Abstract
Objective
To describe seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population.
Design
A longitudinal observational study.
Setting
Most of the study participants were recruited from a health maintenance organization (HMO) in central Massachusetts, USA. Additional individuals of Hispanic descent were recruited from outside of the HMO population to increase the ethnic diversity of this sample.
Subjects
Data from 593 participants, aged 20–70, were used for this investigation. Each participant was followed quarterly (five sampling points: baseline and four consecutive quarters) for 1-year period. Body weight measurements and three 24-h dietary and physical activity recalls were obtained on randomly selected days (including 2 weekdays and 1 weekend day) per quarter. Sinusoidal regression models were used to estimate peak-to-trough amplitude and phase of the peaks.
Results
Daily caloric intake was higher by 86 kcal/day during the fall compared to the spring. Percentage of calories from carbohydrate, fat and saturated fat showed slight seasonal variation, with a peak in the spring for carbohydrate and in the fall for total fat and saturated fat intake. The lowest physical activity level was observed in the winter and the highest in the spring. Body weight varied by about 1/2 kg throughout the year, with a peak in the winter (P<0.001 winter versus summer). Greater seasonal variation was observed in subjects who were male, middle aged, nonwhite, and less educated.
Conclusions
Although there is seasonal variation in diet, physical activity and body weight, the magnitude of the change is generally small in this population.
Keywords: seasons, diet, physical activity, body weight, epidemiology
Introduction
The concept of seasonal variation in nutrient intake has received considerable attention in the literature (Hackett et al., 1985; Van Staveren et al., 1986; Krauchi and Wirz-Justice, 1988; Hartman et al., 1990; De Castro, 1991; Subar et al., 1994; Sasaki et al., 1998; Doyle et al., 1999; Shahar et al., 1999). The findings, however, have been rather inconsistent. The majority of the literature suggests that daily total caloric intake does not vary significantly by season (Hackett et al., 1985; Van Staveren et al., 1986; Subar et al., 1994; Shahar et al., 1999). A few studies have provided a more detailed view of the diet, suggesting that the intake of proteins (Hackett et al., 1985; Krauchi and Wirz-Justice, 1988; De Castro, 1991) and carbohydrates (Hackett et al., 1985) is also constant throughout the year. Others have proposed that dietary intake of total calories, carbohydrates (De Castro, 1991), and fat varies seasonally (Van Staveren et al., 1986; Leonard and Thomas, 1989; De Castro, 1991; Doyle et al., 1999; Shahar et al., 1999). This may be more pronounced in less developed countries where nutrient intakes depend on both seasonal availability and price of locally produced foods (Leonard and Thomas, 1989). Still, differences may be subtle, as a recent analysis of carefully collected data from two regions of rural India indicates (Hebert et al., 2000).
Seasonal variation of recreational physical activity also has been reported in several studies (Van Staveren et al., 1986; Bergstralh et al., 1990; Uitenbroek, 1993; Haggarty et al., 1994; Physical Activity and Health, 1996). In addition, our group (Matthews et al., 2001a) has described seasonal variation of recreational physical activity, as well as household and occupational physical activity in this healthy population in Central Massachusetts where temperature, hours of daylight, and monthly precipitation change significantly by season.
The prevalence of obesity in the US has increased dramatically over the last two decades (Kuczmarski et al., 1994, US Department of Health and Human Sciences, 2001; Flegal et al., 2002). Previous research has shown that weight change depends on energy balance, defined as the relationship between caloric intake and caloric expenditure due to the thermic effect of food, resting metabolic rate, and total physical activity (PA). Studies have also indicated that body weight fluctuates by season (Van Staveren et al., 1986; Sasaki et al., 1998; Shahar et al., 1999). However, the relationship between seasonal variation in body weight and seasonal changes in caloric intake and physical activity, and in particular the relative importance of these factors in determining weight change, has not been as well studied. It may be helpful for future studies to examine changes over the years and identify periods where people eat more, exercise less, and add weight. In addition, the presence of seasonal variation would need to be taken into account when counseling patients about healthy habits as well as when designing studies involving observation of diet and physical activity.
To date, few studies have reported on the simultaneous seasonal variations in caloric intake, caloric expenditure due to PA, and body weight. Using data from an observational study, we used sinusoidal regression models to evaluate seasonal variation of nutrient intake, PA, and body weight as amplitude and phase (a time while the measure was at peak).
Materials and methods
Subject recruitment and study design
Main study
Subjects from this investigation were from the Seasonal Variation of Blood Cholesterol (SEASONS) study, a large prospective study, which was designed to quantify the magnitude and timing of seasonal changes in blood lipids and to identify the major factors contributing to this variation including diet and PA (Merriam et al., 1999; Ockene et al., 2004). Most participants were recruited from the Fallon Healthcare Systems, a Health Maintenance Organization (HMO) in the Central Massachusetts/Worcester area. In addition, a recruiter was used to recruit members of ethnic minority groups who were not Fallon members to maximize the ethnic diversity of the study population. To be eligible, individuals had to be residents of Worcester County, aged between 20 and 70 years old, have telephone service, not have been taking cholesterol-lowering medications, not be actively on lipid-lowering or weight-control diets, be free of possible causes of secondary hypercholesterolemia, and be free of serious chronic illness (e.g., cancer, renal disease, heart failure, and diabetes). Detailed recruitment information has been described elsewhere (Merriam et al., 1999; Ockene et al., 2004). Subjects were recruited between December 1994 and February 1997. All subject recruitment and data collection procedures were approved by the Institutional Review Boards of the Fallon Healthcare System and the University of Massachusetts Medical School. Each subject signed an approved informed consent form prior to entering the study.
