Abstract
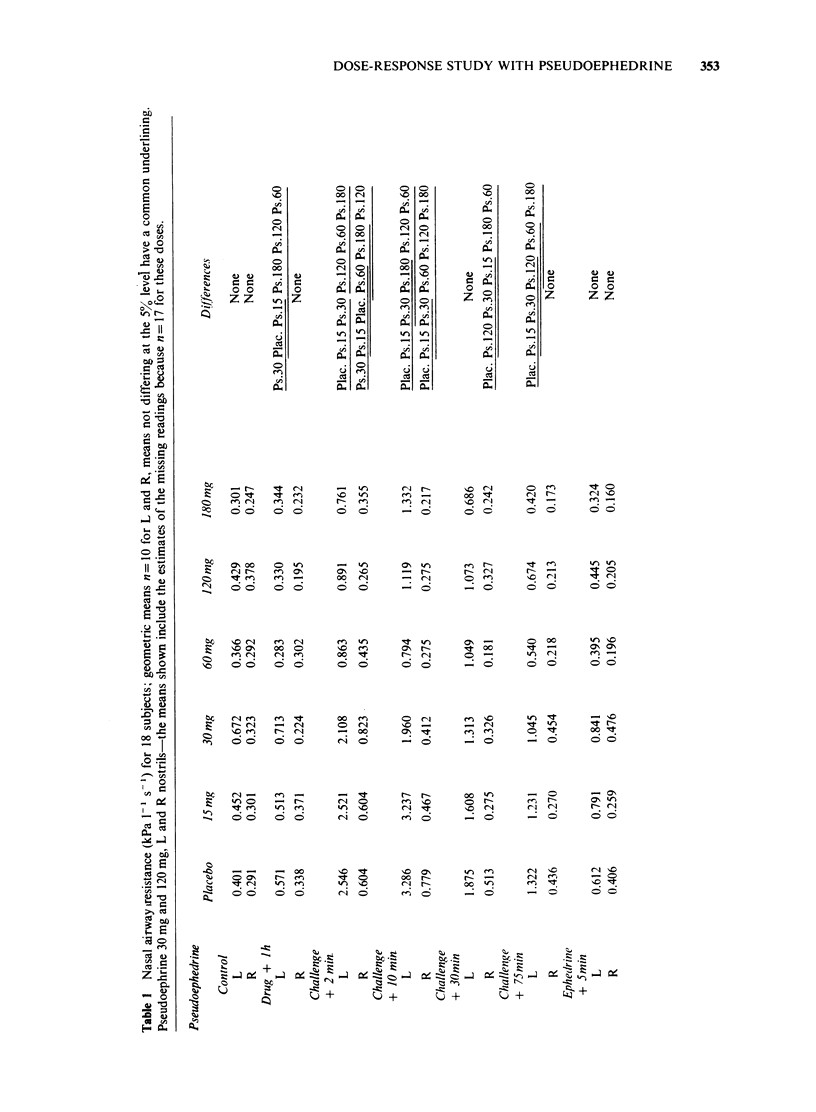
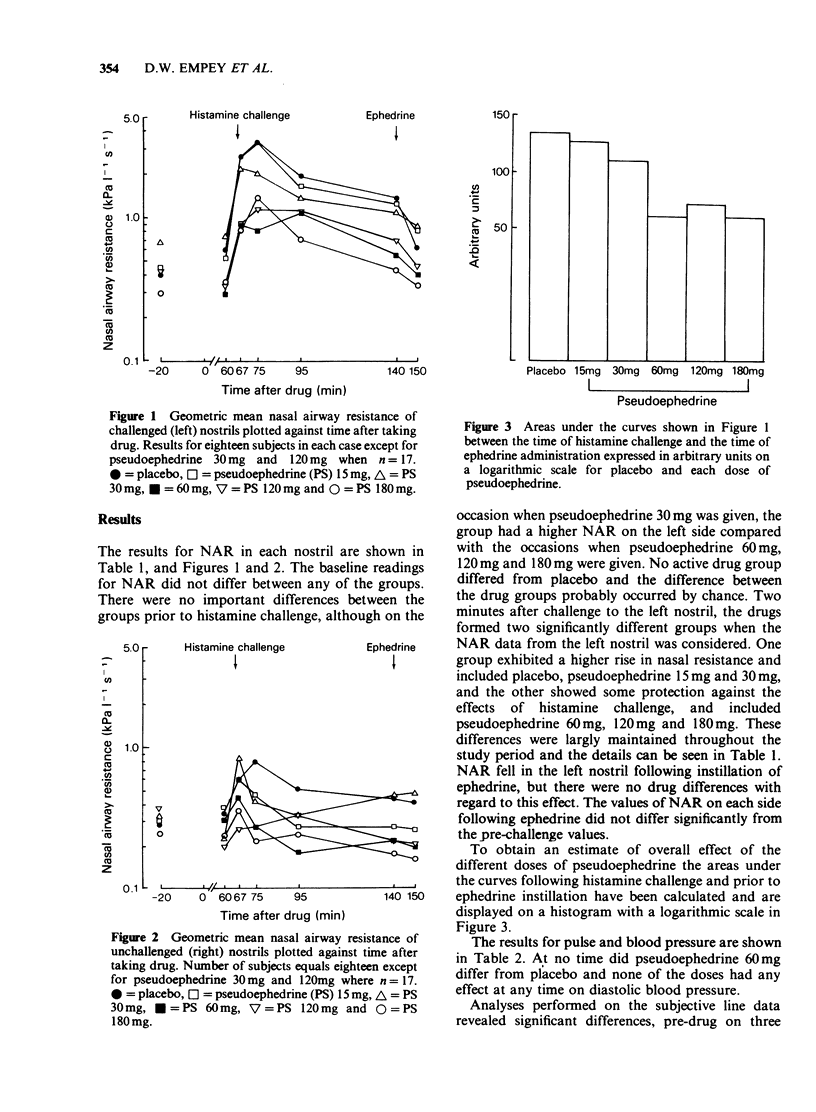
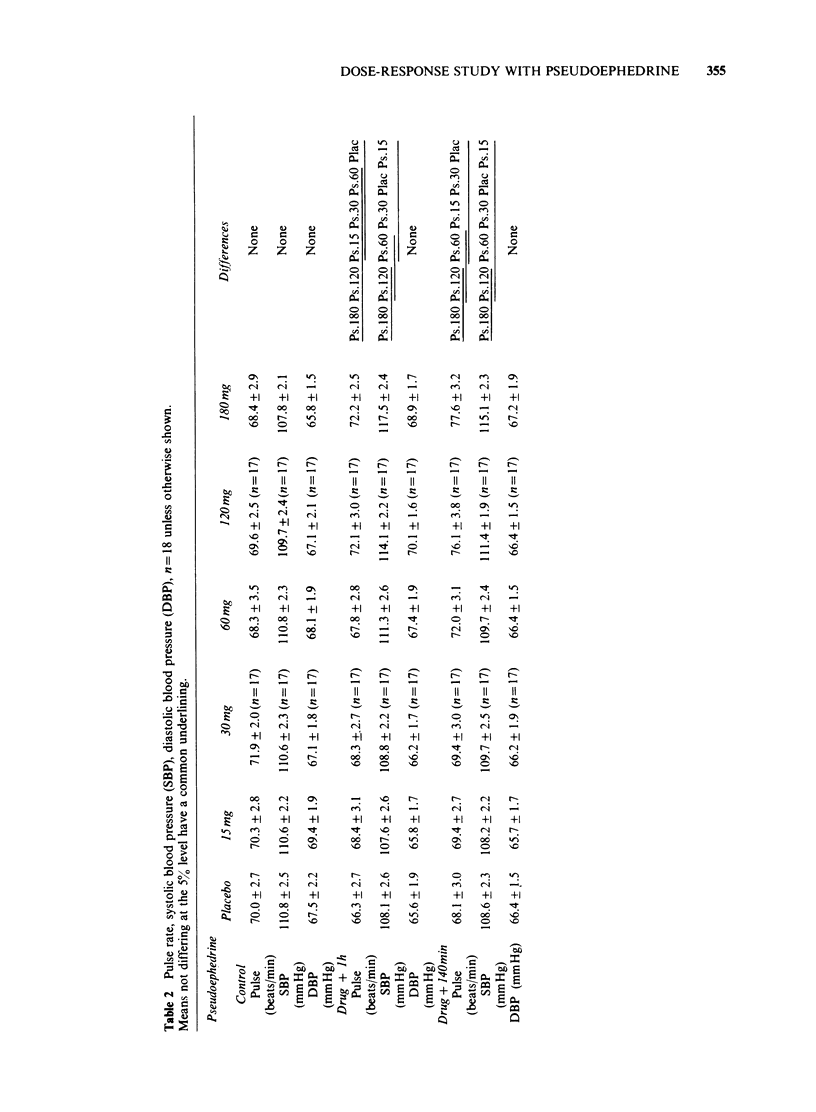
1 The effects of different doses of orally administered pseudoephedrine on nasal airway resistance (NAR) were studied in a group of eighteen healthy subjects using double-blind conditions with drugs administered in a series of cross-over experiments according to a Latin-square design. 2 Challenge with 1% histamine diphosphate to one nostril 1 h after administration of the drugs produced increases in NAR. 3 The effects of pre-treatment with both placebo and increasing doses of pseudoephedrine on this histamine-induced increase in NAR were examined. Pseudoephedrine 60 mg, 120 mg and 180 mg significantly (P less than 0.05) reduced the effect of histamine on NAR compared with the placebo, and the protective effects of these doses did not differ significantly from each other. Pseudoephedrine 15 mg and 30 mg did not differ from placebo in their effects on NAR. 4 Small, but statistically significant increases in pulse and systolic blood pressure occurred after pseudoephedrine 120 mg and 180 mg, but not