Abstract
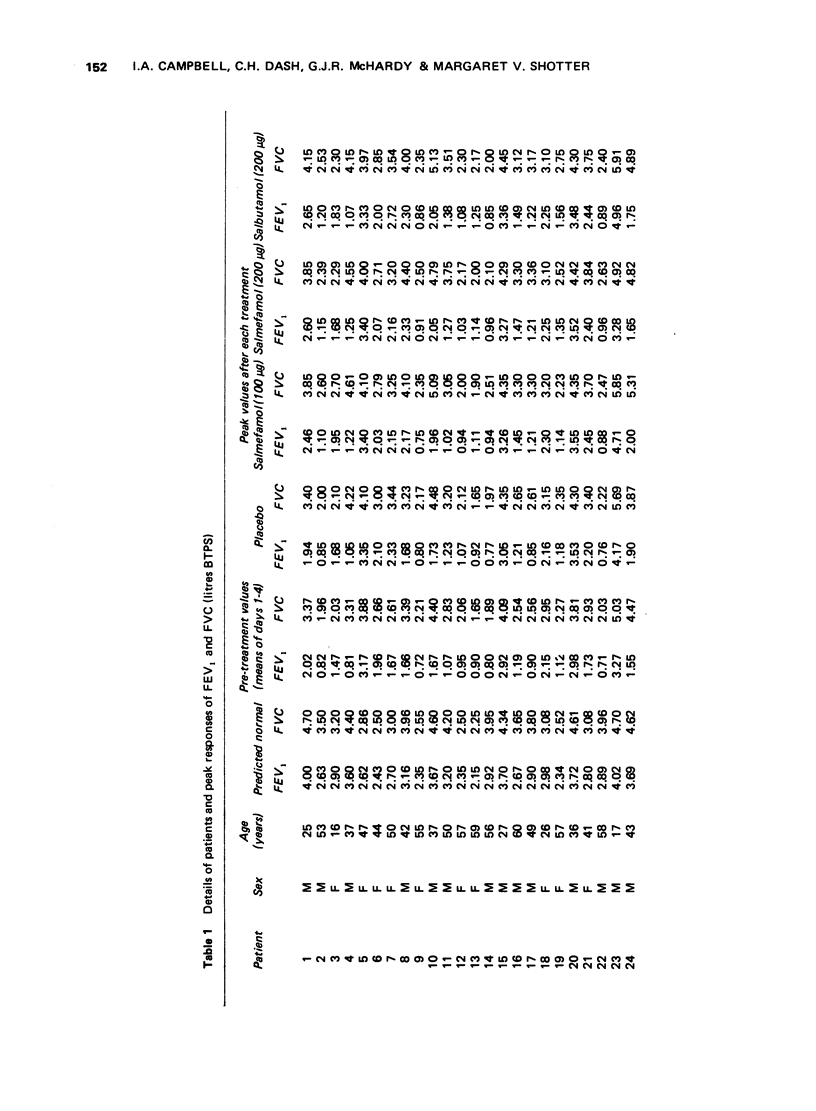
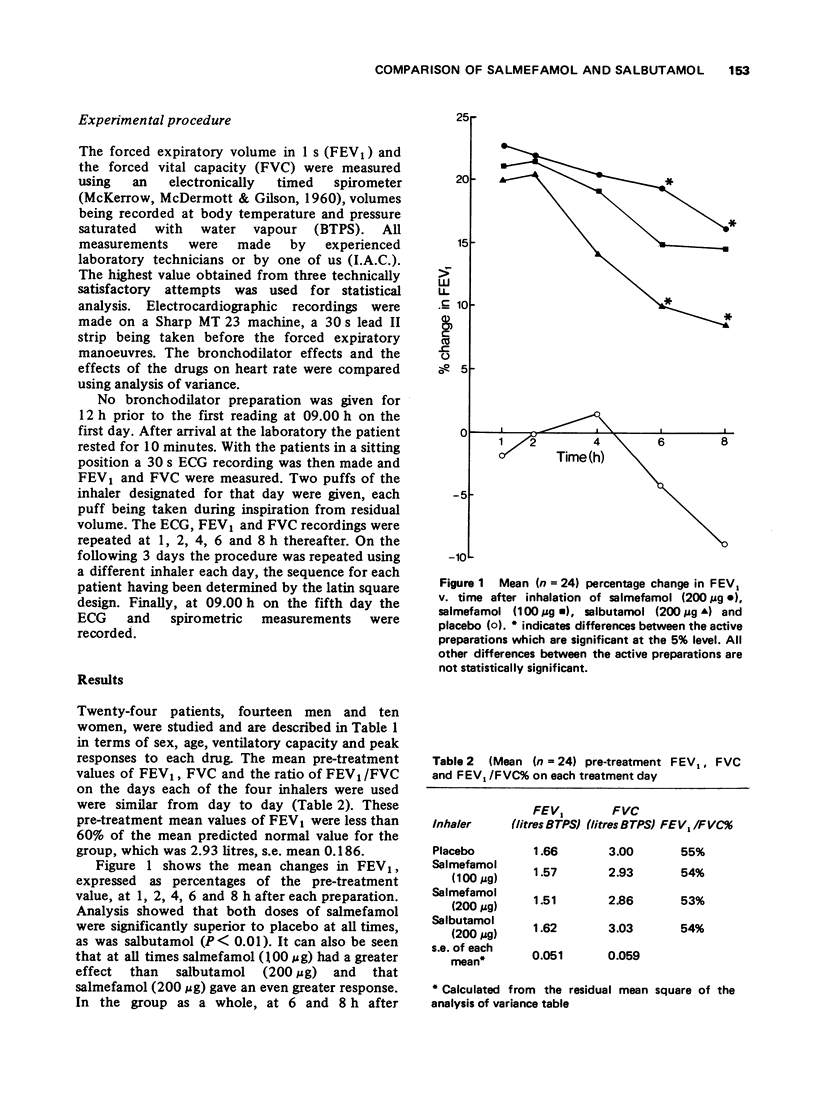
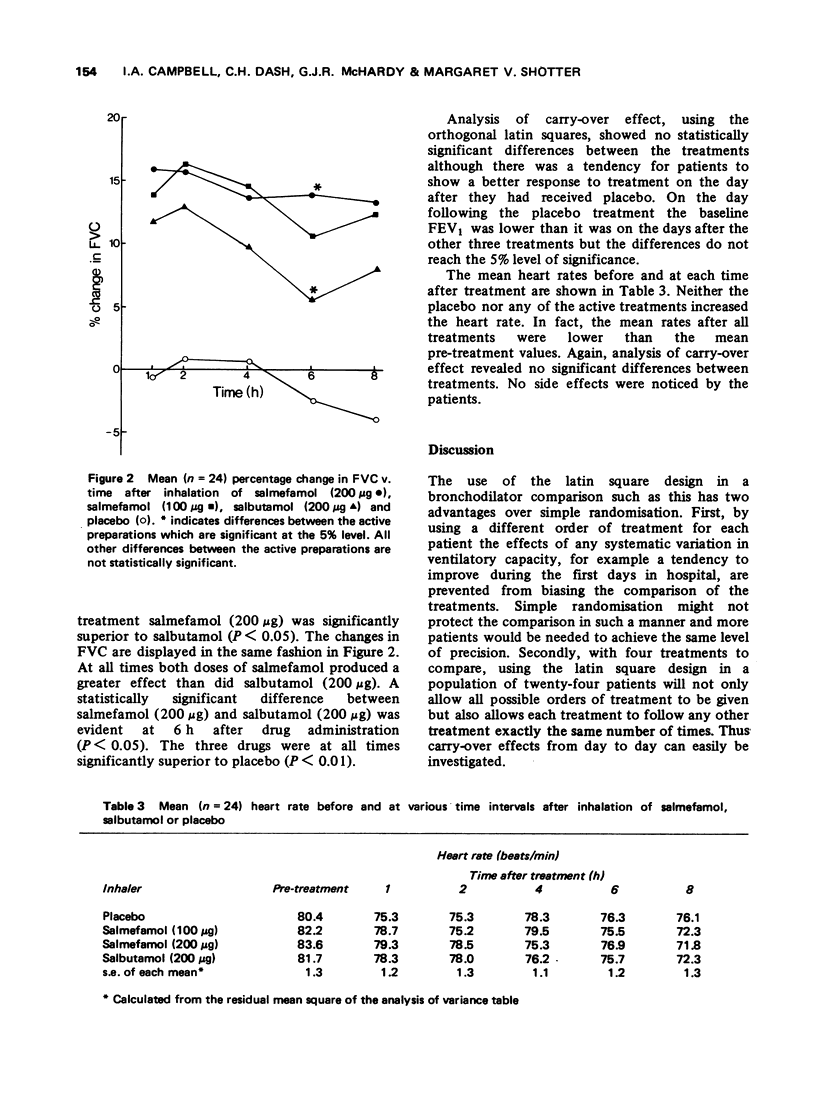
1 Inhaled salmefamol, in doses of 100 mug and 200 mug has been compared with inhaled salbutamol, in a dose of 200 mug, and with placebo in patients with airways obstruction. 2 Both salmefamol and salbutamol are potent bronchodilators with a signficantly superior action over placebo at all times up to 8 h after treatment. 3 The mean peak percentage increases in FEV produced by the three active preparations were similar. The decline from peak values was significantly slower with salmefamol than with salbutamol. Neither drug produced tachycardia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainbridge D., McHardy G. J., Hoare M. V., Dash C. H. A double-blind trial of salmefanol, a new bronchodilator. Postgrad Med J. 1975 Sep;51(599):627–630. doi: 10.1136/pgmj.51.599.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley D., Jack D., Lunts L. H., Ritchie A. C. New class of selective stimulants of beta-adrenergic receptors. Nature. 1968 Aug 24;219(5156):861–862. doi: 10.1038/219861a0. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Dash C. H. The bronchodilator effect of a new adrenergic aerosol--Salmefamol. Acta Allergol. 1972 Feb;27(1):22–26. doi: 10.1111/j.1398-9995.1972.tb01639.x. [DOI] [PubMed] [Google Scholar]
- Lal S., Dash C. H., Gribben M. D. An economical method of comparing inhaled bronchodilators in reversible diffuse airways obstruction. With special reference to a beta-2 stimulant--salmefamol. Thorax. 1974 May;29(3):317–322. doi: 10.1136/thx.29.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]


