Abstract
1 The pharmacokinetic behaviour of the psychotropic drug clobazam, a 1,5 benzodiazepine, and its metabolism were studied with the 14C-labelled compound in rats, dogs, monkeys and man. The absorption was practically complete in all three animal species. Clobazam was not excreted in the unchanged form by all species. The main metabolite in plasma of monkeys, dogs and man was N-demethylclobazam. The metabolites were partially in the conjugated form.
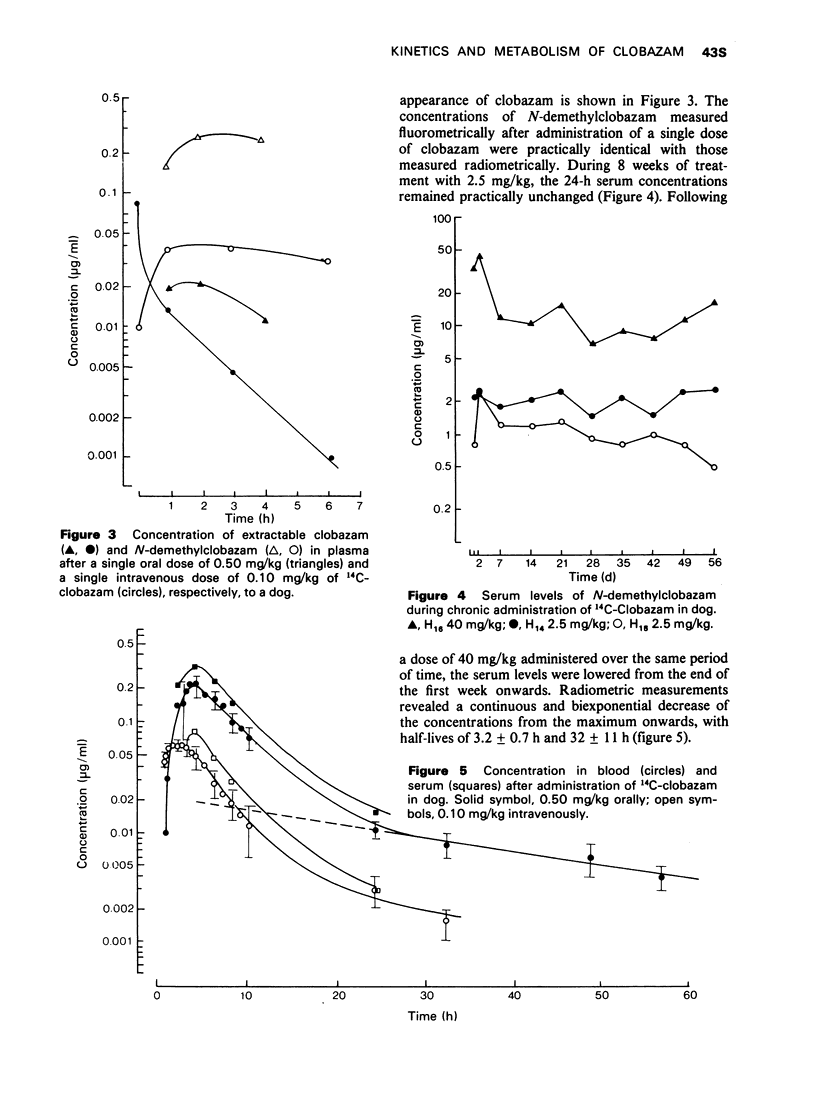
2 The binding to serum proteins (concentration range 0.05-10 μg/ml serum) amounted to between 66% (in rats) and 85% (in man). The maximal levels of total radioactivity (original compound and metabolites) in blood were 0.24 ± 0.043 μg Equ/ml (2-4 h) in doses and 0.67-0.82 μg Equ/ml (0.5-1 h) in rhesus monkeys. These levels were markedly higher than those in rats with values of 0.064 ± 0.012 μg Equ/ml (∼0.5 h). The elimination of radioactivity from blood occurred in two phases.
3 After repeated daily administration of oral doses, the 24-h blood levels accumulated in rats to about three times the initial value. In dogs the 24-h serum concentrations remained practically unchanged. Long-term treatment with clobazam in monkeys neither caused enzyme induction nor other processes retarding metabolism and elimination.
