 38). Though a Fur homologue has been identified in P. aeruginosa (38), it does not directly regulate genes involved in pyoverdine biosynthesis. Rather, an alternative sigma factor, PvdS (7, 21, 26, 51), which positively regulates the expression of several pyoverdine biosynthetic genes (20, 52), is itself regulated by Fur (7, 20, 26). Pyoverdine control of FpvA expression has also been reported (11, 35), reminiscent of siderophore-dependent receptor gene expression in Escherichia coli (14) and Pseudomonas putida (17). In E. coli, ferric dicitrate upregulates its receptor, FecA, and, via a two-component system, FecIR (49), which is responsive to FecA binding of its cognate siderophore (4, 49). Similarly, pseudobactin BN7/8 stimulates expression of its receptor, PupB, via FecIR homologues, dubbed PupIR (17). FecI is an extracytoplasmic-function (ECF) sigma factor (1) whose activity is controlled by FecR (30). Recently, a third example of this type of regulatory system was reported in Bordetella bronchiseptica, where bupIR gene products were shown to control expression of a putative siderophore receptor, BfrZ (37). In an effort to understand the basis of pyoverdine control of FpvA production in P. aeruginosa, then, FecIR homologues were sought in the pvd locus of the available PAO genome (http://www.pseudomonas.com) (47).
MATERIALS AND METHODS
Bacterial strains and growth media.
Bacterial strains and plasmids used in this study are listed in Table 1. Routine growth for both P. aeruginosa and E. coli was performed in Luria-Bertani (LB) medium (Luria broth base; Difco). Growth under iron-limited conditions was performed by using iron-free BM2 succinate or glucose medium (35), which was made to be iron sufficient, as necessary, through the addition of 100 μM FeSO4. Antibiotic selections used for P. aeruginosa included tetracycline (70 μg/ml in LB and 30 μg/ml in BM2 succinate), chloramphenicol (200 μg/ml in LB and 30 μg/ml in BM2 succinate) and kanamycin (for ΔaphA strains only, 100 μg/ml in LB). For E. coli, tetracycline was used at 10 μg/ml (in LB) or 5 μg/ml (in BM2 glucose), chloramphenicol was used at 50 μg/ml (in LB) or 20 μg/ml (in BM2 glucose), and kanamycin was used at 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| P. aeruginosa | ||
| K767 | PAO1 prototroph | N. Gotoh, Kyoto Pharmaceutical University |
| K1120 | PAO1 ΔaphA | N. Gotoh, Kyoto Pharmaceutical University |
| K1203 | K1120 ΔpvdD | A. Meldrum, unpublished |
| K2100 | K1120 ΔfpvI | This study |
| K2102 | K1203 ΔfpvI | This study |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 2 |
| S17-1 | thi pro hsdR recA Tra+ | 43 |
| Plasmids | ||
| pEX18tc | Broad-host-range gene replacement vector; Tcr | 15 |
| pK18mobsacB | Broad-host-range gene replacement vector; Kanr | 40 |
| pRK415 | Broad-host-range, low-copy-number cloning vector carrying MCS downstream of Plac; Tcr | 16 |
| pMP190 | Broad-host-range, low-copy-number lacZ fusion vector; Camr Smr | 44 |
| pEJB3 | pMP190::fpvA-lacZ; Camr | E. J. Blouin, unpublished |
| pAR001 | pRK415::fpvI | This study |
| pAR002 | pEX18tc::ΔfpvI | This study |
| pAR003 | pK18mobsacB::ΔfpvI | This study |
| pAR005 | pRK415 digested with DraI, religated (ΔDraI) | This study |
Camr, chloramphenicol resistance; Kanr, kanamycin resistance; Tcr, tetracycline resistance; Smr, streptomycin resistance; MCS, multiple cloning site; Plac, lac promoter.
DNA techniques.
