Abstract
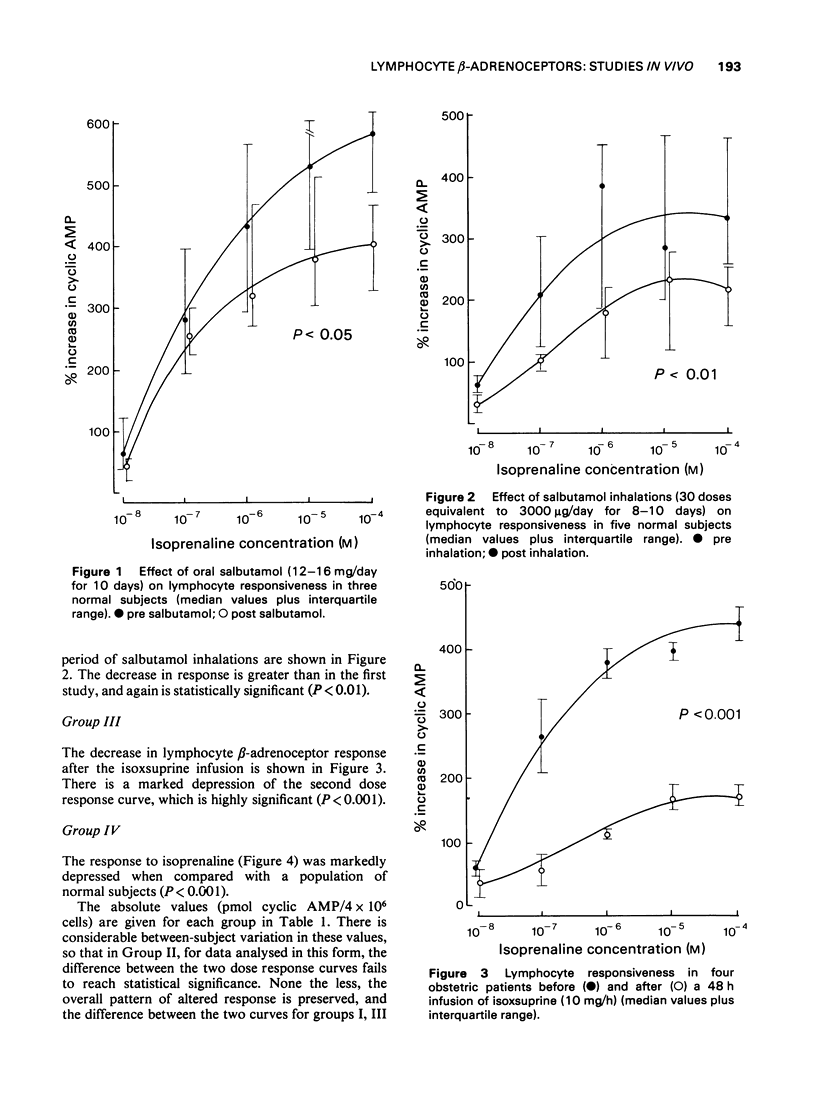
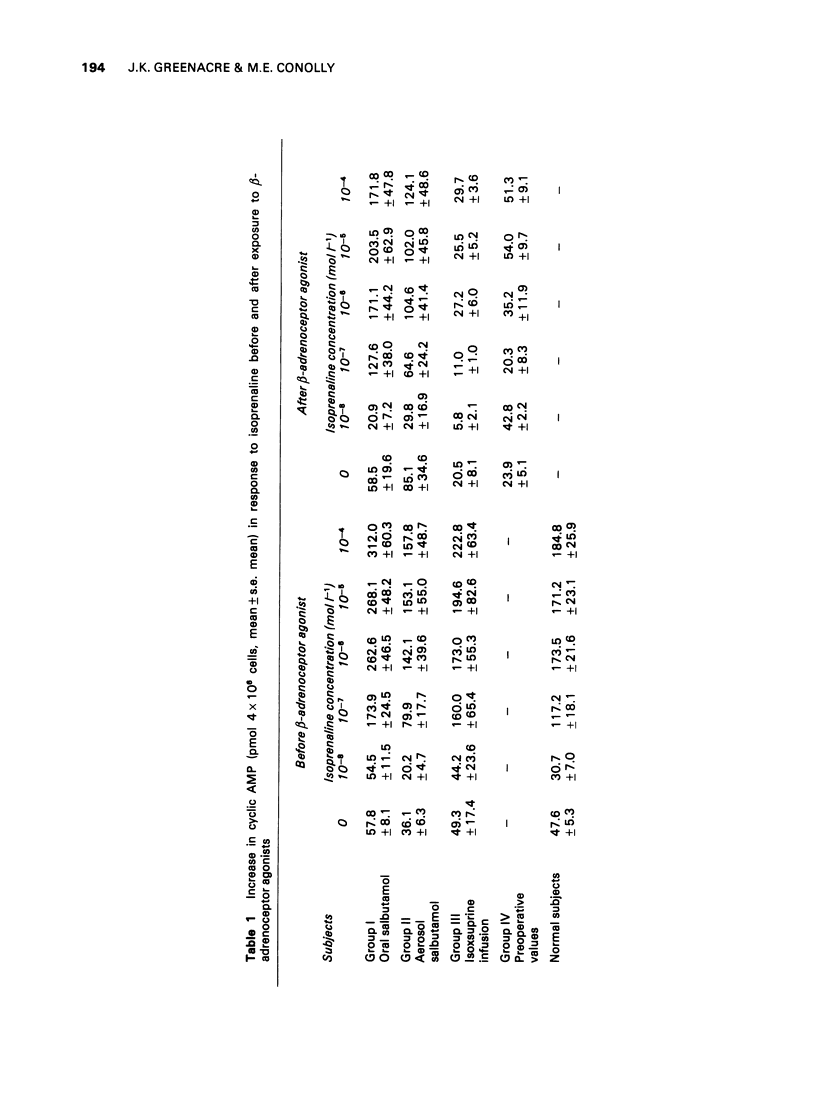
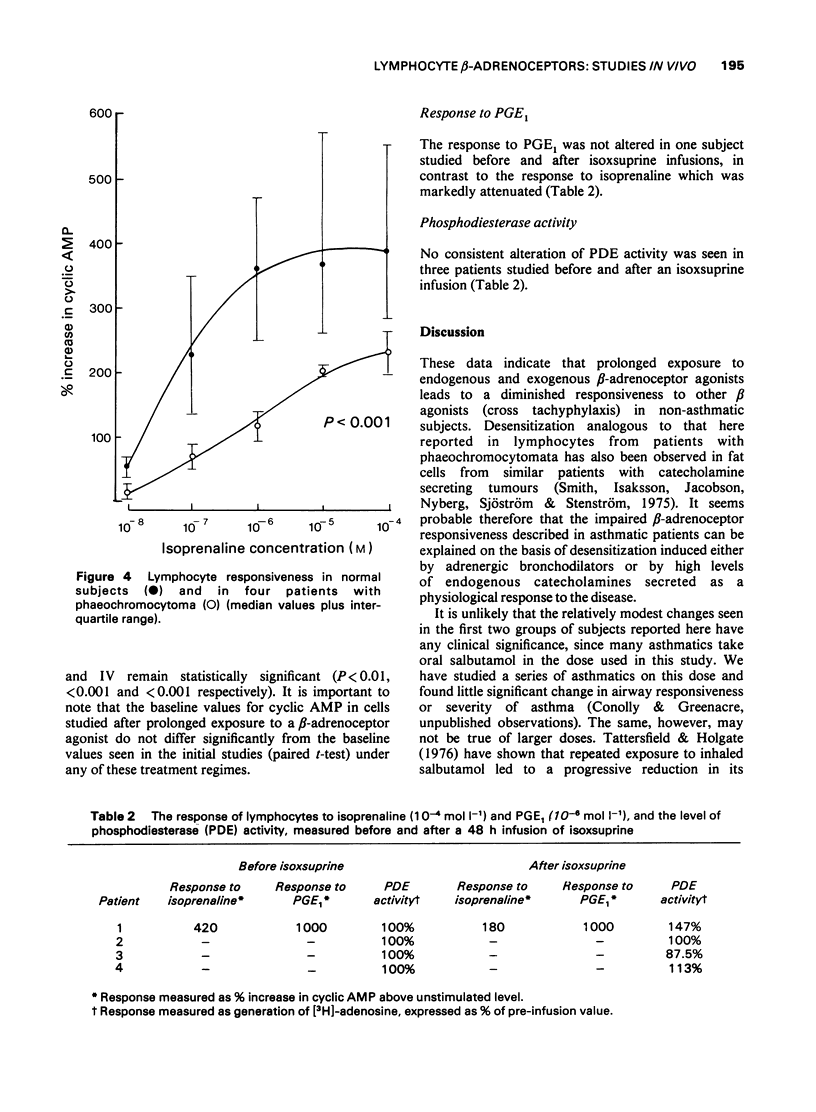
1 Following the observation that lymphocyte beta-adrenoceptor responsiveness was not depressed in asthmatics treated only with non-adrenergic drugs we have explored the effects of prolonged exposure to beta-adrenoceptor agonists in normal subjects. 2 Treatment with oral salbutamol (12-16 mg/kg/day for 10 days), or with inhaled salbutamol (3000 microgram/day for 8-10 days) resulted in a significant reduction in lymphocyte beta-adrenoceptor responsiveness. 3 A 48 h infusion of isoxsuprine (10 mg/h) resulted in a marked depression of lymphocyte beta-adrenoceptor responsiveness (P less than 0.001). 4 Prolonged elevation of endogenous catecholamines caused by phaeochromocytoma was also associated with a marked depression of lymphocyte beta-adrenoceptor responsiveness (P less than 0.001). 5 There was no evidence that an increase in phosphodiesterase activity could explain the reduced cyclic AMP response. 6 It is concluded that diminished beta-adrenoceptor response occurs as a response to prolonged exposure to beta-adrenoceptor agonists. It is likely that the diminished response seen in asthmatic subjects can be explained on a similar basis and does not indicate an inherent cellular defect. 7 The possible clinical significance of such changes in asthmatics are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alston W. C., Patel K. R., Kerr J. W. Response of leucocyte adenyl cyclase to isoprenaline and effect of alpha-blocking drugs in extrinsic bronchial asthma. Br Med J. 1974 Jan 19;1(5898):90–93. doi: 10.1136/bmj.1.5898.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne H. R., Tomkins G. M., Dion S. Regulation of phosphodiesterase synthesis: requirement for cyclic adenosine monophosphate-dependent protein kinase. Science. 1973 Sep 7;181(4103):952–954. doi: 10.1126/science.181.4103.952. [DOI] [PubMed] [Google Scholar]