Data collection methods
Study participants were seen in the Fallon clinic for a baseline visit, then one visit every 3 months over the next year (five visits in total). In a 42-day sampling window around the clinic visit (−28 to + 14 days), three 24-h dietary recall interviews (24HR) were conducted on randomly selected days. These included two weekdays and one weekend day, and employed the Nutrition Data System data entry and nutrient database software developed and maintained by the Nutrition Coordinating Center at the University of Minnesota, Minneapolis, MN (NDS, 1996). The 24HR is the most widely used dietary assessment method (Willett, 1990). It has been used in large-scale epidemiologic studies such as US Department of Health and Human Services’ National Health and Nutrition Examination Survey (NHANES) (Dwyer et al., 2003), and USDA’s Continuing Survey of Food Intakes by Individuals (CSFII) (Kant et al., 1995). The interviewers employed standardized probing techniques, as directed by the NDS system (NDS, 1996), to collect additional information on all foods listed, including details on food preparation, size of portions eaten and items added to foods. The food data collected from the 24-h dietary recalls were converted to energy and nutrient data (e.g., total calories, percentage of calories from fat, saturated fat, carbohydrates, and protein) using the University of Minnesota’s Nutrition Coordinating Center’s Nutrition Data System for Research software. Glycemic index (GI) (the quality of carbohydrate) was determined from the 24HRs using published tables (Foster-Powell et al., 2002; Brand-Miller et al., 2003). Glycemic load (GL) (GI of a food × the amount of carbohydrate eaten)/100) was also calculated, as has been previously reported (Ma et al., 2005).
The 24-h PA data collection was administered together with the diet recall. We adapted the general data collection approach from the 7-day recall of physical activity (Sallis et al., 1985) to the 24-h time frame. Instead of asking about physical activity from the past seven days, participants were asked to recall hours they spent the previous day at different types and intensities of activities. MET values from type of physical activity were obtained from a physical activity compendium published by Ainsworth et al. 2000. The 24-h physical activity recall method was validated against both accelerometers and standard questionnaires (Matthews et al., 2000) and results were comparable to published data (Matthews et al., 2000). All interviews were conducted by registered dietitians trained specifically in the use of this 24-h interview methodology. Body weight was measured at each clinic visit, with the subject removing her/his shoes and wearing minimal layers of clothing. Height (in centimeters) was measured at baseline. Relative mass is expressed as body mass index (BMI = weight(kg)/height(m)2). Data on demographic variables were collected by a self-administered questionnaire at the baseline clinic visit. A total of fifteen 24-h diet and physical activity recalls were potentially available for each subject, with body weight measured for each subject up to five times.
Exclusion criteria for analyses
Of the 641 subjects entered into the study, 44 subjects who were diagnosed with seasonal affective disorder by the Seasonal Pattern Assessment Questionnaire (Rosenthal et al., 1984; Harmatz et al., 2000) were excluded. Additionally, four subjects with two or fewer 24-h diet recalls also were excluded from the analyses. Therefore, the total number of the subjects included in this analysis was 593. The average number of recalls was 11.0 (standard deviation (s.d.) = 4.7) per subject. The average number of body weight measures was 4.6 (s.d. = 1.2) per subject.
Statistical analyses
Participants’ demographic characteristics, as well as relevant dietary factors, PA, and body weight, were described as mean (s.d.) or n (%), for nonparametric variables. Two methods were used to evaluate seasonal effects using mixed models (Littell et al., 1996). First, season of the year was defined using the common season definition: Winter: December 21–March 20; Spring: March 21–June 20; Summer: June 21–September 20; and Fall September 21–December 20. Season-specific means (SE) were obtained using the mixed model by fitting dietary factors, PA, and body weight as dependent variables, season of the year as a fixed effect, and subject as a random effect. Secondly, sinusoidal regression models were used to estimate peak-to-trough amplitude and phase of the peaks for diet, PA, and body weight (Nam, 1995). Amplitude was calculated as peak-to-trough distance, or the maximal difference between the highest and lowest values during the year. Timing of the peak value reached during the year, or the phase, also was identified and reported as the calendar date. This method has been applied successfully in previous reports on seasonal variation in physical activity and blood lipids by our group (Matthews et al., 2001a; Ockene et al., 2004). For diet and physical activity variables, the date of 24-h recalls was used to define sine and cosine coefficients for a sine-shaped seasonal model that assumed a period of 365 days (Koopmans, 1974). Models were fit while controlling for gender, age, education, race/ethnicity, and weekday versus weekend 24HR. Estimates of fixed-effect regression coefficients for the sine and cosine terms in the mixed model were transformed to estimate the amplitude and phase of the seasonal effects. A first-order Taylor series expansion was used to construct estimates of the variance of the amplitude and phase from the variance estimates of the sine and cosine coefficients (see appendix from the article by Matthews et al. (2001a) and the website for SAS codes http://www-unix.oit.umass.edu/~seasons/se35.pdf). SAS version 9.1 for Windows was used for the sinusoidal analyses. Amplitude and phase for body weight were estimated similarly, but the date used for the analysis was the clinic visit date. Gender, age category, education level, and race-specific mixed models were fit to estimate amplitude and phase for each group.
We checked the residual distribution for the models. They were approximately normally distributed, indicating that the models were well fit.
Results
Subjects’ average age was 47.6 years (s.d. = 12.4) at baseline. The study group was predominantly white (85.6%) and employed full-time, with the great majority having completed at least a high school education. Of the 83 nonwhite subjects, 48 were Hispanic and 15 were Black non-Hispanic. Gender distribution was fairly even, with females comprising 46.7% of the group. Most of the participants were overweight or obese; the average BMI was 27.2 kg/m2 with a s.d. of 5.24 kg/m2. See Table 1 for detailed demographic information.
Table 1.