Basic DNA procedures, including restriction endonuclease digestions, ligations, transformations, and agarose gel electrophoresis, were performed as described previously (24). Plasmid DNA isolation was performed by using the alkaline lysis method (24) or by using a plasmid Midi kit (Qiagen, Mississauga, Ontario, Canada). Genomic DNA was extracted from P. aeruginosa by using the method of Barcak et al. (3). DNA fragments for use in cloning were extracted from agarose gels by using Prep-A-Gene (Bio-Rad Labs, Richmond, Calif.) in accordance with the manufacturer's instructions. Nucleotide sequencing of plasmid-borne DNA was carried out by Cortec DNA Services, Inc. (Kingston, Ontario, Canada). Nucleotide sequence alignments were performed using the GENESTREAM website (http://xylian.igh.cnrs.fr/bin/align-guess.cgi) (33).
Cloning of fpvI and construction of a ΔfpvI mutant.
The fpvI gene was amplified by PCR by utilizing primers pff (5′-CATGGAATTCTGGTAGTTGGAAGGAATCCAGC-3′; the EcoRI site is underlined) and pfr (5′-AGCTGAATTCCAGTTGCCTGAGTCAATTCCAG-3′; the EcoRI site is underlined). The PCR mixture contained 50 ng of P. aeruginosa chromosomal DNA, 30 pmol of each primer, 0.2 mM (each) deoxynucleoside triphosphate, 1 mM MgSO4, and 3% (vol/vol) dimethyl sulfoxide in 1× ThermoPol buffer (New England Biolabs, Mississauga, Ontario, Canada), which was heated for 3 min at 95°C before the addition of 2 U of VentR DNA polymerase (New England Biolabs). The reaction was processed for 30 cycles of 1 min at 95°C, 20 s at 58°C, and 1 min at 72°C, followed by 10 min at 72°C. The resulting amplicon was purified by a Qiaquick PCR purification kit (Qiagen), digested with EcoRI, and cloned into EcoRI-digested pRK415, to yield pAR001. Nucleotide sequencing confirmed that the fpvI gene was cloned in the same orientation as the lac promoter.
To construct ΔfpvI mutants of P. aeruginosa, an internal deletion of the fpvI gene was first constructed in the gene replacement vector pK18mobsacB. This was accomplished by amplifying PCR products (by using the conditions and parameters described above) corresponding to sequence upstream and downstream of the deletion end points in fpvI by using primer pairs pflaeco (5′-GATCGAATTCATGCTGCCTCTCGCGATGTC-3′; the EcoRI site is underlined) and pflakpn (5′-CGTAGGTACCGGCACTGAGGAATCGCAG-CA-3′; the KpnI site is underlined) and pfrakpn (5′-CGTAGGTACCAACGCGATGAAGCACTGTC-3′; the KpnI site is underlined) and pfrahind (5′-GACTAAGCTTGTGTTCCAGGTACTGGCTCTG-3′; the HindIII site is underlined), respectively. The downstream fragment was purified by using a Qiaquick PCR purification kit (Qiagen), digested with KpnI and HindIII, and cloned into KpnI-HindIII-restricted pEX18Tc. The resulting vector was then digested with EcoRI and KpnI, and the EcoRI-KpnI-digested upstream fragment was cloned into this vector, yielding the ΔfpvI plasmid pAR002. The ΔfpvI gene was excised from pAR002 by digestion with EcoRI and XmnI, and the resultant 1.8-kb fragment was cloned into EcoRI-XmnI-restricted pK18mobsacB to yield pAR003. This vector was then transformed into E. coli S17-1 and mobilized into P. aeruginosa strains K1120 and K1203 by using a previously described protocol (45). By using chloramphenicol (5 μg/ml) as a counterselective agent, kanamycin-resistant transconjugants were recovered and subsequently streaked onto LB agar containing 10% (wt/vol) sucrose. Sucrose-resistant colonies arising after 24 h of growth at 37°C were screened for the presence of a ΔfpvI chromosomal deletion by colony PCR (42) with primers pff and pfr by using the conditions described above.
