Abstract
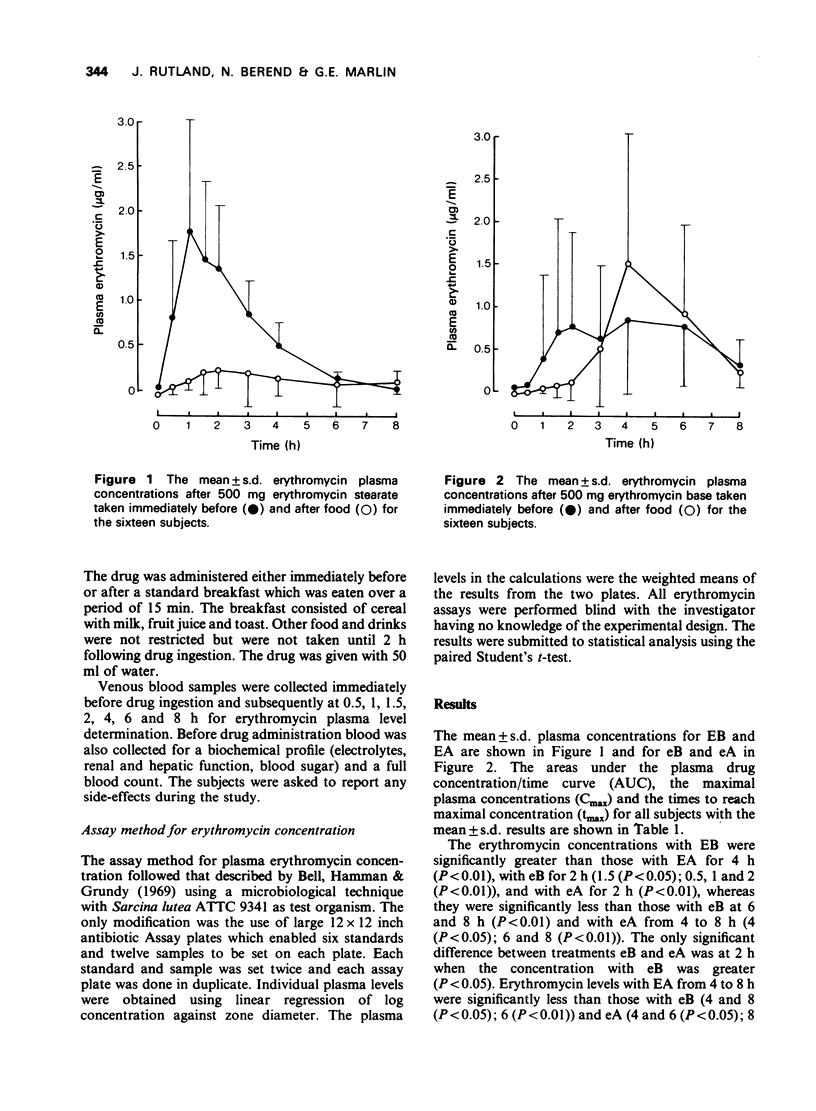
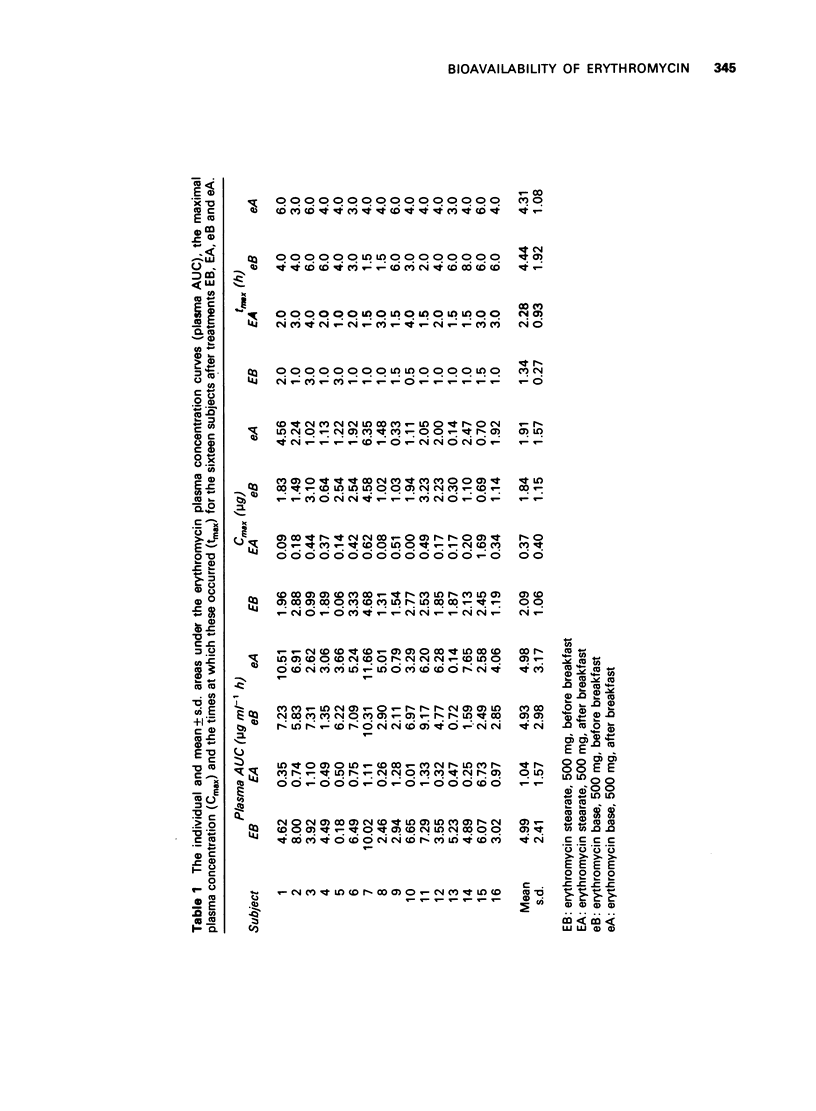
1. The effect of food on the bioavailability of two new formulations of erythromycin, 1) erythromycin stearate, 500 mg (Erythrocin, 250 mg capsule-shaped tablets) and 2) erythromycin base, 500 mg (Eryc, 250 mg capsules containing enteric-coated pellets) was studied in 16 healthy subjects. 2. The study was a balanced, randomized Latin square design and was conducted on 4 days. The four treatments were erythromycin stearate immediately before (EB) and after EA) breakfast and erythromycin base immediately before (eB) and after (eA) breakfast. 3. The mean +/- s.d. maximal plasma erythromycin concentrations were 2.09 +/- 1.06, 0.37 +/- 0.40, 1.8+ +/- 1.15 and 1.91 +/- 1.57 micrograms/ml and the mean +/- s.d. times at which these occurred were 1.3 +/- 0.7, 2.3 +/- 0.9, 4.4 +/- 1.9 and 4.3 +/- 1.1 h for EB, EA, eB and eA respectively. 4. The mean +/- s.d. areas under the curves (0 to 8 h) were 4.99 +/- 2.41, 1.04 +/- 1.57, 4.93 +/- 2.98 and 4.98 +/- 3.14 for EB, EA, eB and eA respectively. 5. The bioavailability of erythromycin stearate was significantly reduced by the prior administration of food, whereas the absorption of the base was not inhibited by food.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell S. C., Hamman J. W., Grundy W. E. Micromethod for assaying serum levels of erythromycin. Appl Microbiol. 1969 Jan;17(1):88–92. doi: 10.1128/am.17.1.88-92.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. M. A comparison of absorption after oral administration of erythromycin estolate and erythromycin stearate. Med J Aust. 1971 Dec 18;2(25):1280–1283. doi: 10.5694/j.1326-5377.1971.tb92846.x. [DOI] [PubMed] [Google Scholar]
- Berend N., Rutland J., Marlin G. E. Erythromycin serum levels. Med J Aust. 1978 Feb 25;1(4):222–223. doi: 10.5694/j.1326-5377.1978.tb107847.x. [DOI] [PubMed] [Google Scholar]