- Browning E. T., Brostrom C. O., Groppi V. E., Jr Altered adenosine cyclic 3',5'-monophosphate synthesis and degradation by C-6 astrocytoma cells following prolonged exposure to norepinephrine. Mol Pharmacol. 1976 Jan;12(1):32–40. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COOKSON D. U., REED C. E. A COMPARISON OF THE EFFECTS OF ISOPROTERENOL IN THE NORMAL AND ASTHMATIC SUBJECT. A PRELIMINARY REPORT. Am Rev Respir Dis. 1963 Nov;88:636–643. doi: 10.1164/arrd.1963.88.5.636. [DOI] [PubMed] [Google Scholar]
- Conolly M. E., Greenacre J. K. The beta-adrenoceptor of the human lymphocyte and human lung parenchyma. Br J Pharmacol. 1977 Jan;59(1):17–23. doi: 10.1111/j.1476-5381.1977.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conolly M. E., Greenacre J. K. The lymphocyte beta-adrenoceptor in normal subjects and patients with bronchial asthma: the effect of different forms of treatment on receptor function. J Clin Invest. 1976 Dec;58(6):1307–1316. doi: 10.1172/JCI108586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul-Micallef R., Fenech F. F. Effect of intravenous prednisolone in asthmatics with diminished adrenergic responsiveness. Lancet. 1975 Dec 27;2(7948):1269–1271. doi: 10.1016/s0140-6736(75)90608-x. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Hamberg M., Samuelsson B. Inhibition of adenylate cyclase in adipocyte ghosts by the prostaglandin endoperoxide PGH2. Adv Prostaglandin Thromboxane Res. 1976;1:325–330. [PubMed] [Google Scholar]
- Greenacre J. K., Schofield P., Conolly M. E. Desensitization of the beta-adrenoceptor of lymphocytes from normal subjects and asthmatic patients in vitro. Br J Clin Pharmacol. 1978 Mar;5(3):199–206. doi: 10.1111/j.1365-2125.1978.tb01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M. H., Pierson R. N., Jr, Pi-Sunyer F. X. Comparison of the circulatory and metabolic effects of isoproterenol, epinephrine and methoxamine in normal and asthmatic subjects. Am J Med. 1968 Jun;44(6):863–872. doi: 10.1016/0002-9343(68)90086-7. [DOI] [PubMed] [Google Scholar]