Whole-cell extracts.
Whole-cell protein extracts were prepared from cultures of P. aeruginosa grown overnight in iron-sufficient or iron-deficient BM2 succinate medium following subculture (1:99 dilution) into the same medium and growth to an optical density at 600 nm (OD600) of approximately 0.7. Two 1.5-ml aliquots were centrifuged sequentially in the same microcentrifuge tube at 13,000 rpm (Biofuge pico; Heraeus Instruments) and resuspended in 400 μl of phosphate-buffered saline (1.7 mM NaH2PO4, 8.1 mM Na2HPO4, 145 mM NaCl). An equal volume of loading buffer (4% [wt/vol] sodium dodecyl sulfate [SDS], 20% [vol/vol] glycerol, 250 mM Tris-HCl [pH 6.8]) was then added, after which the samples were boiled for 5 min and sonicated on ice for 10 s (by using setting 40 on a Vibra Cell sonicator [Sonic and Materials Inc., Danbury, Conn.]). Total cell protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, Ill.) according to the manufacturer's instructions.
SDS-polyacrylamide gel electrophoresis and immunoblotting.
Whole-cell extract samples were electrophoresed on 10% (wt/vol) SDS-polyacrylamide gels (53) and electrotransferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore) as described previously (54). Equal loading of protein in all wells was confirmed by rapid Coomassie staining of duplicate gels (9). Membranes were probed with a polyclonal anti-FpvA antibody as described previously (36).
β-Galactosidase assay.
P. aeruginosa strains containing promoter-lacZ fusions were grown in iron-limited BM2 succinate medium supplemented with the appropriate antibiotics for approximately 18 h at 37°C. In some experiments, pyoverdine was included at 100 μg/ml. Cultures were then diluted 1:49 into the same medium without antibiotics and grown at 37°C to an OD600 of ∼0.7 before being assayed for β-galactosidase activity as described previously (25). To assess the impact of the cloned fpvI gene on fpvA-lacZ expression in a heterologous host, E. coli DH5α harboring pEJB3 and either pAR001 (which carries fpvI) or pAR005 (vector control) was grown in iron-limited BM2 glucose minimal medium with the appropriate antibiotics to an OD600 of ∼0.5 before being assayed for β-galactosidase activity.
RNA isolation and RT-PCR.
RNA was isolated by using the RNeasy RNA isolation kit (Qiagen) in accordance with the manufacturer's instructions. Samples were then treated with RQ1 DNase (Promega, Madison, Wis.) in accordance with the manufacturer's instructions, and RNA was quantitated by spectrophotometry. Reverse transcription (RT)-PCR was carried out by using intragenic primers specific for fpvI (namely, piff [5′-ACTGGAATTCCAGCGAGCAGGAGTCGTCTT-3′] and pifr [5′-ACTGGAATTCTTGCGCAACAGGAAGGAAC-3′]) and rpsL (namely, rpsL1 [5′-GCAACTATCAACCAGCTG-3′] and rpsL2 [5′-GCTGTGCTCTTGCAGGTTGTG-3′]) and the One Step RT-PCR kit (Qiagen) by following the manufacturer's instructions. PCR conditions were as described above, except that the annealing temperature was 59°C. Non-RT controls were run to ensure that no DNA contaminated the RNA samples.
RESULTS AND DISCUSSION
Role of FpvI in fpvA expression.
