Abstract
1 Nadolol excretion was studied in 24 patients with chronic renal failure.
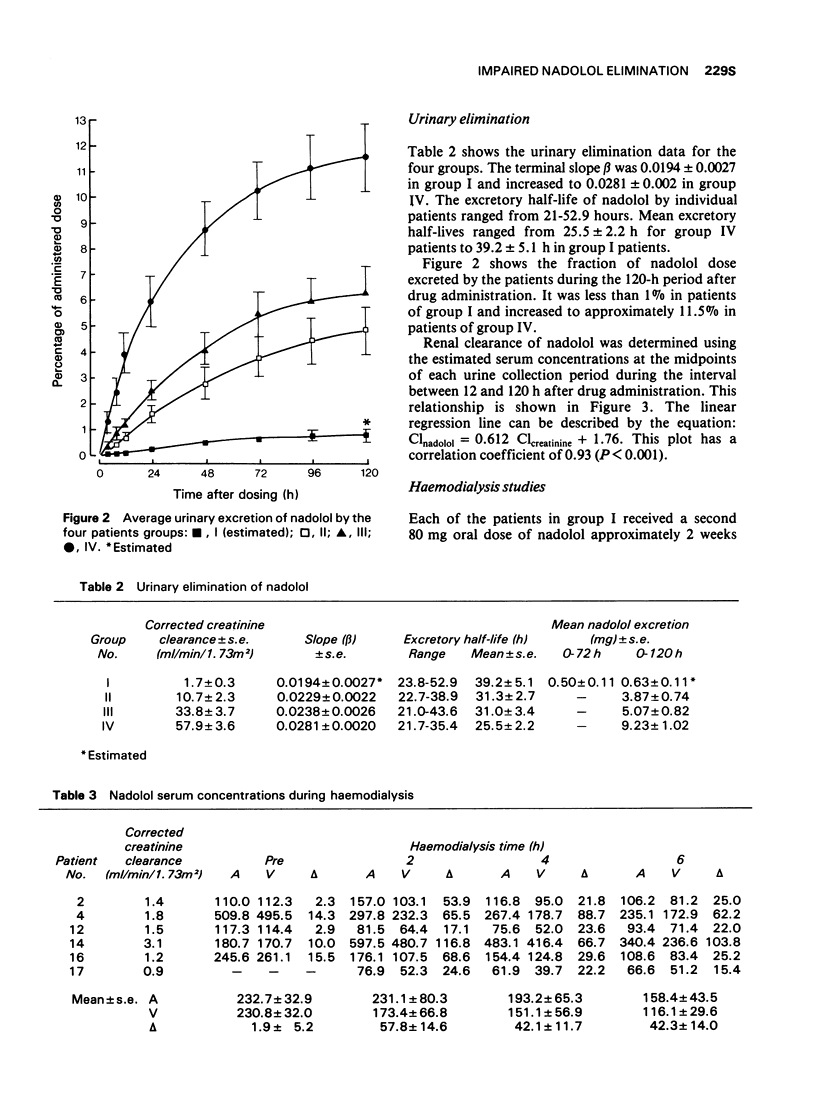
2 The amount of nadolol excreted during the 120-h period after receiving the drug ranged from less than 1% in functionally anephric patients up to 11.5% in patients with average creatinine clearance of 57.9 ± 3.6 ml/min/1.73 m2.