Characteristics of study participants at baseline, Seasonal Variation of Blood Cholesterol study, Worcester, Massachusetts, 1994–1998
| Variable | Mean/frequency | s.d./% |
|---|---|---|
| Age (years) | 47.6 | 12.4 |
| Gender | ||
| Male | 316 | 53.3 |
| Female | 277 | 46.7 |
| Ethnicity/race | ||
| White | 494 | 85.6 |
| Other | 83 | 14.4 |
| Education | ||
| HS or less | 151 | 25.6 |
| Some college or associates degree | 215 | 36.4 |
| College/graduate | 224 | 38.0 |
| Employment | ||
| Full-time | 407 | 68.9 |
| Part-time | 85 | 14.4 |
| Unemployed/retired | 99 | 16.8 |
| Occupational category | ||
| Unemployed/retired | 116 | 19.7 |
| Blue collar | 95 | 16.2 |
| Service work | 161 | 27.4 |
| White collar | 216 | 36.7 |
| Current smoking | ||
| Yes | 88 | 15.9 |
| No* | 465 | 84.1 |
| BMI classification | ||
| Normal (18.5–24.9) | 216 | 36.4 |
| Overweight (25–29.9) | 236 | 39.8 |
| Obese (≥30) | 141 | 23.8 |
| Mean | 27.22 | 5.2 |
Due to missing values the total number of subjects differs.
Defined as never having smoked or having quit smoking at least 1 year prior to enrollment.
The average daily total caloric intake was 1963 kcal (s.d. = 805), average percentage of calories from carbohydrate was 51.2 (s.d. = 11.3), from fat was 31.5 (s.d. = 9.5), and from protein was 16.2 (s.d. = 4.5). Average glycemic index, using white bread as the reference, was 83.6 (s.d. = 9.7), and percentage of calories from saturated fat was 11.2 (s.d. = 4.5). Table 2 shows means of dietary factors, physical activity, and body weight by season. The results indicated that percentages of calories from fat and saturated fat, physical activity, and body weight varied by season. Percentages of calories from fat and saturated fat were highest in the fall. The peak of PA was in the spring, and the peak of body weight was in the winter.
Table 2.
Relevant dietary factors and physical activity by season, Seasonal Variation of Blood Cholesterol study, Worcester, Massachusetts, 1994–1998
| Variable | Winter mean (s.e.) | Spring mean (s.e.) | Summer mean (s.e.) | Fall mean (s.e.) | P-valuea |
|---|---|---|---|---|---|
| Dietary factors | |||||
| Caloric intake (kcal/d) | 1958 (22.9) | 1942 (23.4) | 1956 (23.5) | 1987 (23.4) | 0.50 |
| % calories from carbohydrate | 51.3 (0.3) | 51.4 (0.3) | 51.3 (0.3) | 50.6 (0.3) | 0.25 |
| % calories from fatb | 31.3 (0.3) | 31.0 (0.3) | 31.6 (0.3) | 32.0 (0.3) | 0.038 |
| % calories from protein | 16.1(0.1) | 16.3 (0.1) | 16.0 (0.1) | 16.1 (0.1) | 0.53 |
| Type of carbohydrate | |||||
| GI | 83.4 (0.3) | 83.5 (0.3) | 84.0 (0.3) | 83.5 (0.3) | 0.33 |
| GL | 192.7 (2.6) | 190.1 (2.7) | 192.6 (2.7) | 193.1 (2.7) | 0.82 |
| Type of fat | |||||
| % calories from saturated fatc | 11.1 (0.1) | 11.1 (0.1) | 11.2 (0.1) | 11.6 (0.1) | 0.02 |
| Total physical activity (MET-h/d)d | 29.9 (0.2) | 30.7 (0.2) | 30.5 (0.2) | 30.0 (0.2) | 0.007 |
| Body weight (kg)e | 79.0 (0.8) | 78.8 (0.8) | 78.6 (0.8) | 78.8 (0.8) | 0.002 |
P-value was from mixed model comparing means among four seasons, fitting subject as random effect.
Multiple comparisons show difference between winter and fall (0.023), as well as spring and fall (0.007).
Multiple comparisons show difference between winter and fall (P = 0.004), as well as spring and fall (P = 0.007), and summer and fall (P = 0.03).
Multiple comparisons show difference between winter and spring (P = 0.002), and winter and summer (P = 0.03); also spring and fall (P = 0.01).
Multiple comparisons show difference between winter and summer (P<0.001), winter and fall (P = 0.02) and spring and summer (P = 0.008).
Table 3 shows the magnitude of seasonal variation, as well as where peak values occurred for each factor after adjustment for age, gender, education, and race. We observed a seasonal variation in daily caloric intake, with the highest intake occurring in the fall and peaking in early November. The lowest daily caloric intake occurred in the spring, peaking in early May, with an average difference of 86 kcal/day between the two extremes. Percentage of calories from carbohydrate, fat, and saturated fat showed slight seasonal variation, while percentage of calories from protein remained essentially stable throughout the year. Carbohydrate percentage was highest in the spring, while fat percentage was highest in the fall. Average daily glycemic index (GI) showed only very slight variation, peaking in the summer. There also was only modest variation in PA, although we did observe the lowest level of activity in the winter and the highest in the spring, with the peak occurring in late June. Body weight varied by about half a kilogram throughout the year, with a peak in the winter. Finally, the ratio of caloric intake to expenditure was highest during the fall to early winter, which is expected given the increased caloric and fat intake during the fall and the decreased physical activity in the winter. Seasonal variation of selected nutrient variables, PA, and body weight is displayed in Figure 1. We noted that there were time lags between the peak of caloric intake and body weight, the peak of percentage of calories from fat intake and body weight, and the peak of physical activity and body weight. Peak of caloric intake was in early November while peak of percentage of calories from fat intake was in early September. The lowest reported total physical activity did not occur until December, while the peak of body weight occurred in February.
