Abstract
1 Serum, urine and pharmacologic effect (prolongation of the QT interval) kinetics of the antiarrhythmic disopyramide have been investigated in eight volunteers after intravenous administration (2 mg/kg) and oral administration (300 mg) of the two commercially available preparations, Rythmodan (Roussel Laboratories) and Norpace (Searle Laboratories). 2 An open one compartment body model adequately described the kinetics of disopyramide in serum and urine. 3 After intravenous administration, the following average pharmacokinetic parameters were found: biological half-life, 7.8 h; total clearance, 95 ml/min; renal clearance, 54 ml/min; apparent volume of distribution, 60 litres. 4 After oral Rythmodan and Norpace, serum concentration profiles and urinary excretion data revealed significant differences in rates of absorption, times required to achieve peak serum concentrations and biological half-lives. These differences were largely due to the relatively slow absorption characteristics of Norpace. 5 The absence of hysteresis in plots of QT prolongation against disopyramide serum concentration after oral administration indicated that serum and pharmacologic effect kinetics were indistinguishable within a kinetically equivalent compartment. 6 Analysis of both serum and urine data showed that while Norpace had a significantly higher degree of bioavailability (P less than 0.005), the 5--15% difference between the two formulations should not normally be of any clinical significance.
Full text
PDF




Selected References
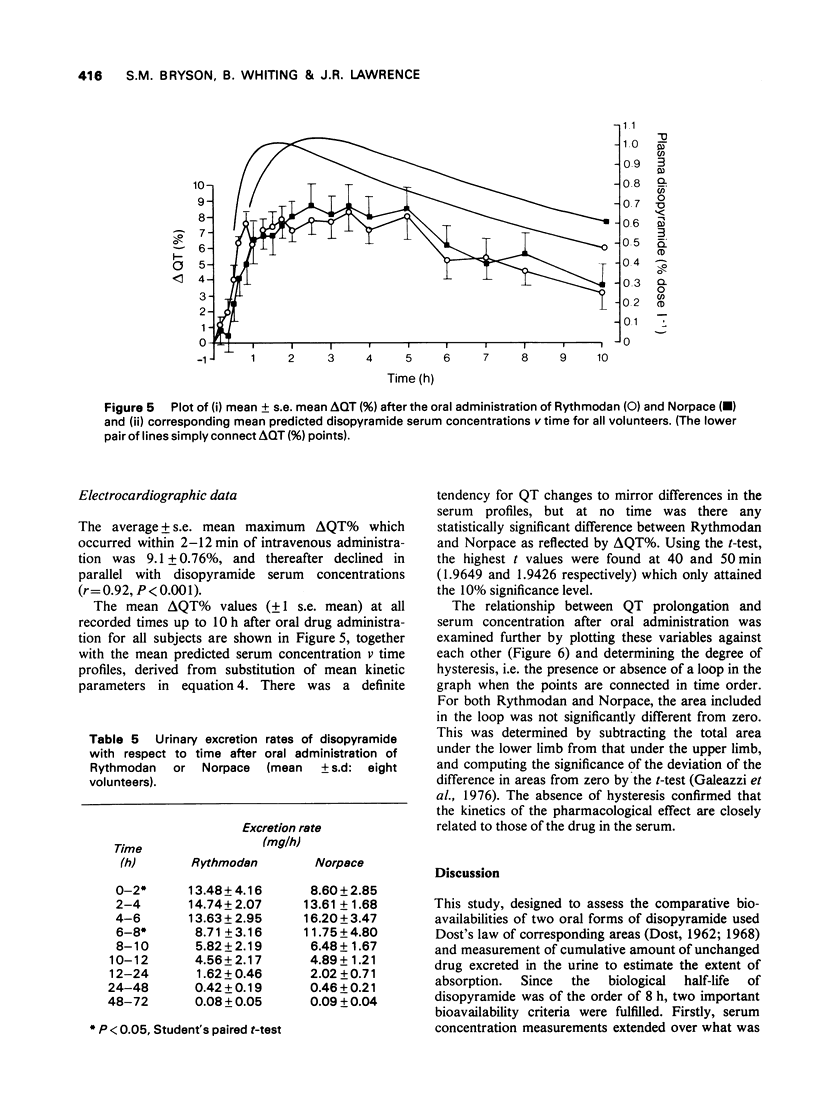
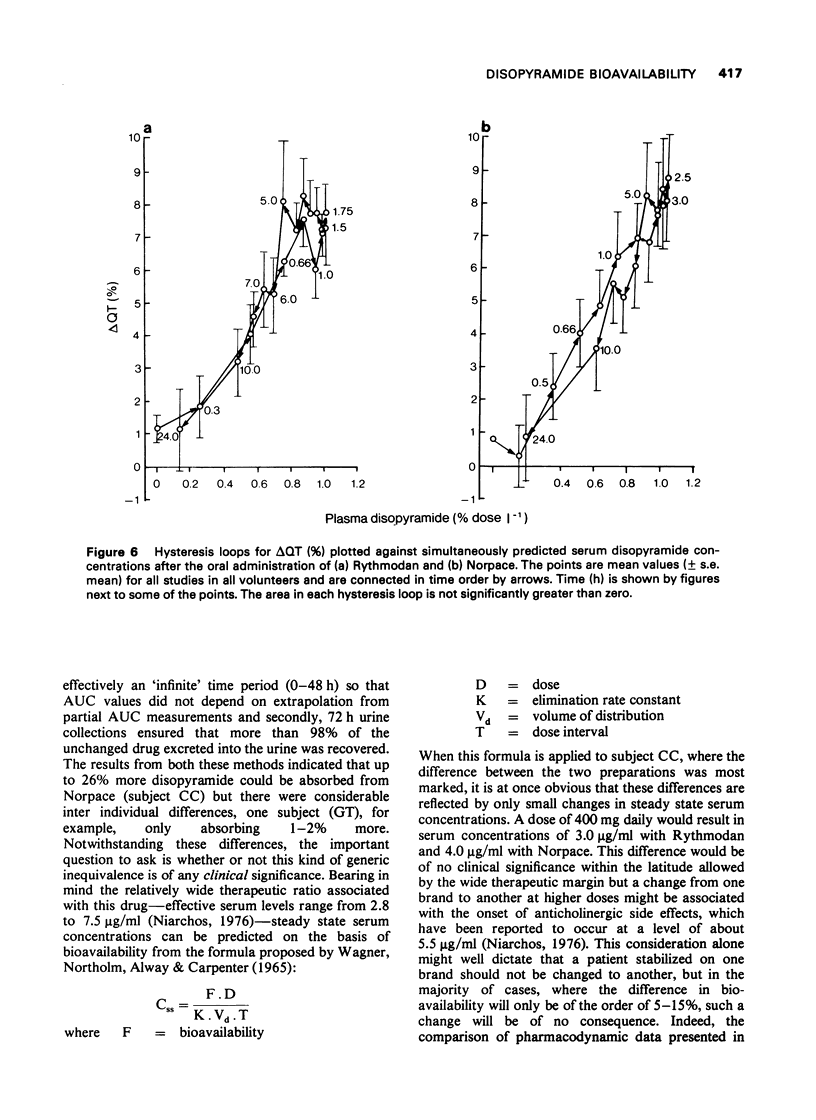
These references are in PubMed. This may not be the complete list of references from this article.