- Harris R., Ukaejiofo E. O. Tissue typing using a routine one-step lymphocyte separation procedure. Br J Haematol. 1970 Feb;18(2):229–235. doi: 10.1111/j.1365-2141.1970.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Sutherland E. W. Formation and release of a hormone antagonist by rat adipocytes. J Biol Chem. 1971 Nov 25;246(22):6822–6827. [PubMed] [Google Scholar]
- Inoue S. Effects of epinephrine on asthmatic children. Effects of epinephrine on blood glucose, pulmonary function, and heart rate of children with asthma of varying severity. J Allergy. 1967 Dec;40(6):337–348. doi: 10.1016/0021-8707(67)90023-8. [DOI] [PubMed] [Google Scholar]
- Lockey S. D., Jr, Glennon J. A., Reed C. E. Comparison of some metabolic responses in normal and asthmatic subjects to epinephrine and glucagon. J Allergy. 1967 Dec;40(6):349–354. doi: 10.1016/0021-8707(67)90024-x. [DOI] [PubMed] [Google Scholar]
- Logsdon P. J., Middleton E., Jr, Coffey R. G. Stimulation of leukocyte adenyl cyclase by hydrocortisone and isoproterenol in asthmatic and nonasthmatic subjects. J Allergy Clin Immunol. 1972 Jul;50(1):45–56. doi: 10.1016/0091-6749(72)90078-4. [DOI] [PubMed] [Google Scholar]
- Mickey J. V., Tate R., Mullikin D., Lefkowitz R. J. Regulation of adenylate cyclase-coupled beta adrenergic receptor binding sites by beta adrenergic catecholamines in vitro. Mol Pharmacol. 1976 May;12(3):409–419. [PubMed] [Google Scholar]
- Miledi R., Potter L. T. Acetylcholine receptors in muscle fibres. Nature. 1971 Oct 29;233(5322):599–603. doi: 10.1038/233599a0. [DOI] [PubMed] [Google Scholar]
- Orange R. P., Kaliner M. A., Laraia P. J., Austen K. F. Immunological release of histamine and slow reacting substance of anaphylaxis from human lung. II. Influence of cellular levels of cyclic AMP. Fed Proc. 1971 Nov-Dec;30(6):1725–1729. [PubMed] [Google Scholar]