Table 3.
Seasonal variationa of dietary intake, physical activity, and body weight, Seasonal Variation of Blood Cholesterol study, Worcester, MA, 1994-1998
| Variable | Amplitude (95% CI) | Phase (95% CI) |
|---|---|---|
| Dietary factors | ||
| Caloric intake (kcal/d) | 86 (41, 131) | November 15 (October 16, December 15) |
| % calories from carbohydrates | 0.80 (0.16, 1.43) | April 03 (February 16, May 20) |
| % calories from fat | 0.67 (0.13, 1.22) | September 27 (August 10, November 14) |
| % calories from protein | 0.11 (−0.19, 0.41) | — |
| Type of carbohydrate | ||
| GI | 0.97 (0.35, 1.60) | July 30 (June 22, September 06) |
| GL | 2.17 (−3.12, 7.46) | — |
| Type of fat | ||
| % calories from saturated fat | 0.43 (0.17, 0.69) | October 30 (September 26, December 03) |
| Physical activity (MET-h/d) | 1.24 (0.78, 1.71) | June 29 (June 07, July 21) |
| Ratio of caloric intake to expenditure | 0.04 (0.01, 0.06) | December 02 (October 27, January 07) |
| Body weight (kg) | 0.48 (0.38, 0.59) | February 10 (January 28, February 23) |
Adjusted for age, gender, education, and race/ethnicity.
Figure 1.
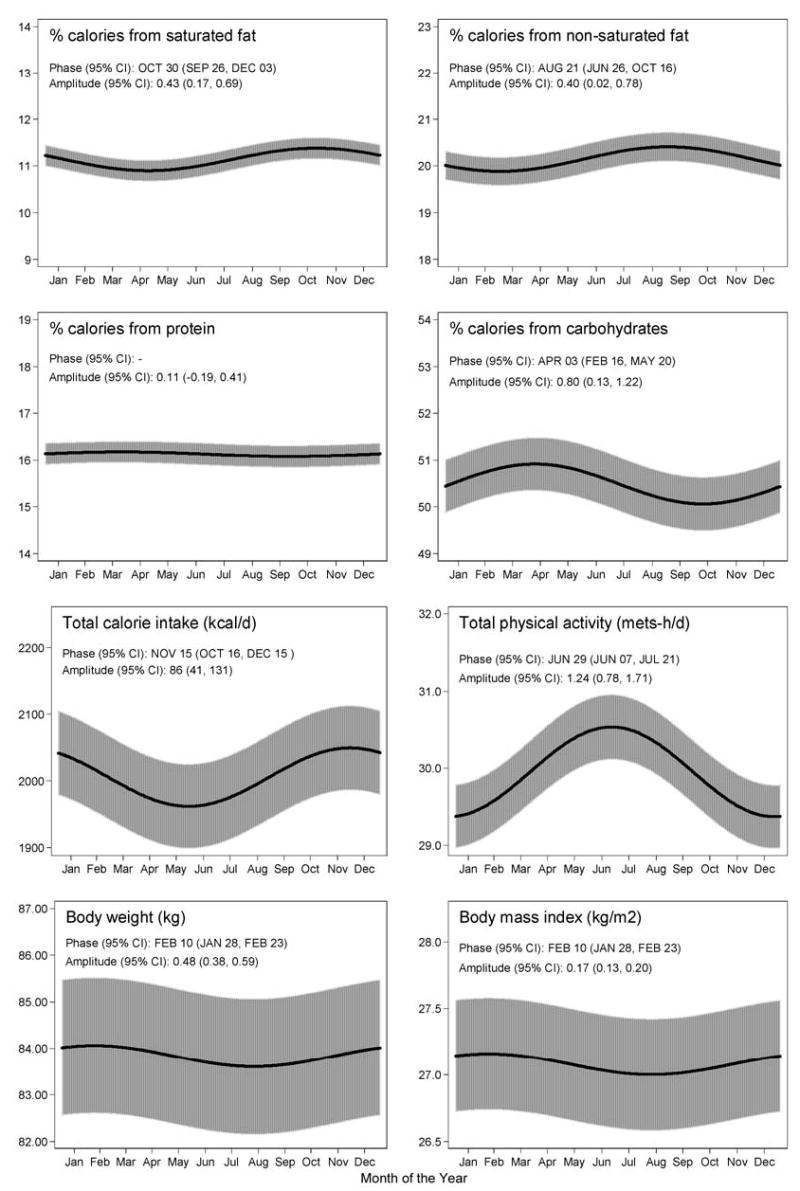
Seasonal variation of dietary composition, caloric intake, physical activity, body weight and body mass index, Seasonal Variation of Blood Cholesterol Study, Worcester, MA, 1994–1998. These graphs show the pattern of seasonal variation in these factors over the 1-year period of the study.
There were some differences in seasonal variation of dietary intake, PA, and body weight by demographic categories. In comparison with female subjects, there appeared to be a slightly greater seasonal variation of daily caloric intake, physical activity, and body weight in males.
The largest seasonal variation of daily caloric intake was observed in the age group between 40 and 50 years old, at 134 kcal/day (95% CI: 46, 222), while caloric intake for subjects in the age group between 50 and 60 years old was relatively stable. Age-group comparisons showed divergence in the timing of the peak value of the log-transformed ratio of caloric intake to expenditure. For subjects in their 60s and 70s, the peak value occurred in early July, while for those in their 50s it was in late November (P = 0.04 for difference). The peak occurred even later, around the winter solstice, for subjects in their 40s (P = 0.01 for difference from 60s to 70s). In addition, the timing of variation in physical activity was different for subjects under 40 and in their 50s than those 60–70 years old – the peak for 60- to 70-year-olds was a few weeks after the summer solstice, but for those under 40 it occurred a few weeks before the winter solstice and for those in their 50s just about the winter solstice (December 24) (P<0.0001 for both differences). The largest seasonal variation in body weight occurred for subjects between 40 and 50 years old, at 0.66 kg (95% CI: 0.46, 0.87).