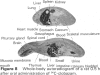
4 Both after a single oral and intravenous dose, more than two-thirds of the radioactivity administered to rats was excreted with the faeces. Dogs, however, excreted about three-quarters of the radioactivity with the urine, irrespective of the route of administration. In monkeys, the excretion also occurred mainly in the urine. In all three species, renal excretion was similarly rapid to that from blood or plasma.
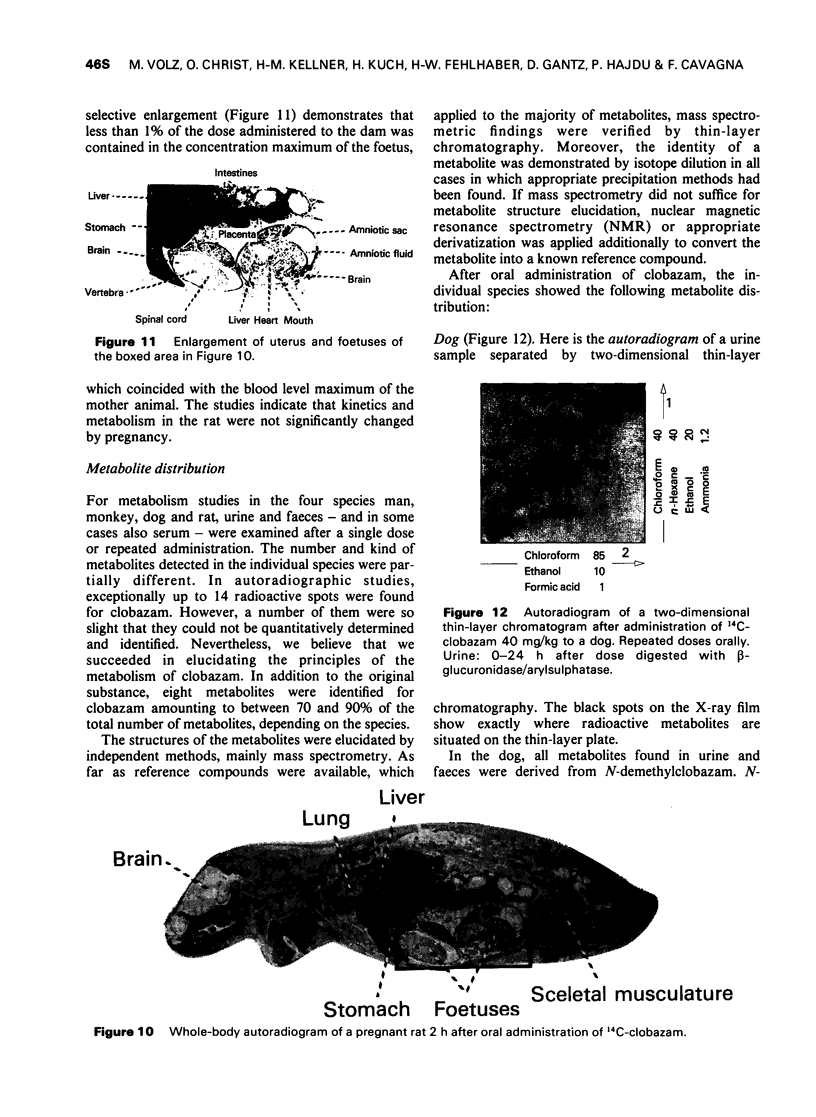
5 Apart from gastro-intestinal tract, liver and kidneys, the distribution in rats and dogs was remarkably even within the range of maximal blood levels. In the rat brain, the concentration amounted to only one-third of that in the blood. Special accumulations were not found. In dogs, the concentration in the brain was as high as that in the blood.