after pseudoephedrine 60 mg, 30 mg or 15 mg. No significant effects were produced by any of the doses of pseudephedrine with regard to diastolic blood pressure. Similarly no dose of pseudoephedrine altered mood or produced any excess of unwanted effects compared with placebo. 5 We conclude that pseudoephedrine 60 mg is the optimal single adult dose since this achieves maximal nasal decongestion without cardiovascular or other unwanted effects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton M. G., Empey D. W., John G. C., McDonnell K. A., Hughes D. T. Histamine challenge and anterior nasal rhinometry: their use in the assessment of pseudoephedrine and triprolidine as nasal decongestants in subjects with hayfever. Br J Clin Pharmacol. 1978 Jul;6(1):51–58. doi: 10.1111/j.1365-2125.1978.tb01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye C., Hill H. M., Hughes D. T., Peck A. W. A comparison of plasma levels of L(+) pseudoephedrine following different formulations, and their relation to cardiovascular and subjective effects in man. Eur J Clin Pharmacol. 1975;8(1):47–53. doi: 10.1007/BF00616414. [DOI] [PubMed] [Google Scholar]
- Dallimore N. S., Eccles R. Changes in human nasal resistance associated with exercise, hyperventilation and rebreathing. Acta Otolaryngol. 1977 Nov-Dec;84(5-6):416–421. doi: 10.3109/00016487709123985. [DOI] [PubMed] [Google Scholar]
- Drew C. D., Knight G. T., Hughes D. T., Bush M. Comparison of the effects of D-(-)-ephedrine and L-(+)-pseudoephedrine on the cardiovascular and respiratory systems in man. Br J Clin Pharmacol. 1978 Sep;6(3):221–225. doi: 10.1111/j.1365-2125.1978.tb04588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. The central rhythm of the nasal cycle. Acta Otolaryngol. 1978 Nov-Dec;86(5-6):464–468. doi: 10.3109/00016487809107526. [DOI] [PubMed] [Google Scholar]
- Empey D. W., Bye C., Hodder M., Hughes D. T. A Double-blind crossover trial of pseudoephedrine and triprolidine, alone and in combination, for the treatment of allergenic rhinitis. Ann Allergy. 1975 Jan;34(1):41–46. [PubMed] [Google Scholar]
- Lader M., Norris H. The effects of nitrous oxide on the human auditory evoked response. Psychopharmacologia. 1969;16(2):115–127. doi: 10.1007/BF00403614. [DOI] [PubMed] [Google Scholar]



