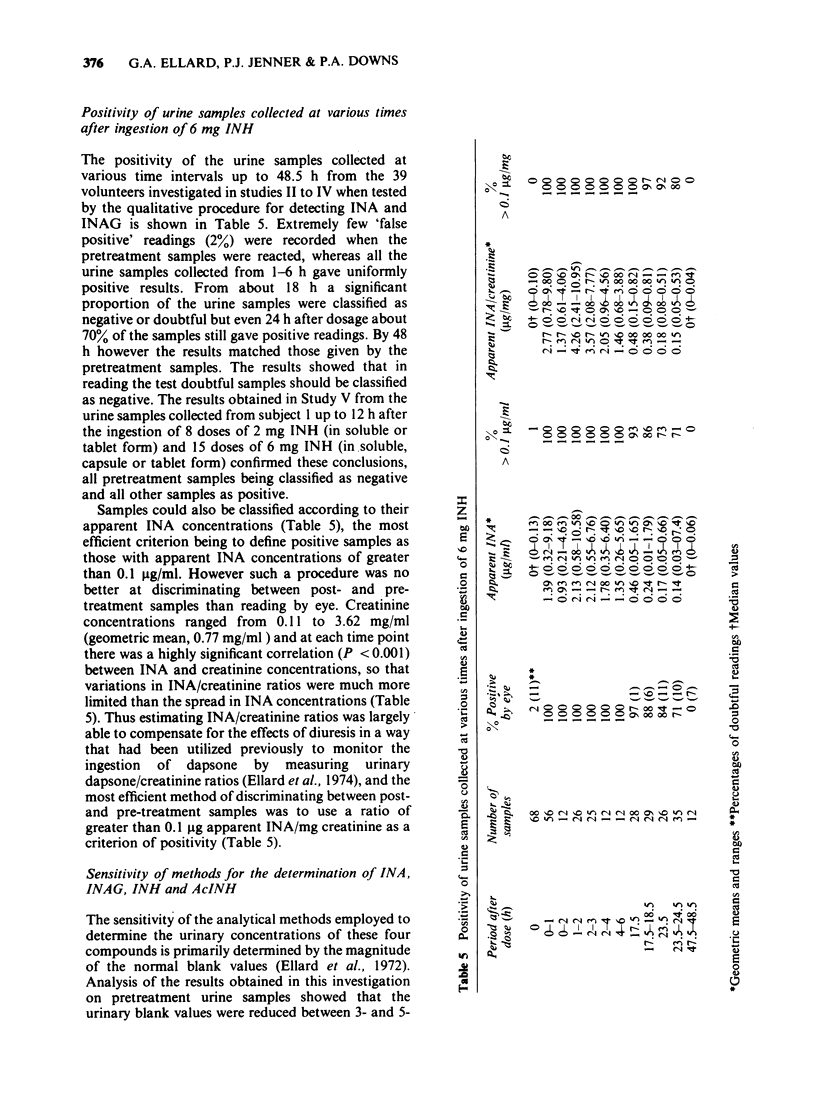
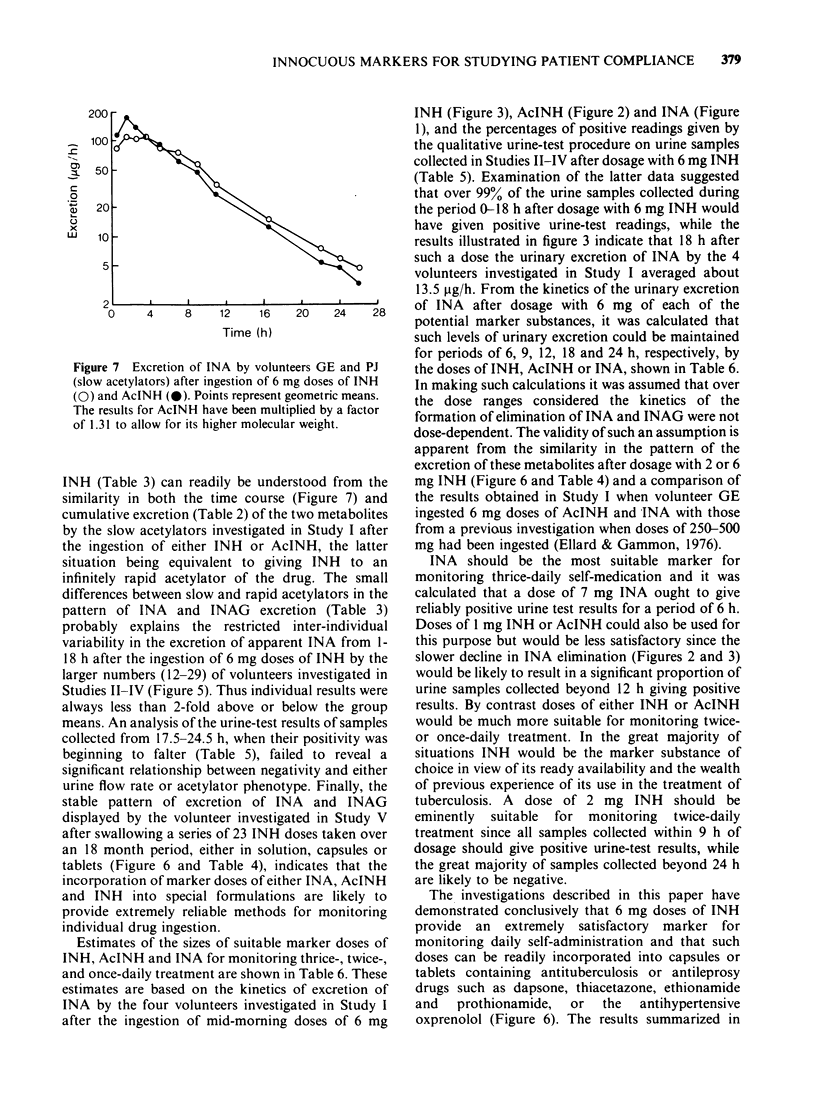
Abstract
1. The possibility of using minute doses of the antituberculosis drug isoniazid (INH) or of its metabolites acetylisoniazid (AcINH) or isonicotinic acid (INA) as innocuous markers for monitoring patient compliance has been investigated. 2. The ingestion of these colourless and tasteless compounds can readily be demonstrated using a sensitive and specific colorimetric method for detecting INA and its metabolite isonicotinylglycine (INAG) in the urine that is rapid and simple to perform. 3. Studies on the kinetics of the urinary elimination of INA and INAG after the ingestion of 6 mg doses of either INH, AcINH or INA by small groups of volunteers indicated the potential suitability of INH or AcINH for monitoring daily or twice-daily self-medication and the appropriateness of INA as a marker for investigating the compliance of drugs prescribed for thrice-daily ingestion. 