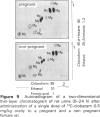
6 In rats, kinetics and metabolism were not significantly changed by pregnancy.
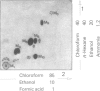
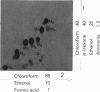
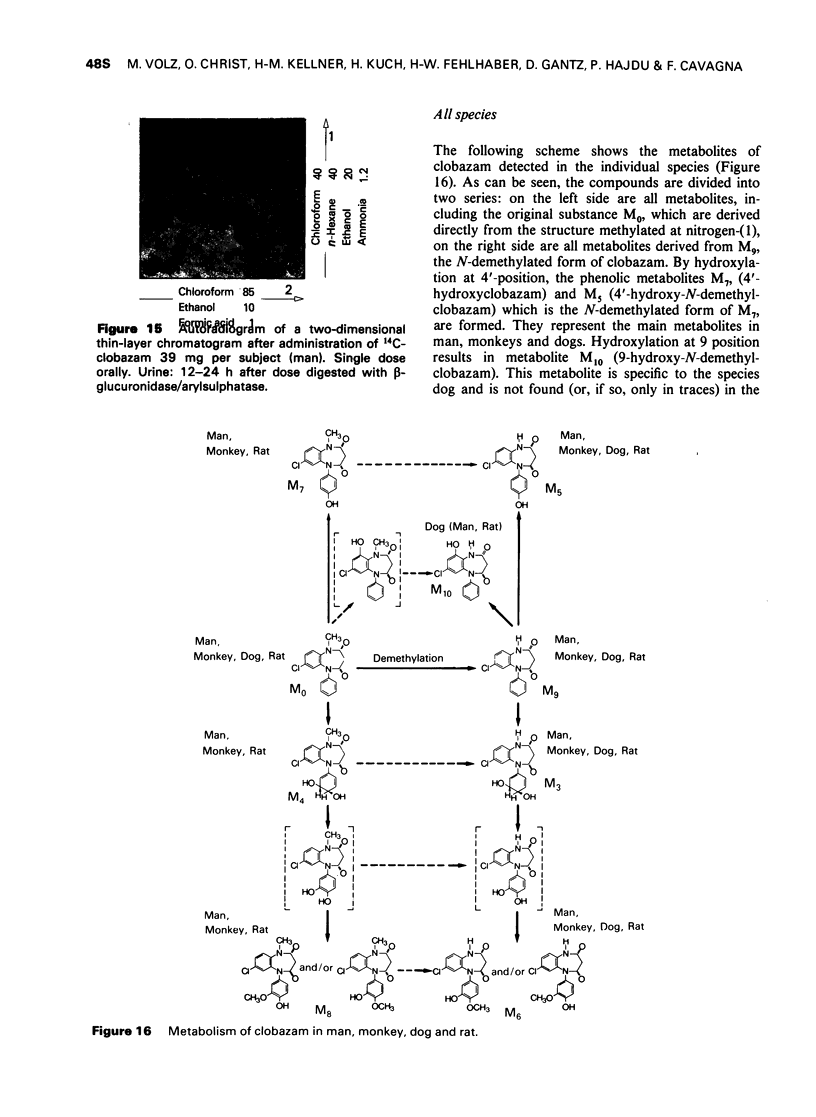
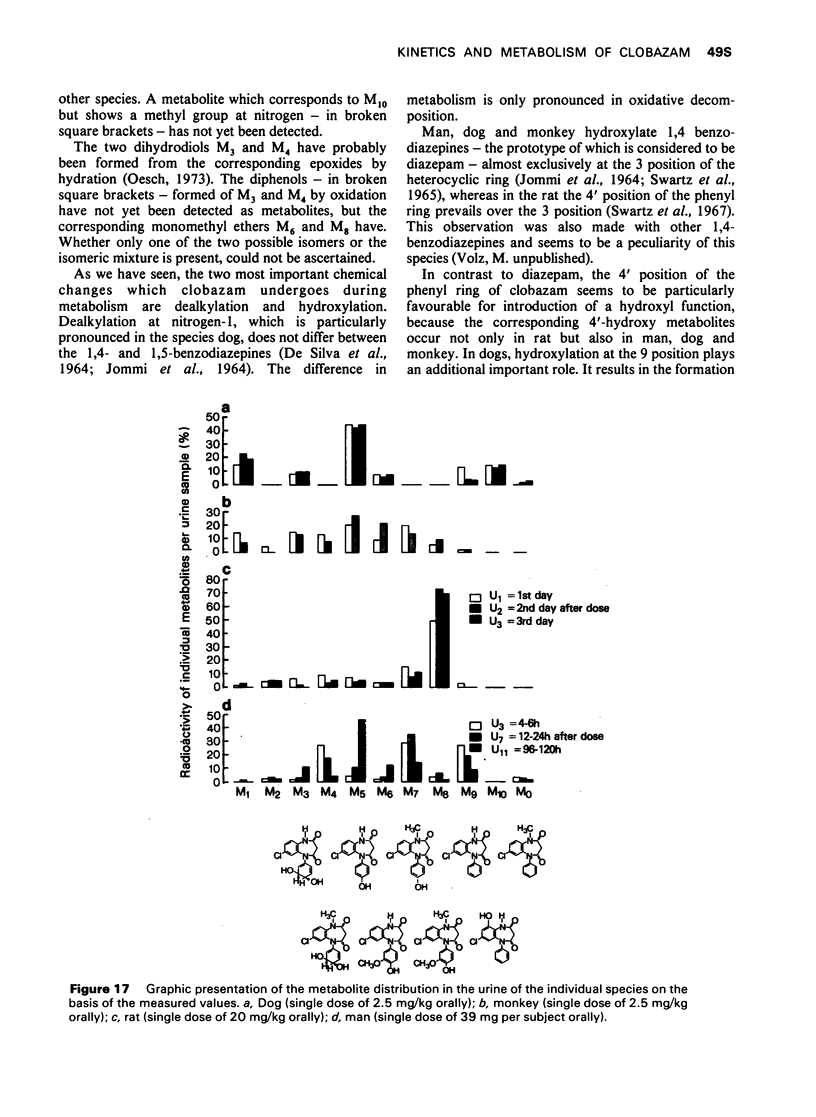
7 For metabolism studies in the four species (man, monkey, dog and rat) urine and faeces (and in some cases also serum) were examined after a single dose or repeated administration. The number and kind of metabolites detected in the individual species were partially different. In autoradiographic studies, exceptionally up to 14 radioactive spots were found for clobazam.
8 The structures of the metabolites were elucidated by independent methods, mainly mass spectrometry. In addition to the original substance, eight metabolites were identified for clobazam amounting to 70-90% of the total number of metabolites, depending on the species.
The two most important chemical changes of clobazam during metabolism are dealkylation and hydroxylation. Dealkylation at nitrogen-(1), particularly pronounced in the species dog, does not differ between the 1,4- and 1,5-benzodiazepines. The difference in metabolism is only pronounced in oxidative decomposition.
9 In contrast to diazepam, the 4′ position of the phenyl ring of clobazam seems to be particularly favourable for introduction of a hydroxyl function. In dogs, hydroxylation at the 9 position plays an additional important role. It results in the formation of the metabolite 9-hydroxy-N-demethylclobazam by which this species is markedly distinguished from the other three species.
