Abstract
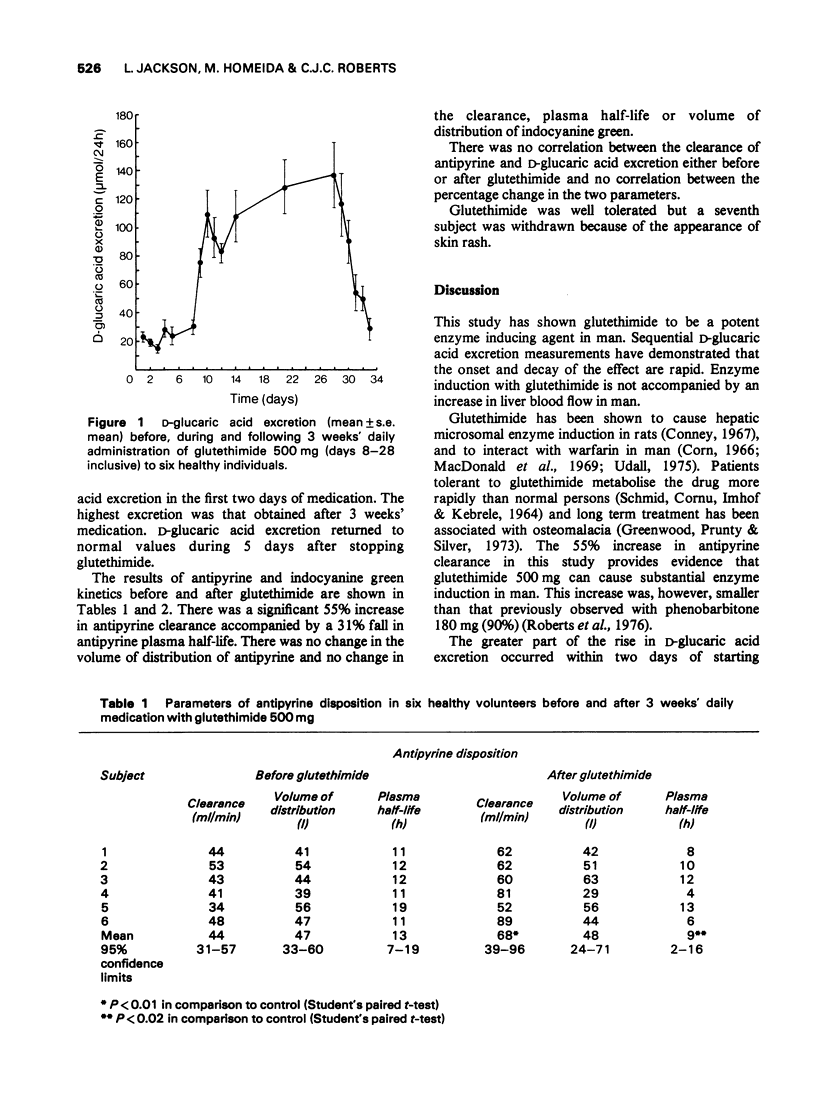
1 Sequential measurements of D-glucaric acid excretion were made in six healthy volunteers before, during and after 3 weeks' daily medication with glutethimide 500 mg. 2 There was a rapid rise in D-glucaric acid excretion within 2 days of starting medication and a rapid decline when it was stopped. 3 Antipyrine clearance and indocyanine green clearance were measured before and at the end of the 3 weeks' medication. 4 There was a 55% increase in antipyrine clearance but no change in indocyanine green clearance. 5 There was no correlation between antipyrine clearance and D-glucaric acid excretion. 6 Glutethimide causes rapid enzyme induction in man without concomitant rise in hepatic blood flow.