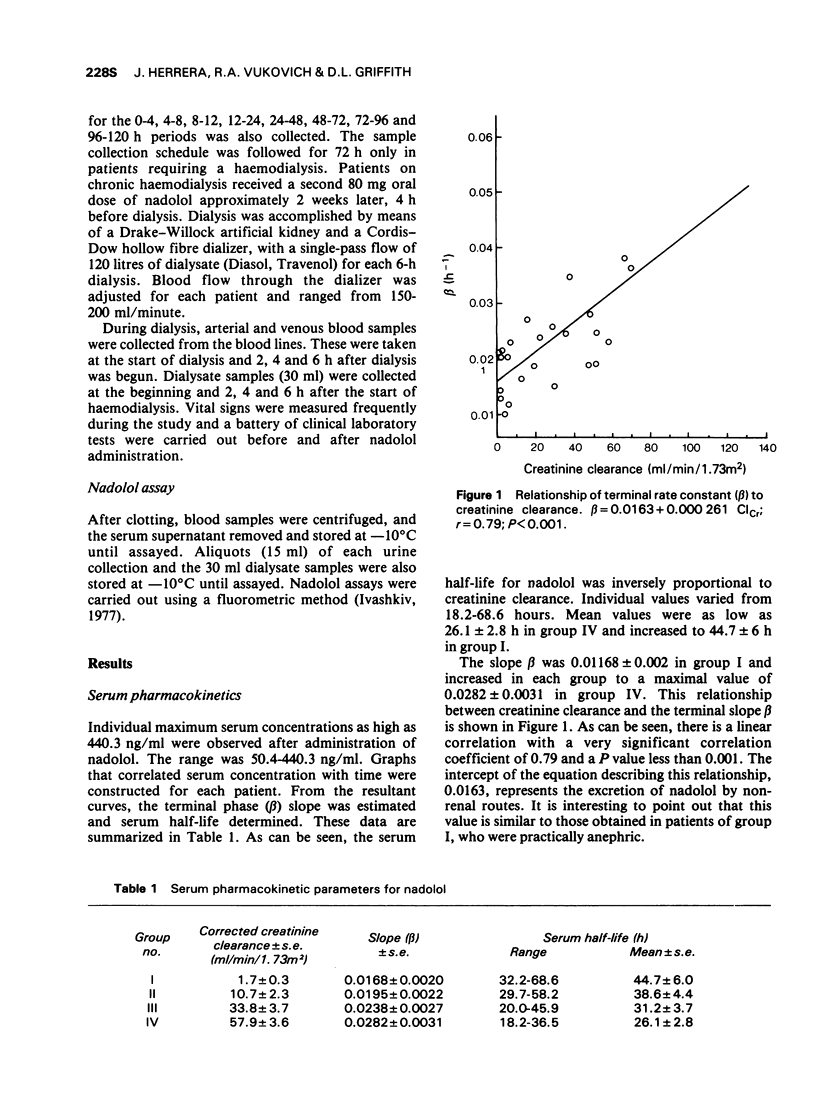
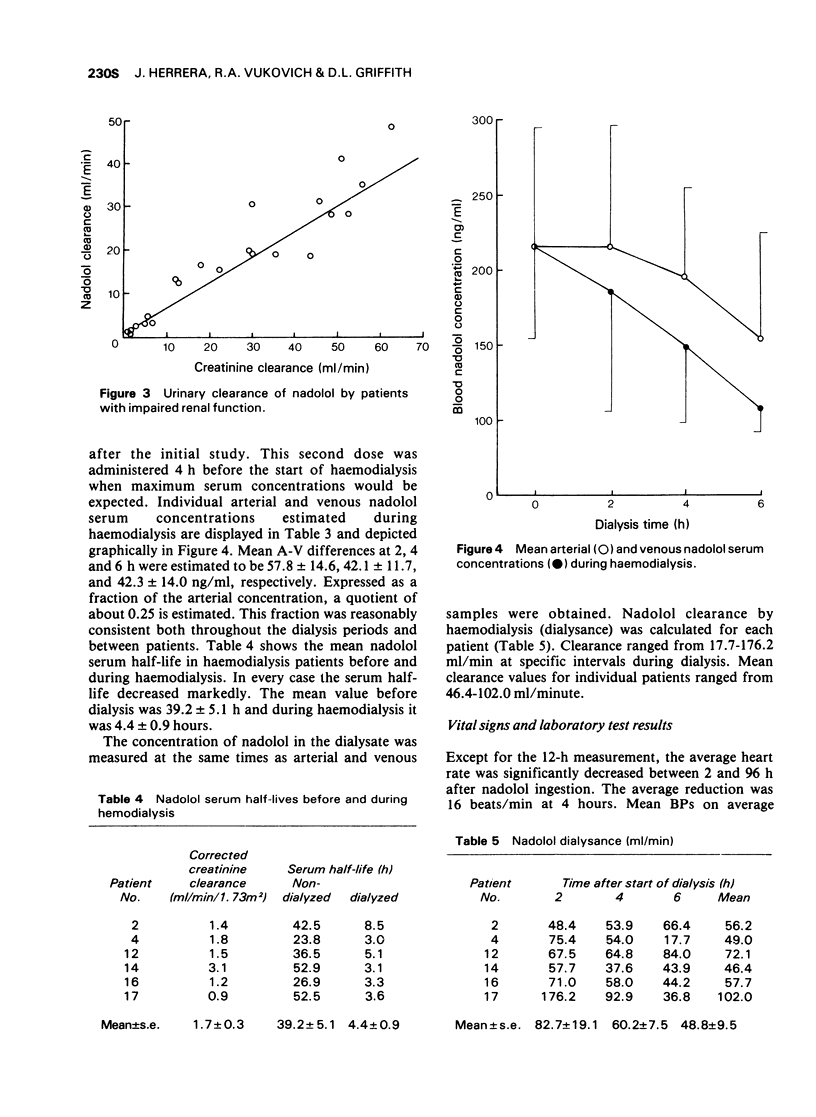
3 Renal clearance of nadolol was found to correlate with creatinine clearance; nadolol elimination is retarded in patients with renal failure.