It is remarkable that clobazam is not hydroxylated at the 3-position. This is obviously a characteristic of the 1,5-benzodiazepines and helps to distinguish them from the 1,4-benzodiazepines such as diazepam.
Full text
PDF





Images in this article
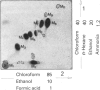
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton K. B., Grimes R. M., Shaw C., Patrick J. E., McGuire J. L. Biotransformation of a 1,5-benzodiazepine, triflubazam, by man. Drug Metab Dispos. 1975 Sep-Oct;3(5):352–360. [PubMed] [Google Scholar]
- JOMMI G., MANITTO P., SILANOS M. A. METABOLISM OF DIAZEPAM IN RABBITS. Arch Biochem Biophys. 1964 Nov;108:334–340. doi: 10.1016/0003-9861(64)90394-7. [DOI] [PubMed] [Google Scholar]
- Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973 May;3(5):305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Bommer P., Vane F. M. Diazepam metabolites in the rat: characterization by high-resolution mass spectrometry and nuclear magnetic resonance. Arch Biochem Biophys. 1967 Aug;121(2):508–516. doi: 10.1016/0003-9861(67)90106-3. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Koechlin B. A., Postma E., Palmer S., Krol G. Metabolism of diazepam in rat, dog, and man. J Pharmacol Exp Ther. 1965 Sep;149(3):423–435. [PubMed] [Google Scholar]