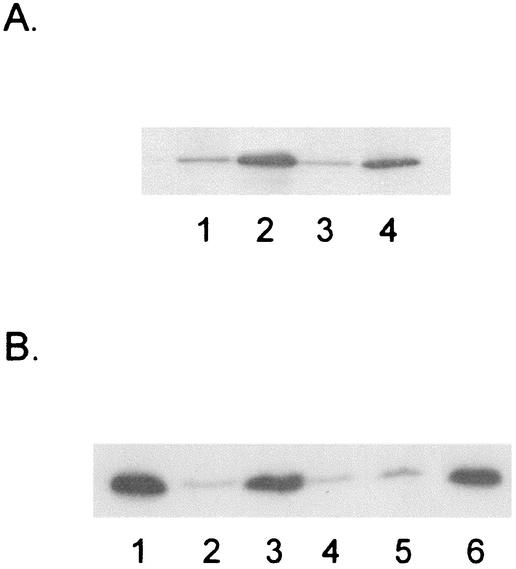
Examination of the Pseudomonas genome (Pseudomonas genome project website, http://www.pseudomonas.com) by using BLASTX (National Center for Biotechnology Information webpage, http://www.ncbi.nlm.nih.gov/BLAST) revealed two open reading frames, PA2387 and PA2388, whose deduced products exhibited homology to FecIR and PupIR, and they were subsequently designated fpvI and fpvR (in accordance with the nomenclature suggested by I. Lamont, University of Otago). The fpvI and fpvR genes were transcribed divergently from one another and were localized within the so-called pvd locus between the pyoverdine biosynthetic genes pvdK (19) and pvdA (50). FpvI showed almost 30.6% identity (59% overall similarity) to FecI (accession no. AAA23766), 31.6% identity (63.3% overall similarity) to BupI (accession no. CAB71123), and 29.5% identity (60.1% overall similarity) to PupI (accession no. CAA54870). To assess its role in FpvA production, then, an in-frame fpvI deletion was constructed in P. aeruginosa strain K1120, yielding K2100. Although FpvA production increased substantially in FpvI+ strains under iron restriction (compare lanes 1 and 2 in Fig. 1A), loss of fpvI in K2100 abolished this iron-limited increase in FpvA (Fig. 1A, lane 3). FpvA production in iron-limited K2100 was, however, restored by the vector-borne fpvI gene present on plasmid pAR001 (Fig. 1A, lane 4), confirming a role for this probable ECF sigma factor in FpvA expression under iron-limiting conditions. To assess whether FpvI was influencing FpvA at the level of gene expression, an fpvA-lacZ fusion vector, pEJB3, was introduced into FpvI+ and FpvI− P. aeruginosa, and β-galactosidase activity was determined for cells cultured under iron-limiting conditions. As seen in Table 2, loss of fpvI in K2100 reduces fpvA expression approximately fivefold relative to that of the FpvI+ parental strain, K1120. Moreover, the cloned fpvI gene restored fpvA expression to wild-type levels in K2100 and stimulated the expression of the fpvA-lacZ fusion in E. coli (Table 2). In contrast, pvdD-lacZ expression, previously demonstrated to be dependent on the PvdS ECF sigma factor (21), was not stimulated by FpvI in E. coli (data not shown). These data are consistent with FpvI directly and positively promoting fpvA gene expression.
FIG. 1.
Immunoblots of iron-limited P. aeruginosa whole-cell extracts developed with anti-FpvA antibodies. (A) Lane 2, K1120 (FpvI+); lane 3, K2100 (FpvI−); lane 4, K2100 carrying pAR001 (FpvI+). An immunoblot of K1120 grown in iron-supplemented minimal medium is shown in lane 1. (B) Lane 1, K1120 (FpvI+); lanes 2 and 3, K1203 (FpvI+ PvdD−); lanes 4 and 5, K2102 (FpvI− PvdD−); lane 6, K2102 carrying pAR001 (FpvI+ PvdD−). Samples in lanes 3, 5, and 6 were prepared from cells supplemented with pyoverdine (100 μg/ml) during growth.
TABLE 2.