Full text
PDF
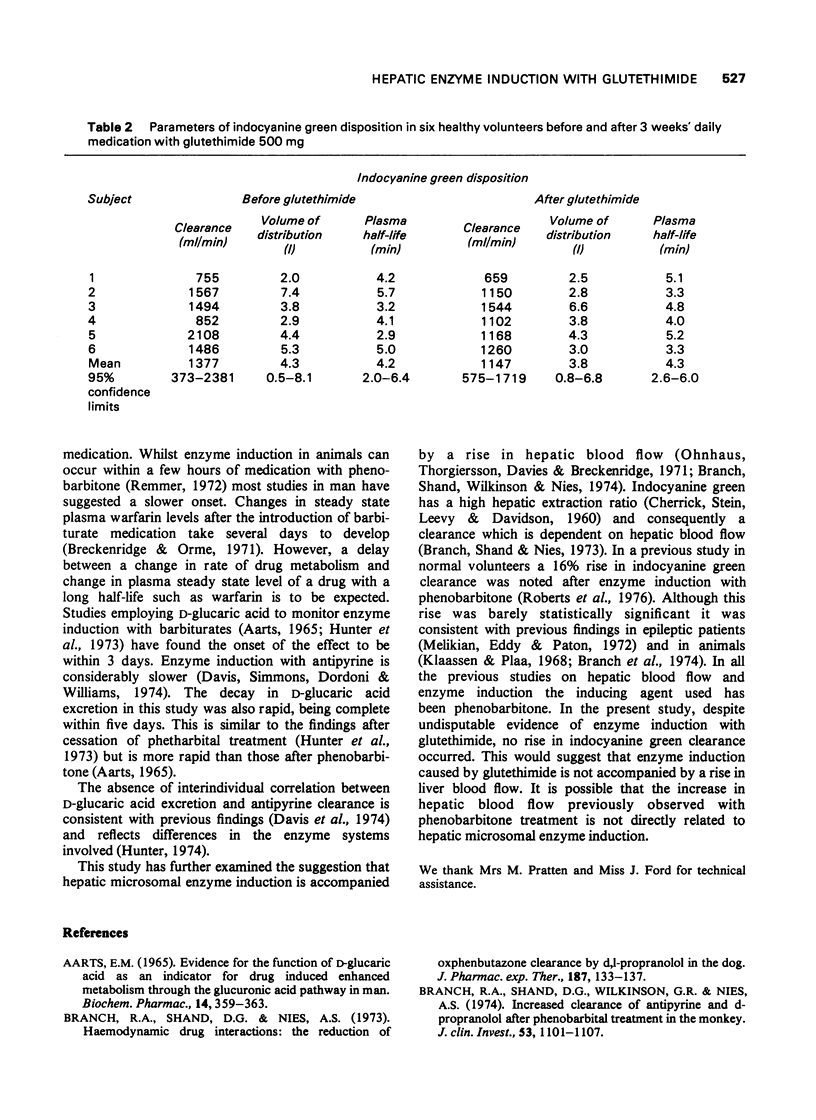

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch R. A., Shand D. G., Nies A. S. Hemodynamic drug interactions: the reduction of oxyphenbutazone clearance by dl-propranol in the dog. J Pharmacol Exp Ther. 1973 Oct;187(1):133–137. [PubMed] [Google Scholar]
- Branch R. A., Shand D. G., Wilkinson G. R., Nies A. S. Increased clearance of antipyrine and d-propranolol after phenobarbital treatment in the monkey. Relative contributions of enzyme induction and increased hepatic blood flow. J Clin Invest. 1974 Apr;53(4):1101–1107. doi: 10.1172/JCI107647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge A., Orme M. Clinical implications of enzyme induction. Ann N Y Acad Sci. 1971 Jul 6;179:421–431. doi: 10.1111/j.1749-6632.1971.tb46919.x. [DOI] [PubMed] [Google Scholar]
- CAESAR J., SHALDON S., CHIANDUSSI L., GUEVARA L., SHERLOCK S. The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci. 1961 Aug;21:43–57. [PubMed] [Google Scholar]
- CHERRICK G. R., STEIN S. W., LEEVY C. M., DAVIDSON C. S. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960 Apr;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Corn M. Effect of phenobarbital and glutethimide on biological half-life of warfarin. Thromb Diath Haemorrh. 1966 Dec 1;16(3):606–612. [PubMed] [Google Scholar]
- Curry S. H., Gordon J. S., Riddall D., Simpson P., Binns T. B., Rondel R. K., McMartin C. Disposition of glutethimide in man. Clin Pharmacol Ther. 1971 Sep-Oct;12(5):849–857. doi: 10.1002/cpt1971125849. [DOI] [PubMed] [Google Scholar]
- Greenwood R. H., Prunty F. T., Silver J. Osteomalacia after prolonged glutethimide administration. Br Med J. 1973 Mar 17;1(5854):643–645. doi: 10.1136/bmj.1.5854.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J. Enzyme induction and medical treatment. J R Coll Physicians Lond. 1974 Jan;8(2):163–174. [PMC free article] [PubMed] [Google Scholar]
- Hunter J., Maxwell J. D., Carrella M., Stewart D. A., Williams R. Urinary D-glucaric-acid excretion as a test for hepatic enzyme induction in man. Lancet. 1971 Mar 20;1(7699):572–575. doi: 10.1016/s0140-6736(71)91166-4. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D., Plaa G. L. Studies on the mechanism of phenobarbital-enhanced sulfobromophthalein disappearance. J Pharmacol Exp Ther. 1968 Jun;161(2):361–366. [PubMed] [Google Scholar]