Large differences were found between white and nonwhite subjects in both timing and magnitude of seasonal variation of daily caloric intake. The amplitude of variation for whites was 67 kcal/day, while for nonwhites it was 409 kcal/day (P<0.0001). The timing of the peak caloric intake also differed, occurring in early December for whites and early October for nonwhites (P<0.0001). Peak physical activity did not occur at the same time in whites and nonwhites – about two months earlier (i.e., early July for whites and late October for nonwhites; P<0.0001). In addition, the peak caloric-intake-to-expenditure ratio occurred at a different time for whites versus nonwhites. The ratio peaked just before the winter solstice for whites and just before the autumnal equinox for nonwhites. When log-transformed, there was essentially no change in both cases. This is logical given the differences in timing of peak caloric intake and PA. The magnitudes of seasonal variation in PA and body weight between white and nonwhites were similar.
When we examined educational categories, we found that subjects with high school education or less had the greatest variation in caloric intake (116 kcal/day (95% CI: 34, 198)) and the least variation in PA (1.02 met-h/day (95% CI: 0.19, 1.86)). However, the greatest variation in body weight was observed in subjects with some college education (0.88 kg (95% CI: 0.68, 1.08)). The timing of peak daily glycemic index (GI) differed by education levels. For college graduates, the peak occurred in mid-December, while for those with less than a high school diploma it occurred in mid-July 18 (P = 0.0411). For high school graduates, GI peaked around September 20, with a P-value of 0.0273 for the difference from college graduates. Subjects who had completed some college showed a peak daily GI in early July (P = 0.0002 for difference from college graduates). In addition, the only observations of differences in the timing of peak physical activity were between educational categories. The peak occurred around December 12 for college graduates, while it was around July 9 for high school graduates and July 24 for subjects who had completed some college (P<0.0001 for the difference of these two groups from college graduates).
Discussion
Small seasonal fluctuations were observed in total calories, carbohydrate, and fat intake, as well as PA and body weight in this cohort. The increase in daily caloric and fat intake during the fall, and decreased PA in the winter coincided with the increase in body weight in the winter. However, these changes were generally small. We did find that seasonal variation was greater in male, middle-aged, non-white, and less-educated subjects, with the most pronounced differences being between white and non-white (mostly Hispanic and Black) subjects. However, the nonwhite group only consisted of 83 subjects, as opposed to 494 white subjects, while race/ethnicity information was not available on 16 participants. The apparent difference may therefore be partially a function of small sample size for nonwhites; this small sample size is a limitation of the study. The results of this study suggest that seasonal variation is not a major factor in the relationship between lifestyle and body weight.
Consistent with results from this study, De Castro (1991) and Doyle et al. 1999 reported that daily caloric intake varied by season, yet this variation was larger than we noted. De Castro observed a 222 kcal/day difference between the total daily intake in the fall versus the spring. De Castro’s study was conducted in late 1980 and average age of subjects was 32 years old, while our study was conducted between 1994 and 1998 and average age was 48 years. The magnitude of difference found by De Castro was much greater than the 86 kcal/day that we observed in our study (though for nonwhites it was much less). On the other hand, several other studies have suggested that daily caloric intake does not vary by season (Hackett et al., 1985; Van Staveren et al., 1986; Subar et al., 1995; Shahar et al., 1999), including in less developed countries such as China (Cai et al., 2004) and India (Hebert et al., 2000).
There is evidence suggesting that the intake of total protein does not change by season (Hackett et al., 1985; Krauchi and Wirz-Justice, 1988; Hebert et al., 2000; Cai et al., 2004), as well as contradictory evidence of increased protein intake during the autumn and winter (Doyle et al., 1999). A separate study reported that the intake of protein showed suggestive, although not significant, seasonal changes (De Castro, 1991). However, our study showed no significant seasonal variation in the percentage of calories from protein.
There has been considerably more agreement regarding the significance of seasonal variation in the intake of fat and carbohydrates. Data have suggested that each nutrient exhibits seasonal differences in intake. Previous studies have shown that carbohydrates have a peak intake during the fall (De Castro, 1991) or winter (Krauchi and Wirz-Justice, 1988), and a minimum in the summer. One hypothesized reason for this phenomenon is that in some individuals there may be a drop in serotonin levels during the winter (Van Staveren et al., 1986). Such a drop in serotonin has a tendency to stimulate an urge to eat more carbohydrate-rich foods. The intake of fats follows a similar pattern (De Castro, 1991), with peaks occurring during the winter (Van Staveren et al., 1986; Shahar et al., 1999) and spring (Van Staveren et al., 1986). However, in our study, we observed the peak percentage of calories from carbohydrates to occur in the spring, which differs from the findings of previous studies. It is important to note that in both China and India variability in micronutrients, such as beta carotene, was larger than macronutrients (Hebert et al., 2000; Cai et al., 2004). This may indicate a reasonable shift to more affordable and accessible sources of carbohydrate, fat, and protein to compensate in seasonal variations in cost and availability in what are large sources of macronutrients in virtually all populations.
Reports on seasonal variation of PA and body weight have been relatively consistent. At temperate latitudes, PA has been found to increase in the spring and to decrease in the winter (Van Staveren et al., 1986; Bergstralh et al., 1990; Uitenbroek, 1993; Haggarty et al., 1994 Haggarty et al., 1996; Matthews et al., 2001a), while body weight has been found to increase during the winter, and to decrease in the summer (Van Staveren et al., 1986; Sasaki et al., 1998; Shahar et al., 1999).