Influence of FpvI on fpvA-lacZ expressiona
| Strain | Relevant phenotype | β-Galactosidase activity (Miller units) withb:
|
|
|---|---|---|---|
| No supplementation | Pyoverdine supplementation | ||
| K1120 | FpvI+ | 1,400 | —c |
| K2100 | FpvI− | 256 | — |
| K2100 (pAR001) | FpvI+ | 1,455 | — |
| DH5α (pAR001) | FpvI+ | 106 | — |
| DH5α (pAR005) | FpvI− | 3 | — |
| K1203 | FpvI+ Pvd−d | 616 | 1,749 |
| K2102 | FpvI− Pvd− | 370 | 298 |
P. aeruginosa and E. coli strains harboring the fpvA-lacZ vector pEJB3 were cultured to log phase in antibiotic-supplemented iron-limited medium with or without the addition of 100 μg of pyoverdine/ml and assayed for β-galactosidase activity.
Data are a representation of experiments performed in triplicate.
—, not done.
Pyoverdine deficient owing to a deletion in the pvdD gene.
Iron regulation of fpvI.
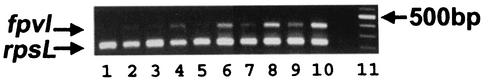
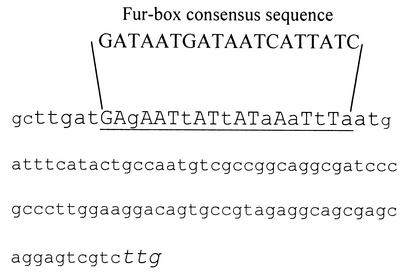
Since many iron uptake and regulatory genes, including that encoding the aforementioned PvdS ECF sigma factor, are themselves regulated by iron, with regulation mediated by the Fur repressor protein, it was of interest to assess regulation of fpvI by iron. By RT-PCR, expression of fpvI was clearly shown to increase under conditions of iron limitation (Fig. 2). Moreover, examination of the nucleotide sequence upstream of fpvI revealed the presence of a putative Fur box (Fig. 3, underlined), with 12 of 19 nucleotides matching the consensus Fur box sequence; indeed, Fur binding to this site has been previously confirmed (fpvI was previously identified as a Fur-regulated gene dubbed pig32 [31]). Thus, the observation that expression of fpvA is governed by an iron-regulated probable sigma factor explains the known iron regulation of this receptor gene despite the absence of a Fur box upstream of the fpvA gene. This indirect iron regulation of gene expression is reminiscent of the iron-regulated expression of the pyoverdine biosynthetic (pvd) genes, which also lack Fur boxes and whose expression is governed by the iron-regulated ECF sigma factor PvdS (20, 21, 32, 52).
FIG. 2.
fpvI expression in P. aeruginosa strain PAO1 K767, measured by RT-PCR of RNA (4 ng) isolated from cells cultivated under iron-sufficient (odd-numbered lanes) and iron-deficient (even-numbered lanes) conditions. The reactions were carried out for 26 (lanes 1 and 2), 27 (lanes 3 and 4), 28 (lanes 5 and 6), 29 (lanes 7 and 8), and 30 (lanes 9 and 10) cycles. fpvI and the rpsL (control) products are highlighted. Lane 11, 100-bp ladder with a 500-bp marker highlighted.
FIG. 3.
Nucleotide sequence of the 5′ upstream region of fpvI, highlighting a putative Fur box. The Fur box (underlined) and FpvI translational initiation codon (italicized) are highlighted. Matches to the consensus Fur box sequence are capitalized.
Involvement of pyoverdine in FpvI-mediated fpvA expression.