- Birkhead J. S., Vaughan Williams E. M. Dual effect of disopyramide on atrial and atrioventricular conduction and refractory periods. Br Heart J. 1977 Jun;39(6):657–660. doi: 10.1136/hrt.39.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. W., Lambert H. J., Karim A. Comparative binding of disopyramide phosphate and quinidine sulfate to human plasma proteins. J Pharm Sci. 1974 Dec;63(12):1877–1879. doi: 10.1002/jps.2600631210. [DOI] [PubMed] [Google Scholar]
- DOST F. H. [A procedure for the determination of the absolute transport capacity of blood in its steady state]. Klin Wochenschr. 1962 Jul 15;40:732–733. doi: 10.1007/BF01482413. [DOI] [PubMed] [Google Scholar]
- Duchateau A. M., Merkus F. W., Schobben F. Rapid gas chromatographic determination of disopyramide in serum using a nitrogen detector. J Chromatogr. 1975 Jun 18;109(2):432–435. doi: 10.1016/s0021-9673(01)91823-0. [DOI] [PubMed] [Google Scholar]
- Galeazzi R. L., Benet L. Z., Sheiner L. B. Relationship between the pharmacokinetics and pharmacodynamics of procainamide. Clin Pharmacol Ther. 1976 Sep;20(3):278–289. doi: 10.1002/cpt1976203278. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Duhme D. W., Koch-Weser J., Smith T. W. Intravenous digoxin as a bioavailability standard: slow infusion and rapid injection. Clin Pharmacol Ther. 1974 May;15(5):510–513. doi: 10.1002/cpt1974155510. [DOI] [PubMed] [Google Scholar]
- Hinderling P. H., Bres J., Garrett E. R. Protein binding and erythrocyte partitioning of disopyramide and its monodealkylated metabolite. J Pharm Sci. 1974 Nov;63(11):1684–1690. doi: 10.1002/jps.2600631103. [DOI] [PubMed] [Google Scholar]
- Hinderling P. H., Garrett E. R. Pharmacodynamics of the antiarrhythmic disopyramide in healthy humans: correlation of the kinetics of the drug and its effects. J Pharmacokinet Biopharm. 1976 Jun;4(3):231–242. doi: 10.1007/BF01063615. [DOI] [PubMed] [Google Scholar]
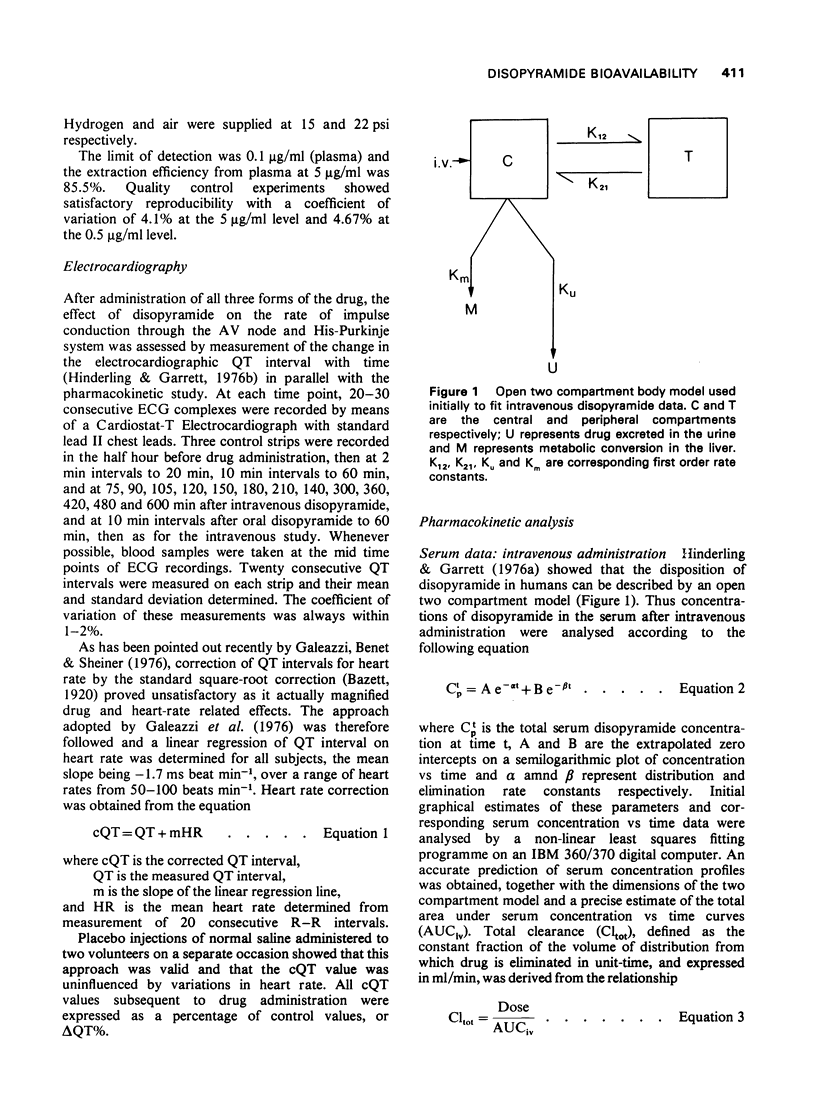
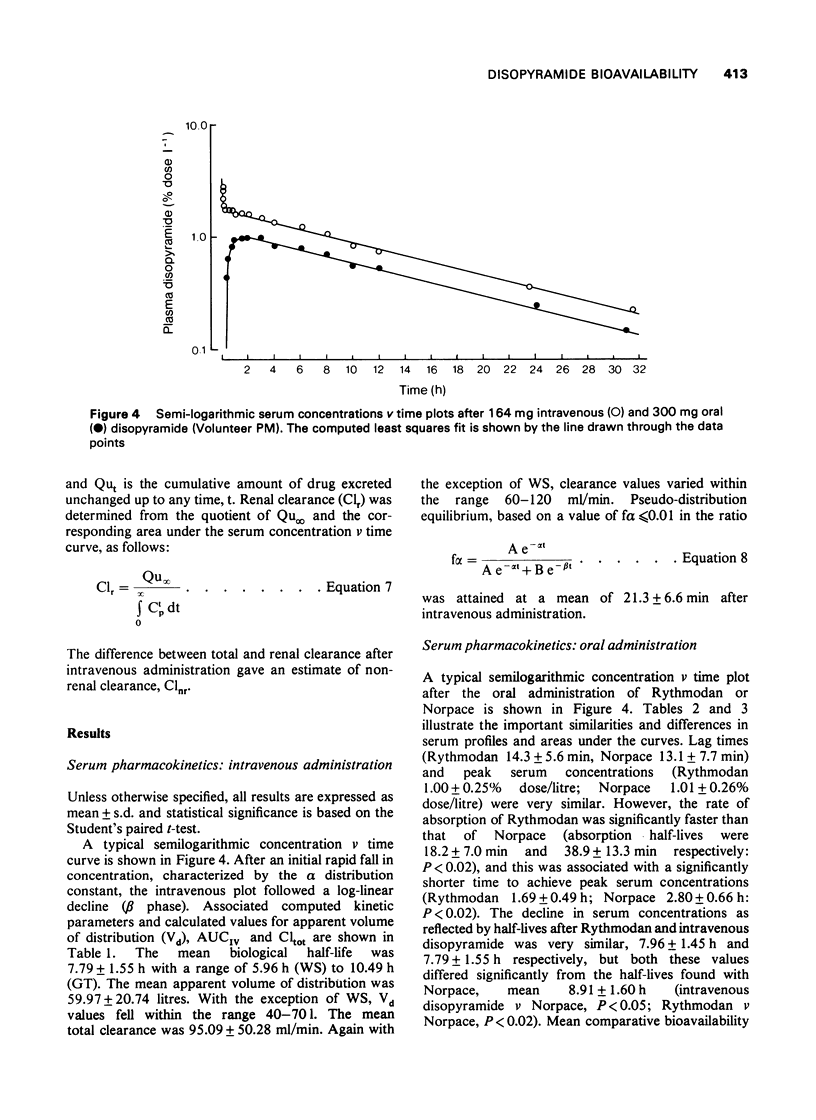
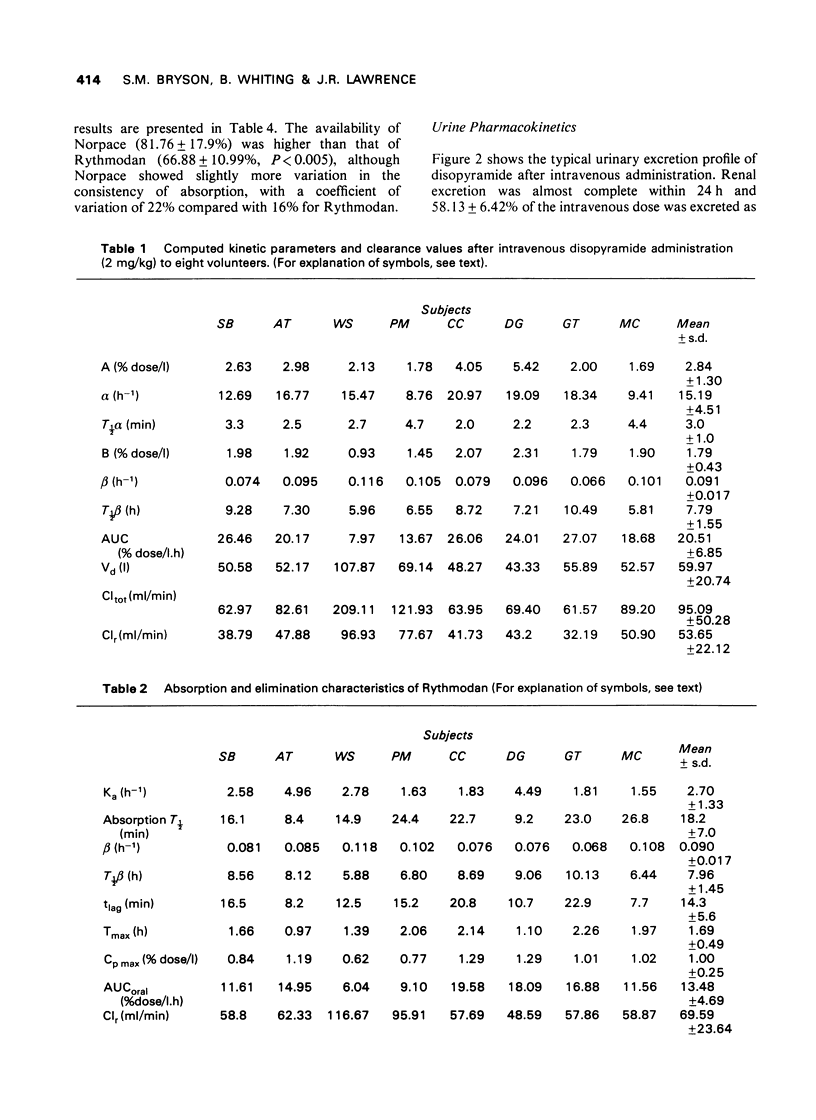