4 Nadolol serum half-life is prolonged in proportion to the remaining renal function. Therefore, dosage intervals in renal patients receiving nadolol should be adjusted to creatinine clearance.
5 Haemodialysis effectively reduced serum concentration of the drug; it may therefore be a useful therapy for drug intoxication.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beermann B., Dalén E., Lindström B. Elimination of furosemide in healthy subjects and in those with renal failure. Clin Pharmacol Ther. 1977 Jul;22(1):70–78. doi: 10.1002/cpt197722170. [DOI] [PubMed] [Google Scholar]
- Craig C. P., Rifkin S. I. Pharmacokinetics and hemodialyzability of cefazolin in uremic patients. Clin Pharmacol Ther. 1976 Jun;19(6):825–829. doi: 10.1002/cpt1976196825. [DOI] [PubMed] [Google Scholar]
- Dettli L., Spring P., Habersang R. Drug dosage in patients with impaired renal function. Postgrad Med J. 1970 Oct;(Suppl):32–35. [PubMed] [Google Scholar]
- Dreyfuss J., Brannick L. J., Vukovich R. A., Shaw J. M., Willard D. A. Metabolic studies in patients with nadolol: oral and intravenous administration. J Clin Pharmacol. 1977 May-Jun;17(5-6):300–307. doi: 10.1002/j.1552-4604.1977.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Frithz G. Dose-ranging study of the new beta-adrenergic antagonist nadolol in the treatment of essential hypertension. Curr Med Res Opin. 1978;5(5):383–387. doi: 10.1185/03007997809111902. [DOI] [PubMed] [Google Scholar]
- Furberg B., Dahlqvist A., Raak A., Wrege U. Comparison of the new beta-adrenoceptor antagonist, nadolol, and propranolol in the treatment of angina pectoris. Curr Med Res Opin. 1978;5(5):388–393. doi: 10.1185/03007997809111903. [DOI] [PubMed] [Google Scholar]
- Ivashkiv E. Fluorometric determination of nadolol in human serum and urine. J Pharm Sci. 1977 Aug;66(8):1168–1172. doi: 10.1002/jps.2600660831. [DOI] [PubMed] [Google Scholar]
- Lavene D., Weiss Y. A., Safar M. E., Loria Y., Agorus N., Georges D., Milliez P. L. Pharmacokinetics and hepatic extraction ratio of pindolol in hypertensive patients with normal and impaired renal function. J Clin Pharmacol. 1977 Aug-Sep;17(8-9):501–508. doi: 10.1002/j.1552-4604.1977.tb05643.x. [DOI] [PubMed] [Google Scholar]
- Lee R. J., Evans D. B., Baky S. H., Laffan R. J. Pharmacology of nadolol (SQ 11725), a beta-adrenergic antagonist lacking direct myocardial depression. Eur J Pharmacol. 1975 Sep-Oct;33(2):371–382. doi: 10.1016/0014-2999(75)90182-x. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Seidl L. G., Cluff L. E. Studies on the epidemiology of adverse drug reactions. V. Clinical factors influencing susceptibility. Ann Intern Med. 1966 Oct;65(4):629–640. doi: 10.7326/0003-4819-65-4-629. [DOI] [PubMed] [Google Scholar]