A small but statistically significant increase in body weight was shown in the winter. Several factors which are positively related to body weight were increased before the peak of body weight: these include total caloric intake, dietary glycemic index, and percentage of calories from fat and saturated fat (Donato and Hegsted, 1985; Pi-Sunyer, 1990; Ludwig, 2000; Ludwig, 2002; Ma et al., 2005). Total PA, which was negatively related to body weight (Ball and Crawford, 2003; Lazzer et al., 2005), was lowest in the winter. In nonwhites the increased caloric intake goes along with an increased PA (i.e., both peak in October), but apparently the increased PA was unable to prevent weight gain. In addition, the ratio of caloric intake to expenditure and body weight peaked in the winter. In the SEASONS study, blood lipid levels exhibited seasonal variation, being higher in the winter and lower in the summer (Ockene et al., 2004). In addition to plasma volume, body weight, physical activity, and saturated fat intake explain some of the variation. Another study found seasonal variation in LDL levels and body mass index, related to variation in dietary fat and saturated fat intake (but not caloric intake, which did not vary significantly); these were all higher in the winter (Shahar et al., 1999). We suggest that in anticipation of the possible weight gain, individuals need to be conscious of their daily total caloric intake in the winter, their percentage of caloric intake from fat and saturated fat in the fall; and total physical activity before and during the winter.
We observed time lags between the peaks of caloric intake and body weight, the peaks for the percent of calories from fat and body weight, and between the lowest reported total physical activity and the peak of body weight. The delay makes sense given that it would take some time for body weight to change in response to changes in diet and/or physical activity. A cumulative deficit of PA and cumulative increase in calories and fat intake may explain the increase of 0.5 kg in body weight during the winter. Specialized statistical methods will need to be developed in order to understand the nature of the time lag and account for it in future analyses.
A previous study by our group demonstrated that participants with a college degree derived the least benefit from a low-fat, low-calorie dietary intervention, whereas those with no more than a high school education showed a much greater effect (Hebert et al., 1999). This may be due to more educated patients having received considerable exposure to lifestyle-improvement messages from peers and from the media, and therefore adhering to dietary recommendation, whereas the less-educated have not received these lifestyle improvement messages.
We found that subjects with high school education or less had the least variation in physical activity. It is possible that less-educated subjects are employed in jobs demanding manual labor, so their physical activity remains more constant over the year. In contrast, more educated people tend to have sedentary jobs, so their PA would tend to vary according to the seasonal demands of yard work, availability of outside exercise facilities, and willingness to exercise indoors.
There are several strengths to our investigation. First, both sinusoidal and fixed season approaches were used in the analyses. Sinusoidal method decomposes date into two variables with sine and cosine values to fit into the model, allowing for a continuous function rather than simply four seasons. This retains the maximum amount of information on date when measures were collected. The sinusoidal approach appeared to be more powerful. Secondly, the 24-h dietary recalls were used in the collection of dietary intake, and this provides more accurate data than food frequency questionnaires (FFQs), as recall bias is less of an issue with the 24-h dietary recalls (Hebert et al., 2002). Thirdly, all participants were not within the same socioeconomic status as indicated by educational levels. Finally, the current study collected information on diet, physical activity, and body weight at the same time over 1-year period, which allows us to examine seasonal variation of these variables simultaneously.
Our study also has several potential limitations. First, information on diet and physical activity was obtained from self-report via 24-h recalls. Although a single 24 h recall is unlikely to capture a person’s true diet and physical activity and could result in misclassification, three recalls (the number per time point) begins to approximate an individual’s usual intake of macronutrients – caloric intake, carbohydrate, protein, and fat (Hebert et al., 1998; Willett, 1998) and PA (Matthews et al., 2001a). Although there is always the potential for misclassification due to error in self-reporting, the error is minimized by using trained dietitians and asking participants to recall the previous day only. It is true that 24HRs are subject to bias in comparison with biochemical markers; however, biochemical marker testing such as the doubly labeled water method is very expensive and therefore impractical for large epidemiological studies. Secondly, prediction of GI and GL values of foods based on existing international tables has some variability between populations, so our GI and GL calculation could be biased. Thirdly, our participants are a generally healthy population in the Central Massachusetts/Worcester area consisted largely of white, middle-class members of an HMO, from a single geographic location. Also, as the study protocol involved a lengthy series of clinic visits, and diet and physical activity assessments, participants needed to have been relatively highly motivated. Selection factors relating to the participants’ interest in their own health and time availability for participation may have contributed to creating a fairly homogeneous study group. For these reasons, our findings may not be fully generalizable to other socio-economic strata, and to other cultures and ethnic groups. Finally, this study was conducted using existing data collected during 1994–1998; it would be useful to do a similar study at this time to examine these factors. However, we believe the data we are presenting are still very useful given the need to understand contributors leading to the current state of obesity, diet, and PA and their variations.
In conclusion, the present study revealed that small seasonal variations of daily caloric intake, diet composition, physical activity, and body weight are in fact present in normal individuals in the United States. These variations, however, do not appear great enough to take into account in the design of longitudinal studies and cross-sectional studies for the collection of dietary, PA, and body weight data. These fluctuations were greater in subjects who were male, middle aged, nonwhite, and less educated. Results of the study lend support to the well-established evidence-based advice to maintain PA and caloric intake at a steady state during the winter in order to avoid weight gain.