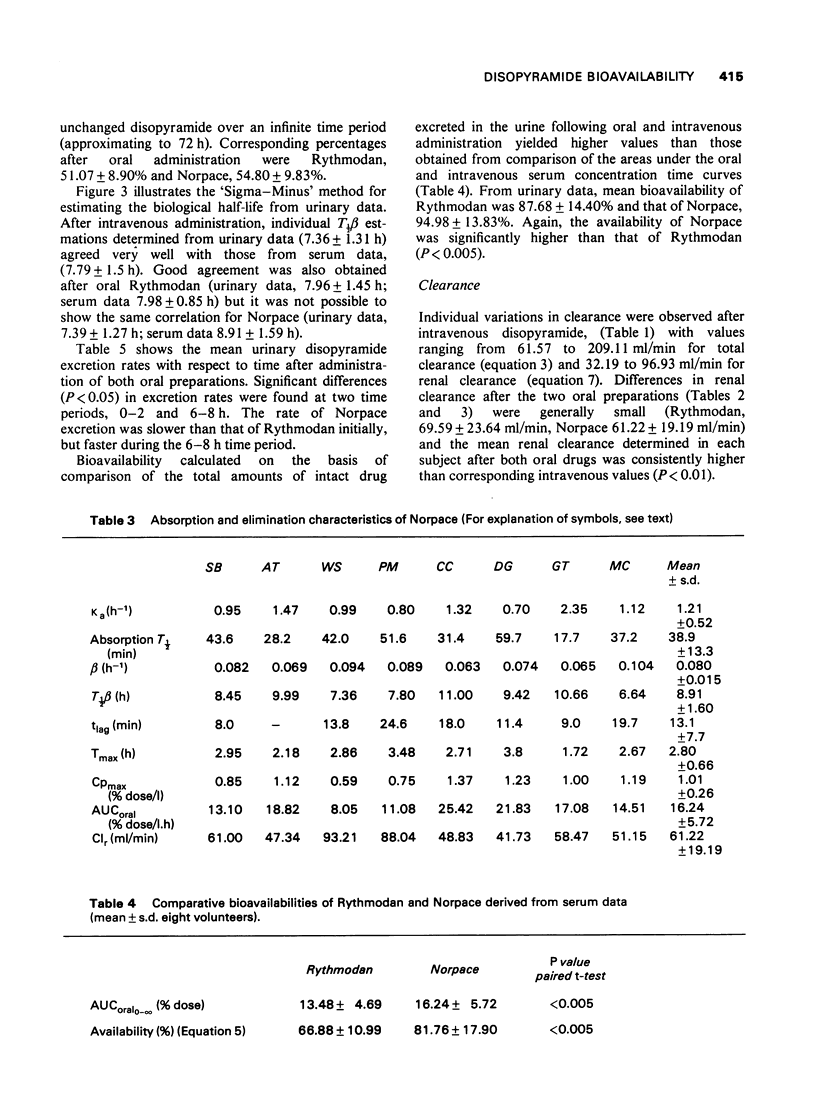
- Hinderling P. H., Garrett E. R. Pharmacokinetics of the antiarrhythmic disopyramide in healthy humans. J Pharmacokinet Biopharm. 1976 Jun;4(3):199–230. doi: 10.1007/BF01063614. [DOI] [PubMed] [Google Scholar]
- Härtel G., Louhija A., Konttinen A. Disopyramide in the prevention of recurrence of atrial fibrillation after electroconversion. Clin Pharmacol Ther. 1974 Jun;15(6):551–555. doi: 10.1002/cpt1974156551. [DOI] [PubMed] [Google Scholar]
- Jennings G., Jones M. S., Besterman E. M., Model D. G., Turner P. P., Kidner P. H. Oral disopyramide in prophylaxis of arrhythmias following myocardial infarction. Lancet. 1976 Jan 10;1(7950):51–54. doi: 10.1016/s0140-6736(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Karim A., Ranney R. E., Kraychy S. Species differences in the biotransformation of a new antiarrhythmic agent: disopyramide phosphate. J Pharm Sci. 1972 Jun;61(6):888–893. doi: 10.1002/jps.2600610612. [DOI] [PubMed] [Google Scholar]
