Abstract
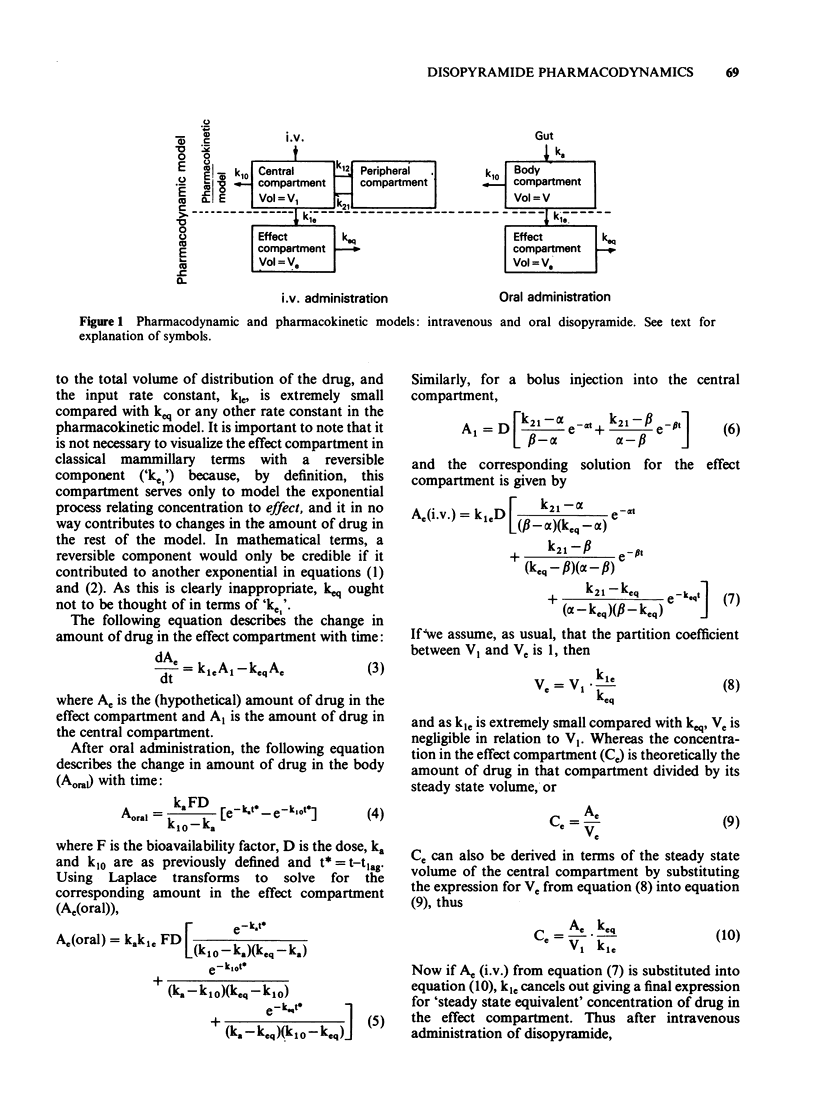
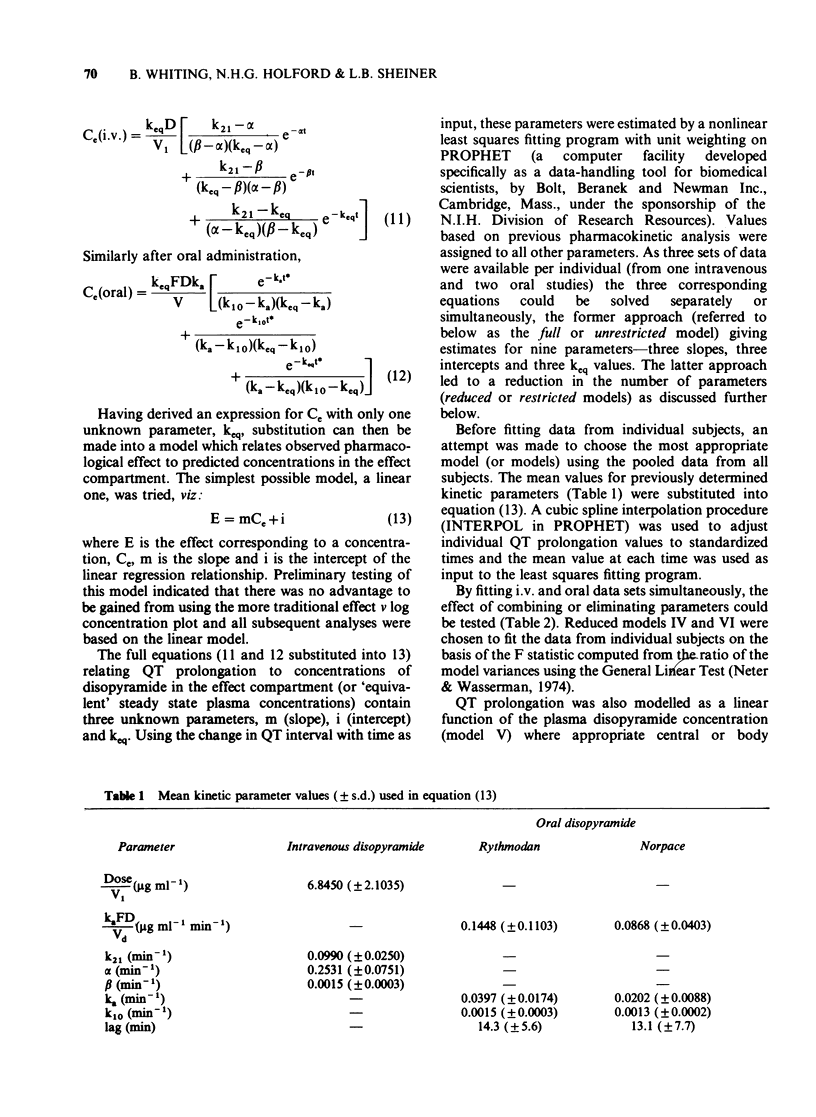
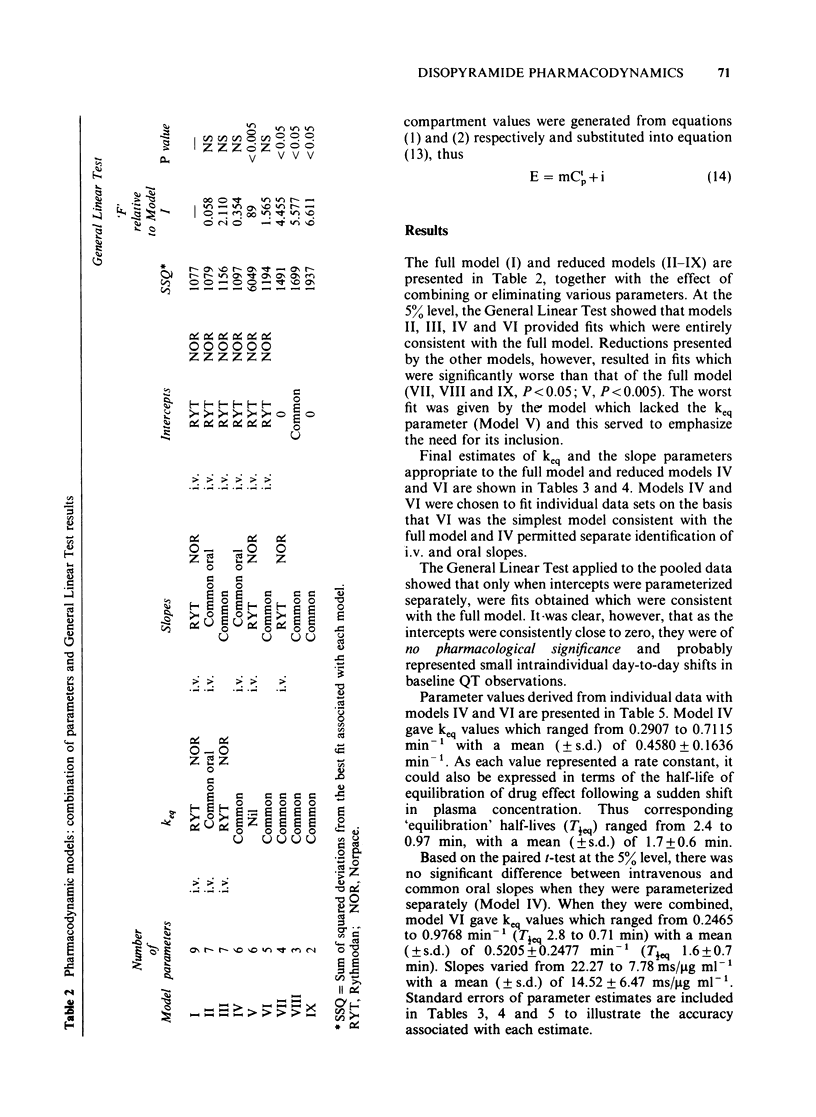
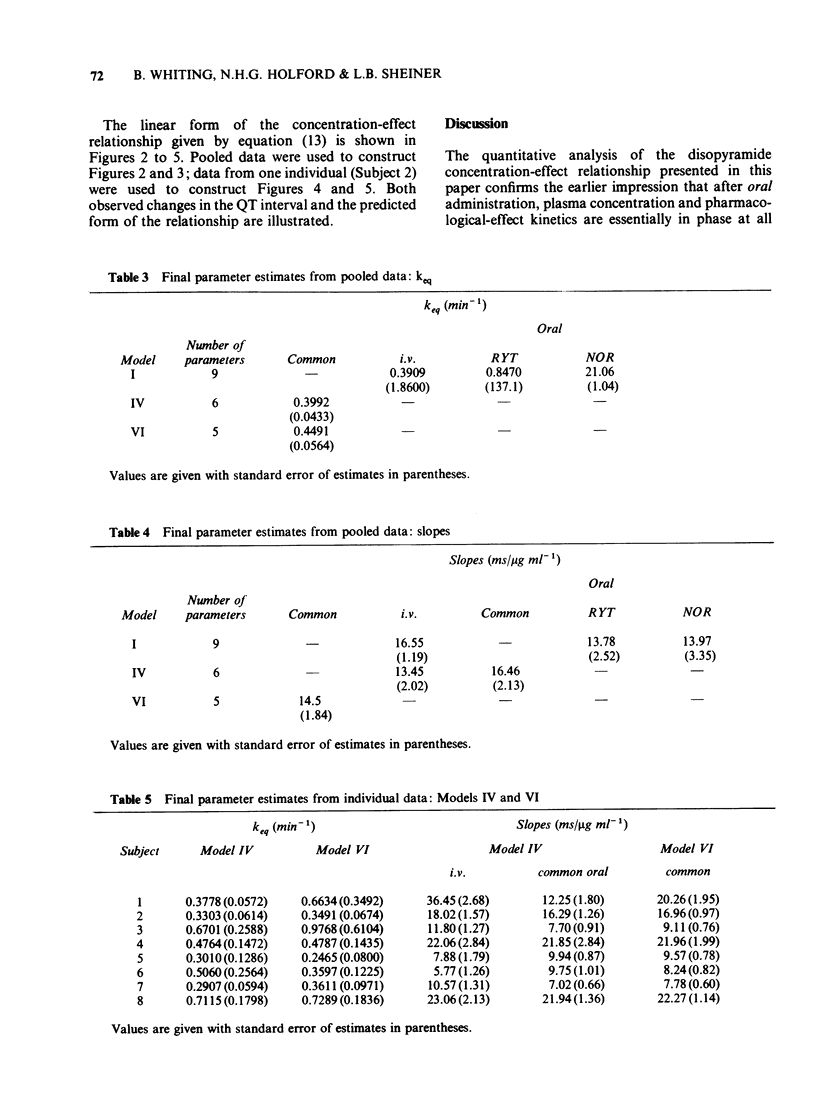
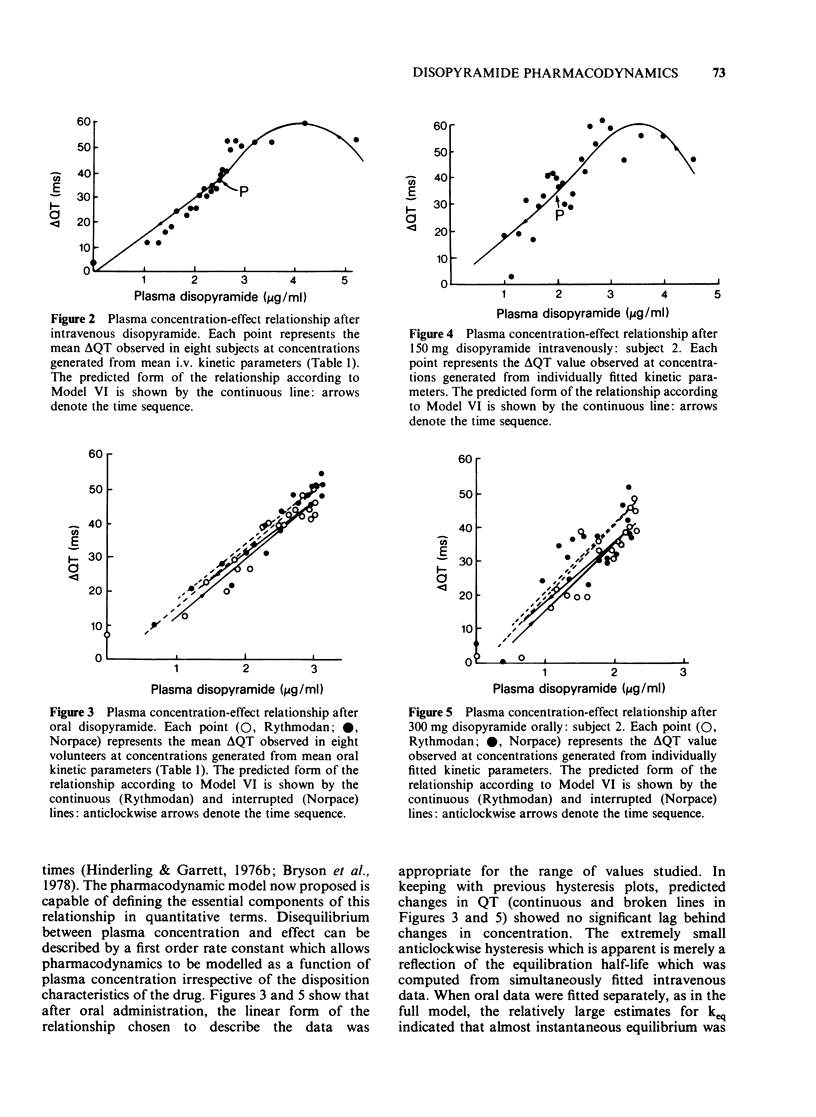
1. A combined pharmacokinetic-pharmacodynamic model has been used to analyse the relationship between QT prolongation and changes in plasma concentration which occurred after disopyramide was given intravenously and orally to eight healthy subjects. 2. The pharmacokinetic models appropriate to intravenous and oral disopyramide have been extended by an 'effect compartment' which has no influence on the predetermined mass of drug in the