4. More extensive studies showed that over 99% of the urine samples collected within 18h of dosage with 6 mg INH would give positive results when tested for the presence of INA and INAG, and that doses of 2-6 mg INH could readily by incorporated into capsules or tablets and used as markers for monitoring the ingestion of the antituberculosis or antileprosy drugs dapsone, thiacetazone, ethionamide or prothionamide, or the antihypertensive oxprenolol. Such doses are less than a fiftieth of the normal therapeutic INH dose used in the treatment of tuberculosis. 5. Evidence is presented that INH, AcINH and INA possess most of the characteristics that one would hope to find in a marker for monitoring compliance including very limited inter-individual variability in the rates at which they are converted to the compounds being detected in the urine.
Full text
PDF


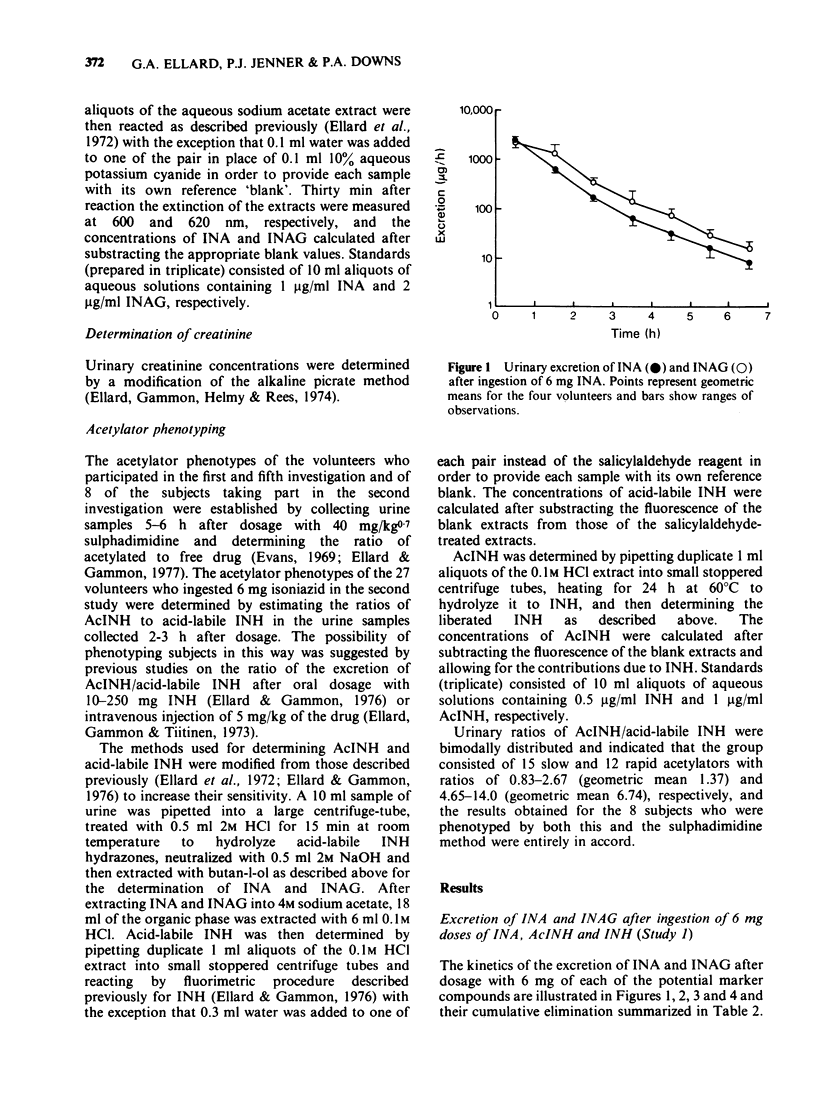
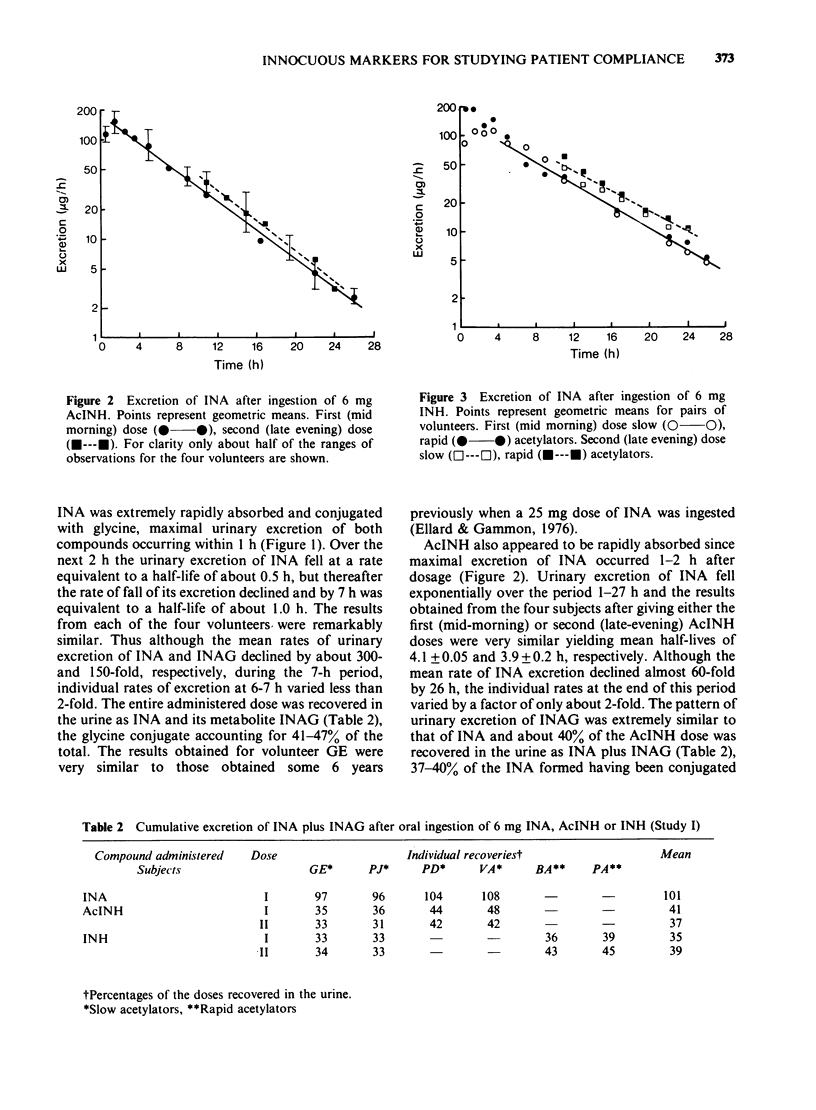
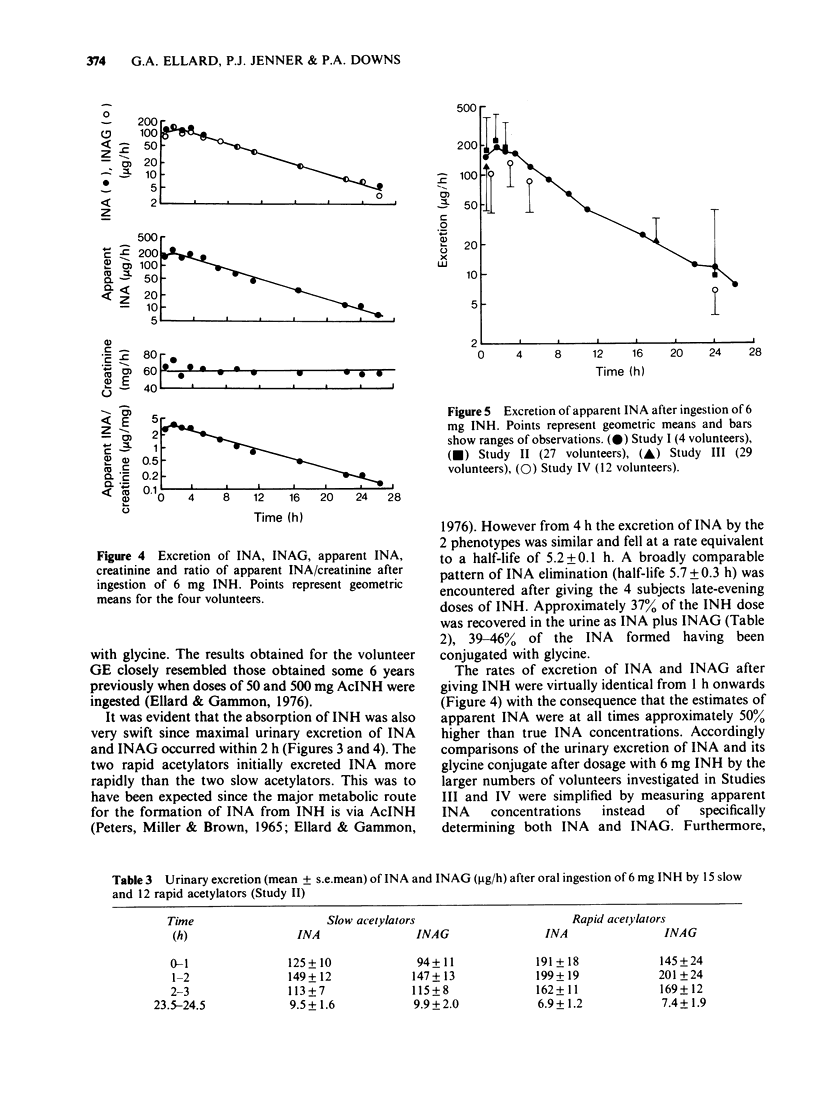
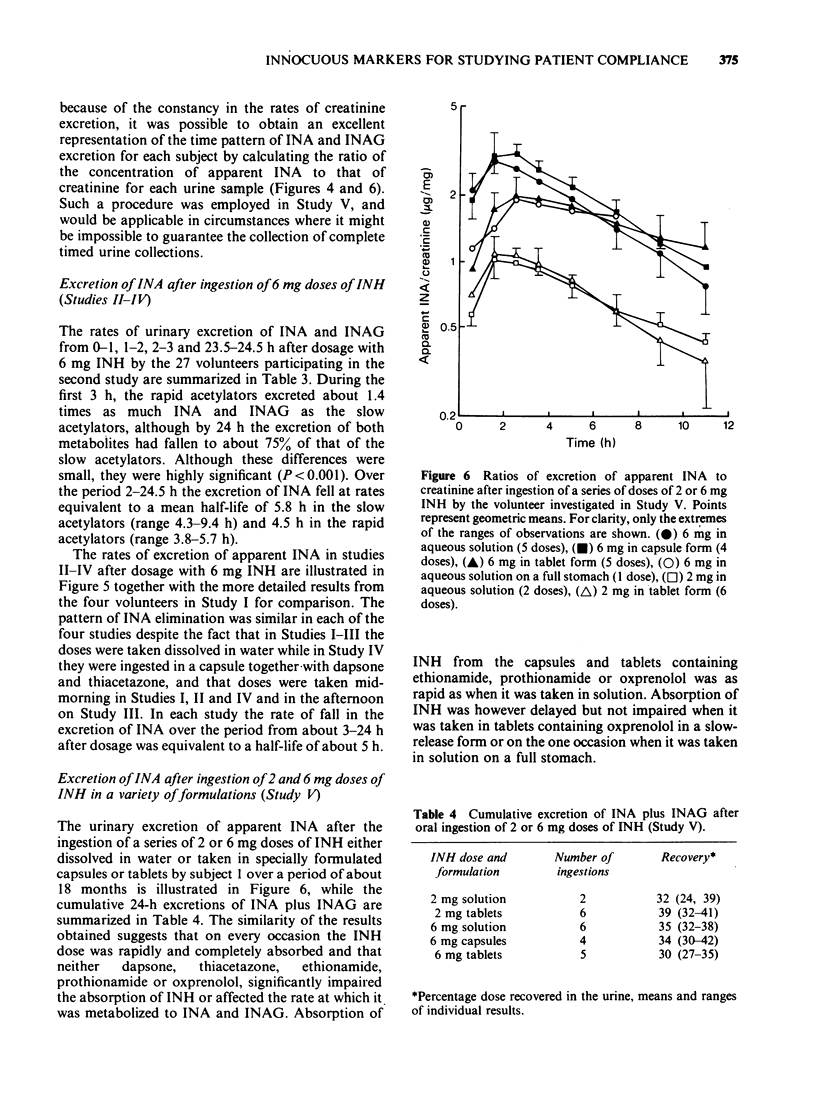