Acknowledgments
The project described was supported by Grant No. R01-HL52745 to Dr Ira S Ockene and 1 R21 HL074895-01 to Dr Yunsheng Ma from the National Heart, Lung and Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI. The authors thank Philip Merriam, Laura Robidoux, and Priscilla Cirillo for their assistance with study recruitment and data collection; Kelly Scribner for coordination of the 24-h recalls; and Seasons dieticians who conducted the 24-h recalls: Susan Nelson, Christine Singelton, Pat Jeans, Karen Lafayette, Deborah Lamb, Stephanie Olson and Eileen Capstraw. The authors also thank Drs Charles Matthews and Patty Freedson for their contribution on physical activity measurements.
Footnotes
Sponsorship: US National Heart, Lung and Blood Institute.
Guarantor: Y Ma.
Contributors: Study concept and design: YM, BCO, WL, and ISO. Statistical analysis: WL, and ARH. Drafting of the manuscript: YM and BCO. Critical revision of the manuscript for important intellectual contents: WL, ARH, DC, JRH, MC, MS, and ISO.
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32 (Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Ball K, Crawford D. The obesity epidemic: contextual influences on physical activity and body weight. J Sci Med Sport. 2003;6:377–378. doi: 10.1016/s1440-2440(03)80263-3. [DOI] [PubMed] [Google Scholar]
- Bergstralh EJ, Sinaki M, Offord KP, Wahner HW, Melton LJ., III Effect of season on physical activity score, back extensor muscle strength, and lumbar bone mineral density. J Bone Miner Res. 1990;5:371–377. doi: 10.1002/jbmr.5650050410. [DOI] [PubMed] [Google Scholar]
- Brand-Miller J, Wolever TM, Foster-Powell K, Colagiuri S (2003). The New Glucose Revolution: The Authoritative Guide to the Glycemic Index–the Dietary Solution for Lifelong Health. Marlowe & Company: New York, NY.
- Cai H, Shu XO, Hebert JR, Jin F, Yang G, Liu DK, et al. Variation in nutrient intakes among women in Shanghai, China. Eur J Clin Nutr. 2004;58:1604–1611. doi: 10.1038/sj.ejcn.1602013. [DOI] [PubMed] [Google Scholar]
- De Castro J. Seasonal rhythms of human nutrient intake and meal pattern. Physiol Behav. 1991;50:243–248. doi: 10.1016/0031-9384(91)90527-u. [DOI] [PubMed] [Google Scholar]
- Donato K, Hegsted DM. Efficiency of utilization of various sources of energy for growth. Proc Natl Acad Sci USA. 1985;82:4866–4870. doi: 10.1073/pnas.82.15.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle W, Crawley H, Robert H, Bates C. Iron deficiency in older people: interactions between food and nutrient intakes with biochemical measures of iron; further analysis of the National Diet and Nutrition Survey of people aged 65 years and over. Eur J Clin Nurt. 1999;53:552–559. doi: 10.1038/sj.ejcn.1600787. [DOI] [PubMed] [Google Scholar]
- Dwyer J, Picciano MF, Raiten DJ. Collection of food and dietary supplement intake data: what we eat in America-NHANES. J Nutr. 2003;133:590S–600S. doi: 10.1093/jn/133.2.590S. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Hackett A, Appleton D, Rugg-Gunn A, Eastoe J. Some influences on the measurement of food intake during a dietary survey of adolescent. Hum Nutr Appl Nutr. 1985;39:167–177. [PubMed] [Google Scholar]
- Haggarty P, McNeill G, Manneh MK, Davidson L, Milne E, Duncan G, et al. The influence of exercise on the energy requirements of adult males in the UK. Br J Nutr. 1994;72:799–813. doi: 10.1079/bjn19940086. [DOI] [PubMed] [Google Scholar]
- Harmatz GM, Well AD, Overtree CE, Kawamura KY, Rosal MC, Ockene IS. Seasonal variation of depression and other moods: a longitudinal approach. J Biol Rhythms. 2000;15:344–350. doi: 10.1177/074873000129001350. [DOI] [PubMed] [Google Scholar]
- Hartman AM, Brown CC, Palmgren J, Pietinen P, Verkasalo M, Myer D, et al. Variability in nutrient and food intakes among older middle-aged men. Implications for design of epidemiologic and validation studies using food recording. Am J Epidemiol. 1990;132:999–1012. doi: 10.1093/oxfordjournals.aje.a115743. [DOI] [PubMed] [Google Scholar]
- Hebert J, Ebbeling C, Matthews C, Ma Y, Clemow L, Hurley T, et al. Systematic errors in middle-aged women’s estimates of energy intake: comparing three self-report measures to total energy expenditure from doubly labeled water. Ann Epidemiol. 2002;12:577–586. doi: 10.1016/s1047-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Hebert J, Ebbeling C, Ockene I, Ma Y, Rider L, Merriam P, et al. A dietitian-delivered group nutrition program leads to reductions in dietary fat, serum cholesterol, and body weight: the Worcester Area Trial for Counseling in Hyperlipidemia (WATCH) J Am Diet Assoc. 1999;99:544–552. doi: 10.1016/s0002-8223(99)00136-4. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Gupta PC, Mehta H, Ebbeling CB, Bhonsle RR, Varghese F. Sources of variability in dietary intake in two distinct regions of rural India: implications for nutrition study design and interpretation. Eur J Clin Nutr. 2000;54:479–486. doi: 10.1038/sj.ejcn.1601042. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Hurley TG, Chiriboga DE, Barone J. A comparison of selected nutrient intakes derived from three diet assessment methods used in a low-fat maintenance trial. Public Health Nutr. 1998;1:207–214. doi: 10.1079/phn19980032. [DOI] [PubMed] [Google Scholar]
- Kant AK, Ballard-Barbash R, Schatzkin A. Evening eating and its relation to self-reported body weight and nutrient intake in women, CSFII 1985–86. J Am Coll Nutr. 1995;14:358–363. doi: 10.1080/07315724.1995.10718521. [DOI] [PubMed] [Google Scholar]
- Koopmans LH (1974). The Spectral Analysis of Time Series. Academic Press: New York.