In order to ascertain whether FpvI mediates the positive influence of pyoverdine on FpvA production, an fpvI deletion was created in a pyoverdine-deficient derivative of P. aeruginosa, K1203, and the ability of exogenously added pyoverdine to promote FpvA production in the resulting strain, K2102, was assessed. Immunoblotting with an anti-FpvA antibody confirmed previous findings that FpvA production was reduced in a pyoverdine-deficient (but FpvI+) strain (Fig. 1B, lane 2; compare with lane 1) and can be restored by the addition of exogenous pyoverdine (Fig. 1B, lane 3). In contrast, pyoverdine did not restore FpvA production in the FpvI− strain K2102 (Fig. 1B, lane 5; compare with lane 4). Introduction of the cloned fpvI gene on plasmid pAR001 did, however, restore pyoverdine stimulation of FpvA production in this mutant (Fig. 1B, lane 6). Again, this effect occurred at the level of fpvA gene expression, with pyoverdine enhancing expression of the fpvA-lacZ fusion in the FpvI+ strain, K1203, but not in the FpvI− strain, K2102 (Table 2). The observation that FpvI mediates the positive influence of pyoverdine on fpvA gene expression is reminiscent of PvdS and its mediation of the positive influence of this siderophore on pvd gene expression, the latter in a process involving the receptor itself and an anti-sigma factor, FpvR (18, 41). Pyoverdine-dependent FpvI-mediated stimulation of fpvA expression appears also to be controlled by FpvA and FpvR (I. L. Lamont, personal communication).
Acknowledgments
The Δpvd strain K1203 was engineered by using a ΔpvdD plasmid provided by I. L. Lamont (University of Otago).
This work was supported by an operating grant from the Canadian Institutes of Health Research (formerly the Medical Research Council of Canada) to K.P. K.P. is a Canadian Cystic Fibrosis Foundation (CCFF) Scholar. G.A.R. is the recipient of a CCFF graduate student scholarship.
REFERENCES
- 1.Angerer, A., S. Enz, M. Ochs, and V. Braun. 1995. Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. FecI belongs to a new subfamily of sigma 70-type factors that respond to extracytoplasmic stimuli. Mol. Microbiol. 18:163-174. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1992. Short protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Barcak, G. J., M. S. Chandler, R. J. Redfield, and J. F. Tomb. 1991. Genetic systems in Haemophilus influenzae. Methods Enzymol. 204:321-342. [DOI] [PubMed] [Google Scholar]
- 4.Braun, V. 1997. Surface signaling: novel transcription initiation mechanism starting from the cell surface. Arch. Microbiol. 167:325-331. [DOI] [PubMed] [Google Scholar]
- 5.Cox, C. D. 1980. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J. Bacteriol. 142:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox, C. D., and P. Adams. 1985. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect. Immun. 48:130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunliffe, H. E., T. R. Merriman, and I. L. Lamont. 1995. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J. Bacteriol. 177:2744-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, J. F., R. L. Bennett, and L. I. Rothfield. 1978. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J. Bacteriol. 135:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faguy, D. M., D. P. Bayley, A. S. Kostyukova, N. A. Thomas, and K. F. Jarrell. 1996. Isolation and characterization of flagella and flagellin proteins from the thermoacidophilic archaea Thermoplasma volcanium and Sulfolobus shibatae. J. Bacteriol. 178:902-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fick, R. 1993. Psudomonas aeruginosa the opportunist: pathogenisis and disease. CRC Press, Boca Raton, Fla.