- Boggiano B. G., Gleeson M. Gastric acid inactivation of erythromycin stearate in solid dosage forms. J Pharm Sci. 1976 Apr;65(4):497–502. doi: 10.1002/jps.2600650406. [DOI] [PubMed] [Google Scholar]
- Cooksley W. G., Powell L. W. Erythromycin jaundice: diagnosis by an in vitro challenge test. Aust N Z J Med. 1977 Jun;7(3):291–293. doi: 10.1111/j.1445-5994.1977.tb03689.x. [DOI] [PubMed] [Google Scholar]
- GRIFFITH R. S., BLACK H. R. COMPARISON OF THE BLOOD LEVELS OBTAINED AFTER SINGLE AND MULTIPLE DOSES OF ERYTHROMYCIN ESTOLATE AND ERYTHROMYCIN STEARATE. Am J Med Sci. 1964 Jan;247:69–74. doi: 10.1097/00000441-196401000-00010. [DOI] [PubMed] [Google Scholar]
- HUNT J. N. Gastric emptying and secretion in man. Physiol Rev. 1959 Jul;39(3):491–533. doi: 10.1152/physrev.1959.39.3.491. [DOI] [PubMed] [Google Scholar]
- Levine R. R. Factors affecting gastrointestinal absorption of drugs. Am J Dig Dis. 1970 Feb;15(2):171–188. doi: 10.1007/BF02235648. [DOI] [PubMed] [Google Scholar]
- McDonald P. J., Mather L. E., Story M. J. Studies on absorption of a newly developed enteric-coated erythromycin base. J Clin Pharmacol. 1977 Oct;17(10 Pt 1):601–606. doi: 10.1177/009127007701701007. [DOI] [PubMed] [Google Scholar]
- Prescott L. F. Gastrointestinal absorption of drugs. Med Clin North Am. 1974 Sep;58(5):907–916. doi: 10.1016/s0025-7125(16)32088-0. [DOI] [PubMed] [Google Scholar]
- Stephens V. C., Pugh C. T., Davis N. E., Hoehn M. M., Ralston S. A study of the behavior of propionyl erythromycin in blood by a new chromatographic method. J Antibiot (Tokyo) 1969 Nov;22(11):551–557. doi: 10.7164/antibiotics.22.551. [DOI] [PubMed] [Google Scholar]
- Welling P. G., Huang H., Hewitt P. F., Lyons L. L. Bioavailability of erythromycin stearate: influence of food and fluid volume. J Pharm Sci. 1978 Jun;67(6):764–766. doi: 10.1002/jps.2600670608. [DOI] [PubMed] [Google Scholar]
- Wiegand R. G., Chun A. H. Serum protein binding of erythromycin and erythromycin 2'-propionate ester. J Pharm Sci. 1972 Mar;61(3):425–428. doi: 10.1002/jps.2600610322. [DOI] [PubMed] [Google Scholar]