- Krauchi K, Wirz-Justice A. The four seasons: food intake frequency in seasonal affective disorder in the course of a year. Psychiatr Res. 1988;25:323–338. doi: 10.1016/0165-1781(88)90102-3. [DOI] [PubMed] [Google Scholar]
- Kuczmarski R, Flegal K, Campbell S, Johnson C. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- Lazzer S, Boirie Y, Poissonnier C, Petit I, Duche P, Taillardat M, et al. Longitudinal changes in activity patterns, physical capacities, energy expenditure, and body composition in severely obese adolescents during a multidisciplinary weight-reduction program. Int J Obes. 2005;29 (1):37–46. doi: 10.1038/sj.ijo.0802845. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Thomas RB. Biosocial responses to seasonal food stress in highland Peru. Hum Biol. 1989;61:65–85. [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R (1996). SAS System for Mixed Models. SAS Institute Inc.: Cary, NC.
- Ludwig DS. Dietary glycemic index and obesity. J Nutr. 2000;130 (Suppl):280S–283S. doi: 10.1093/jn/130.2.280S. [DOI] [PubMed] [Google Scholar]
- Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- Ma Y, Olendzki B, Chiriboga D, Hebert J, Li Y, Li W, et al. Association between dietary carbohydrates and body weight. Am J Epidemiol. 2005;161:359–367. doi: 10.1093/aje/kwi051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews CE, Freedson P, Hebert J, Stanek E, Ockene I, Merriam P. Comparison of physical activity assessment methods in the Seasonal Variation of Blood Cholesterol Levels Study. Med Sci Sports Exerc. 2000;32:976–984. doi: 10.1097/00005768-200005000-00015. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Freedson PS, Stanek EJ, Hebert JR, Merriam PA, Rosal MC, et al. Seasonal variation of household, occupational, and leisure-time physical activity: longitudinal analyses from the seasonal variation of cholesterol study. Am J Epidemiol. 2001a;153:172–183. doi: 10.1093/aje/153.2.172. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Hebert JR, Freedson PS, Stanek EJ, III, Merriam PA, Ebbeling CB, et al. Sources of variance in daily physical activity levels in the seasonal variation of blood cholesterol study. Am J Epidemiol. 2001b;153:987–995. doi: 10.1093/aje/153.10.987. [DOI] [PubMed] [Google Scholar]
- Merriam PA, Ockene IS, Hebert JR, Rosal MC, Matthews CE. Seasonal variation of blood cholesterol levels: study methodology. J Biol Rhythms. 1999;14:330–339. doi: 10.1177/074873099129000669. [DOI] [PubMed] [Google Scholar]
- Nam J. Interval estimation and significance testing for cyclic trends in seasonality studies. Biometrics. 1995;51:1411–1417. [PubMed] [Google Scholar]
- NDS (1996). Version 2.9 The Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN.
- Ockene IS, Chiriboga DE, Stanek EJI, Harmatz MG, Nicolosi R, Saperia G, et al. Seasonal variation in serum cholesterol: treatment implications and possible mechanisms. Arch Intern Med. 2004;164:863–870. doi: 10.1001/archinte.164.8.863. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX. Effect of the composition of the diet on energy intake. Nutr Rev. 1990;48:94–105. doi: 10.1111/j.1753-4887.1990.tb02911.x. discussion 114–131. [DOI] [PubMed] [Google Scholar]
- Physical Activity and Health (1996). A report of the Surgeon General. Washington, DC, U.S. Department of Health and Human Services, President’s Council on Physical Fitness and Sports.
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- Sallis J, Haskell W, Wood P, Fortmann S, Rodgers T, Blair S, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Sakamoto K, Akaho R, Nakajima T, Takahashi K. Familial transmission of seasonal changes in sleep and eating function in the general population. Psychiatr Res. 1998;81:211–217. doi: 10.1016/s0165-1781(98)00093-6. [DOI] [PubMed] [Google Scholar]
- Shahar D, Froom P, Harari G, Yerushalmi N, Lubin F, Kristal-Boneh E. Changes in dietary intake account for seasonal changes in cardiovascular disease risk factors. Eur J Clin Nutr. 1999;53:395–400. doi: 10.1038/sj.ejcn.1600761. [DOI] [PubMed] [Google Scholar]
- Subar A, Thompson F, Smith A, Jobe J, Ziegler R, Potischman N, et al. Improving food frequency questionnaires: a qualitative approach using cognitive interviewing. J Am Diet Assoc. 1995;95:781–788. doi: 10.1016/s0002-8223(95)00217-0. [DOI] [PubMed] [Google Scholar]
- Subar AF, Frey CM, Harlan LC, Kahle L. Differences in reported food frequency by season of questionnaire administration: The 1987 National Health Interview Survey. Epidemiology. 1994;5:226–233. doi: 10.1097/00001648-199403000-00013. [DOI] [PubMed] [Google Scholar]
- Uitenbroek DG. Seasonal variation in leisure time physical activity. Med Sci Sports Exerc. 1993;25:755–760. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Sciences (2001). The Surgeon General’s call to action to prevent and decrease overweight and obesity. Rockville, MD, U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General. [PubMed]
- Van Staveren W, Deurenberg P, Burema J, De Groot L, Hautvast J. Seasonal variation in food intake, pattern of physical activity and change in body weight in a group of young adult Dutch women consuming self-selected diets. Int J Obes. 1986;10:133–145. [PubMed] [Google Scholar]
- Willett WC (1990). Nutritional Epidemiology. Oxford University Press: Oxford.
- Willett WC (1998). Nutritional Epidemiology. Oxford University Press: Oxford.