- 11.Gensberg, K., K. Hughes, and A. W. Smith. 1992. Siderophore-specific induction of iron uptake in Pseudomonas aeruginosa. J. Gen. Microbiol. 138(Pt 11):2381-2387. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell, B., and J. M. Gutteridge. 1986. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch. Biochem. Biophys. 246:501-514. [DOI] [PubMed] [Google Scholar]
- 13.Hantke, K. 1984. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol. Gen. Genet. 197:337-341. [DOI] [PubMed] [Google Scholar]
- 14.Harle, C., I. Kim, A. Angerer, and V. Braun. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J. 14:1430-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 16.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 17.Koster, M., W. van Klompenburg, W. Bitter, J. Leong, and P. Weisbeek. 1994. Role for the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J. 13:2805-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamont, I. L., P. A. Beare, U. Ochsner, A. I. Vasil, and M. L. Vasil. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 99:7072-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehoux, D. E., F. Sanschagrin, and R. C. Levesque. 2000. Genomics of the 35-kb pvd locus and analysis of novel pvdIJK genes implicated in pyoverdine biosynthesis in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 190:141-146. [DOI] [PubMed] [Google Scholar]
- 20.Leoni, L., A. Ciervo, N. Orsi, and P. Visca. 1996. Iron-regulated transcription of the pvdA gene in Pseudomonas aeruginosa: effect of Fur and PvdS on promoter activity. J. Bacteriol. 178:2299-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leoni, L., N. Orsi, V. de Lorenzo, and P. Visca. 2000. Functional analysis of PvdS, an iron starvation sigma factor of Pseudomonas aeruginosa. J. Bacteriol. 182:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merriman, T. R., M. E. Merriman, and I. L. Lamont. 1995. Nucleotide sequence of pvdD, a pyoverdine biosynthetic gene from Pseudomonas aeruginosa: PvdD has similarity to peptide synthetases. J. Bacteriol. 177:252-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer, J. M., F. Halle, D. Hohnadel, P. Lemanceau, and H. Ratefiarivelo. 1987. Siderophores of Pseudomonas—biological properties, p. 189-205. In G. Winkelmann, D. van der Helm, and J. B. Neilands (ed.), Iron transport in microbes, plants and animals. VCH Publishers, New York, N.Y.
- 24.Meyer, J. M., A. Neely, A. Stintzi, C. Georges, and I. A. Holder. 1996. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 64:518-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, J. F. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Miyazaki, H., H. Kato, T. Nakazawa, and M. Tsuda. 1995. A positive regulatory gene, pvdS, for expression of pyoverdin biosynthetic genes in Pseudomonas aeruginosa PAO. Mol. Gen. Genet. 248:17-24. [DOI] [PubMed] [Google Scholar]
- 27.Neilands, J., K. Konopka, B. Schwyn, M. Coy, R. Francis, B. Paw, and A. Bagg. 1987. Comparative biochemistry of microbial iron assimilation, p. 3-33. In G. Winkelmann, D. van der Helm, and J. Neilands (ed.), Iron transport in microbes, plants and animals. VCH Publishers, New York, N.Y.
- 28.Neilands, J. B. 1981. Iron absorption and transport in microorganisms. Annu. Rev. Nutr. 1:27-46. [DOI] [PubMed] [Google Scholar]
- 29.Neilands, J. B. 1981. Microbial iron compounds. Annu. Rev. Biochem. 50:715-731. [DOI] [PubMed] [Google Scholar]
- 30.Ochs, M., S. Veitinger, I. Kim, D. Welz, A. Angerer, and V. Braun. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: FecR is required for transcription activation by FecI. Mol. Microbiol. 15:119-132. [DOI] [PubMed] [Google Scholar]
- 31.Ochsner, U. A., and M. L. Vasil. 1996. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc. Natl. Acad. Sci. USA 93:4409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochsner, U. A., P. J. Wilderman, A. I. Vasil, and M. L. Vasil. 2002. GeneChip(R) expression analysis of the iron starvation response in Pseudomonas aeruginosa: identification of novel pyoverdine biosynthesis genes. Mol. Microbiol 45:1277-1287. [DOI] [PubMed] [Google Scholar]
- 33.Pearson, W. R., T. Wood, Z. Zhang, and W. Miller. 1997. Comparison of DNA sequences with protein sequences. Genomics 46:24-36. [DOI] [PubMed] [Google Scholar]
- 34.Poole, K., C. Dean, D. Heinrichs, S. Neshat, K. Krebes, L. Young, and L. Kilburn. 1996. Siderophore-mediated iron transport in Pseudomonas aeruginosa, p. 371-383. In T. Nakazawa (ed.), Molecular biology of pseudomonads. ASM Press, Washington, D.C.