- Karim A. The pharmacokinetics of Norpace. Angiology. 1975 Jan;26(1 Pt 2):85–98. [PubMed] [Google Scholar]
- Marcus F. I., Dickerson J., Pippin S., Stafford M., Bressler R. Digoxin bioavailability: formulations and rates of infusions. Clin Pharmacol Ther. 1976 Sep;20(3):253–259. doi: 10.1002/cpt1976203253. [DOI] [PubMed] [Google Scholar]
- Mizgala H. F., Huvelle P. R. Acute termination of cardiac arrhythmias with intravenous disopyramide. J Int Med Res. 1976;4(1 Suppl):82–85. [PubMed] [Google Scholar]
- Spurrell R. A., Thorburn C. W., Camm J., Sowton E., Deuchar D. C. Effects of disopyramide on electrophysiological properties of specialized conduction system in man and on accessory atrioventricular pathway in Wolff-Parkinson-White syndrome. Br Heart J. 1975 Aug;37(8):861–867. doi: 10.1136/hrt.37.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. G., Northam J. I., Alway C. D., Carpenter O. S. Blood levels of drug at the equilibrium state after multiple dosing. Nature. 1965 Sep 18;207(5003):1301–1302. doi: 10.1038/2071301a0. [DOI] [PubMed] [Google Scholar]