- Parker C. W., Smith J. W. Alterations in cyclic adenosine monophosphate metabolism in human bronchial asthma. I. Leukocyte responsiveness to -adrenergic agents. J Clin Invest. 1973 Jan;52(1):48–59. doi: 10.1172/JCI107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. W., Evans R. J., Prime F. J. SElectivity of broncholidlator action of salbutamol in asthmatic patients. Br J Dis Chest. 1971 Jan;65(1):21–38. [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. A new kind of drug antagonism: evidence that agonists cause a molecular change in acetylcholine receptors. Mol Pharmacol. 1969 Jul;5(4):394–411. [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Romero J. A., Zatz M., Kebabian J. W., Axelrod J. Circadian cycles in binding of 3H-alprenolol to beta-adrenergic receptor sites in rat pineal. Nature. 1975 Dec 4;258(5534):435–436. doi: 10.1038/258435a0. [DOI] [PubMed] [Google Scholar]
- Russell T. R., Pastan I. H. Cyclic adenosine 3':5'-monophosphate and cyclic guanosine 3':5'-monophosphate phosphodiesterase activities are under separate genetic control. J Biol Chem. 1974 Dec 25;249(24):7764–7769. [PubMed] [Google Scholar]
- Shenfield G. M., Hodson M. E., Clarke S. W., Paterson J. W. Interaction of corticosteroids and catecholamines in the treatment of asthma. Thorax. 1975 Aug;30(4):430–435. doi: 10.1136/thx.30.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U., Isaksson O., Nyberg G., Sjörstrõm L. Human adipose tissue in culture. IV. Evidence for the formation of a hormone antagonist by catecholamines. Eur J Clin Invest. 1976 Jan 30;6(1):35–42. doi: 10.1111/j.1365-2362.1976.tb00491.x. [DOI] [PubMed] [Google Scholar]
- Tattersfield A. E., Holgate S. T. Letter: Intravenous prednisolone in asthma. Lancet. 1976 Feb 21;1(7956):422–422. doi: 10.1016/s0140-6736(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]