body. 3. The model incorporates an adjustment for lag of effect behind any rapid changes in plasma concentration such as occur in the early distributive phase following intravenous administration. This permits calculation of the proportionality constant relating plasma concentration to effect. 4. Irrespective of the route of administration the mean (+/- s.d.) prolongation of the QT interval was 14.5 +/- 6.5 ms/micrograms ml-1. 5. There was no evidence that metabolite produced during first pass after oral administration made any significant contribution to effect. 6. This modelling technique should be applicable to the study of the concentration-effect relationship of a number of other drugs, both in health and in disease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryson S. M., Whiting B., Lawrence J. R. Disopyramide serum and pharmacologic effect kinetics applied to the assessment of bioavailability. Br J Clin Pharmacol. 1978 Nov;6(5):409–419. doi: 10.1111/j.1365-2125.1978.tb04605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeazzi R. L., Benet L. Z., Sheiner L. B. Relationship between the pharmacokinetics and pharmacodynamics of procainamide. Clin Pharmacol Ther. 1976 Sep;20(3):278–289. doi: 10.1002/cpt1976203278. [DOI] [PubMed] [Google Scholar]
- Hinderling P. H., Garrett E. R. Pharmacodynamics of the antiarrhythmic disopyramide in healthy humans: correlation of the kinetics of the drug and its effects. J Pharmacokinet Biopharm. 1976 Jun;4(3):231–242. doi: 10.1007/BF01063615. [DOI] [PubMed] [Google Scholar]
- Hinderling P. H., Garrett E. R. Pharmacokinetics of the antiarrhythmic disopyramide in healthy humans. J Pharmacokinet Biopharm. 1976 Jun;4(3):199–230. doi: 10.1007/BF01063614. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B., Stanski D. R., Vozeh S., Miller R. D., Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979 Mar;25(3):358–371. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]