- 35.Poole, K., S. Neshat, and D. Heinrichs. 1991. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol. Lett. 62:1-5. [PubMed] [Google Scholar]
- 36.Poole, K., S. Neshat, K. Krebes, and D. E. Heinrichs. 1993. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J. Bacteriol. 175:4597-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pradel, E., and C. Locht. 2001. Expression of the putative siderophore receptor gene bfrZ is controlled by the extracytoplasmic-function sigma factor BupI in Bordetella bronchiseptica. J. Bacteriol. 183:2910-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince, R. W., D. G. Storey, A. I. Vasil, and M. L. Vasil. 1991. Regulation of toxA and regA by the Escherichia coli fur gene and identification of a Fur homologue in Pseudomonas aeruginosa PA103 and PA01. Mol. Microbiol. 5:2823-2831. [DOI] [PubMed] [Google Scholar]
- 39.Sawatzki, G. 1987. The role of iron-binding proteins in bacterial infections, p. 477-489. In G. Winkelmann, D. van der Helm, and J. B. Neilands (ed.), Iron transport in microbes, plants, and animals. VCH Publishers, New York, N.Y.
- 40.Schafer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 41.Shen, J., A. Meldrum, and K. Poole. 2002. FpvA receptor involvement in pyoverdine biosynthesis in Pseudomonas aeruginosa. J. Bacteriol. 184:3268-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheu, D. S., Y. T. Wang, and C. Y. Lee. 2000. Rapid detection of polyhydroxyalkanoate-accumulating bacteria isolated from the environment by colony PCR. Microbiology 146(Pt 8):2019-2025. [DOI] [PubMed] [Google Scholar]
- 43.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 44.Spaink, H., R. Okker, C. Wijffelman, E. Pees, and B. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J2. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 45.Stintzi, A., P. Cornelis, D. Hohnadel, J. M. Meyer, C. Dean, K. Poole, S. Kourambas, and V. Krishnapillai. 1996. Novel pyoverdine biosynthesis gene(s) of Pseudomonas aeruginosa PAO. Microbiology 142(Pt 5):1181-1190. [DOI] [PubMed] [Google Scholar]
- 46.Stintzi, A., Z. Johnson, M. Stonehouse, U. Ochsner, J. M. Meyer, M. L. Vasil, and K. Poole. 1999. The pvc gene cluster of Pseudomonas aeruginosa: role in synthesis of the pyoverdine chromophore and regulation by PtxR and PvdS. J. Bacteriol. 181:4118-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 48.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Hove, B., H. Staudenmaier, and V. Braun. 1990. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J. Bacteriol. 172:6749-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Visca, P., A. Ciervo, and N. Orsi. 1994. Cloning and nucleotide sequence of the pvdA gene encoding the pyoverdin biosynthetic enzyme l-ornithine N5-oxygenase in Pseudomonas aeruginosa. J. Bacteriol. 176:1128-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson, M. J., and I. L. Lamont. 2000. Characterization of an ECF sigma factor protein from Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 273:578-583. [DOI] [PubMed] [Google Scholar]
- 52.Wilson, M. J., B. J. McMorran, and I. L. Lamont. 2001. Analysis of promoters recognized by PvdS, an extracytoplasmic-function sigma factor protein from Pseudomonas aeruginosa. J. Bacteriol. 183:2151-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, L., X. Z. Li, and K. Poole. 2000. Multiple antibiotic resistance in Stenotrophomonas maltophilia: involvement of a multidrug efflux system. Antimicrob. Agents Chemother. 44:287-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao, Q., X. Z. Li, A. Mistry, R. Srikumar, L. Zhang, O. Lomovskaya, and K. Poole. 1998. Influence of the TonB energy-coupling protein on efflux-mediated multidrug resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:2225-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]