- MacDonald M. G., Robinson D. S., Sylwester D., Jaffe J. J. The effects of phenobarbital, chloral betaine, and glutethimide administration on warfarin plasma levels and hypoprothrombinemic responese in man. Clin Pharmacol Ther. 1969 Jan-Feb;10(1):80–84. doi: 10.1002/cpt196910180. [DOI] [PubMed] [Google Scholar]
- Marsh C. A. Metabolism of d-glucuronolactone in mammalian systems. Identification of d-glucaric acid as a normal constituent of urine. Biochem J. 1963 Jan;86(1):77–86. doi: 10.1042/bj0860077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian V., Eddy J. D., Paton A. The stimulant effect of drugs on indocyanine green clearance by the liver. Gut. 1972 Oct;13(10):755–758. doi: 10.1136/gut.13.10.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnhaus E. E., Thorgeirsson S. S., Davies D. S., Breckenridge A. Changes in liver blood flow during enzyme induction. Biochem Pharmacol. 1971 Oct;20(10):2561–2570. doi: 10.1016/0006-2952(71)90164-x. [DOI] [PubMed] [Google Scholar]
- Roberts C. J., Jackson L., Halliwell M., Branch R. A. The relationship between liver volume, antipyrine clearance and indocyanine green clearance before and after phenobarbitone administration in man. Br J Clin Pharmacol. 1976 Oct;3(5):907–913. doi: 10.1111/j.1365-2125.1976.tb00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMID K., CORNU F., IMHOF P., KEBERLE H. DIE BIOCHEMISCHE DEUTUNG DER GEWOEHNUNG AN SCHLAFMITTEL. Schweiz Med Wochenschr. 1964 Feb 15;94:235–240. [PubMed] [Google Scholar]
- Simmons C. J., Davis M., Dordoni B., Williams R. Urinary D-glucaric acid assay by an improved enzymatic procedure. Clin Chim Acta. 1974 Feb 28;51(1):47–51. doi: 10.1016/0009-8981(74)90060-6. [DOI] [PubMed] [Google Scholar]
- Stevenson I. H. Factors influencing antipyrine elimination. Br J Clin Pharmacol. 1977 Jun;4(3):261–265. doi: 10.1111/j.1365-2125.1977.tb00710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udall J. A. Clinical implications of warfarin interactions with five sedatives. Am J Cardiol. 1975 Jan;35(1):67–71. doi: 10.1016/0002-9149(75)90560-3. [DOI] [PubMed] [Google Scholar]