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNSTEIN J., LOTT W. A., STEINBERG B. A., YALE H. L. Chemotherapy of experimental tuberculosis. V. Isonicotinic acid hydrazide (nydrazid) and related compounds. Am Rev Tuberc. 1952 Apr;65(4):357–364. doi: 10.1164/art.1952.65.4.357. [DOI] [PubMed] [Google Scholar]
- Bailey L. C., Abdou H. High-performance liquid chromatographic analysis of isoniazid and its dosage forms. J Pharm Sci. 1977 Apr;66(4):564–567. doi: 10.1002/jps.2600660427. [DOI] [PubMed] [Google Scholar]
- Blackwell B. The drug defaulter. Clin Pharmacol Ther. 1972 Nov-Dec;13(6):841–848. doi: 10.1002/cpt1972135part2841. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H. G., Riegelman S. Pharmacokinetics of isoniazid and some metabolites in man. J Pharmacokinet Biopharm. 1976 Aug;4(4):287–325. doi: 10.1007/BF01063121. [DOI] [PubMed] [Google Scholar]
- DEUSCHLE K. W., JORDAHL C., HOBBY G. L. Clinical usefulness of riboflavin-tagged isoniazid for self-medication in tuberculous patients. Am Rev Respir Dis. 1960 Jul;82:1–10. doi: 10.1164/arrd.1960.82.1.1. [DOI] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T. Acetylator phenotyping of tuberculosis patients using matrix isoniazid or sulphadimidine and its prognostic significance for treatment with several intermittent isoniazid-containing regimens. Br J Clin Pharmacol. 1977 Feb;4(1):5–14. doi: 10.1111/j.1365-2125.1977.tb00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T., Helmy H. S., Rees R. J. Urine tests to monitor the self-administration of dapsone by leprosy patients. Am J Trop Med Hyg. 1974 May;23(3):464–470. doi: 10.4269/ajtmh.1974.23.464. [DOI] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T. Pharmacokinetics of isoniazid metabolism in man. J Pharmacokinet Biopharm. 1976 Apr;4(2):83–113. doi: 10.1007/BF01086149. [DOI] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T., Tiitinen H. Determination of the acetylator phenotype from the ratio of the urinary excretion of acetylisoniazid to acid-labile isoniazid: a study in Finnish Lapland. Tubercle. 1973 Sep;54(3):201–210. doi: 10.1016/0041-3879(73)90025-1. [DOI] [PubMed] [Google Scholar]
- Ellard G. A., Gammon P. T., Wallace S. M. The determination of isoniazid and its metabolites acetylisoniazid, monoacetylhydrazine, diacetylhydrazine, isonicotinic acid and isonicotinylglycine in serum and urine. Biochem J. 1972 Feb;126(3):449–458. doi: 10.1042/bj1260449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellard G. A., Greenfield A sensitive urine-test method for monitoring the ingestion of isoniazid. J Clin Pathol. 1977 Jan;30(1):84–87. doi: 10.1136/jcp.30.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A. An improved and simplified method of detecting the acetylator phenotype. J Med Genet. 1969 Dec;6(4):405–407. doi: 10.1136/jmg.6.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A. Genetic variations in the acetylation of isoniazid and other drugs. Ann N Y Acad Sci. 1968 Jul 31;151(2):723–733. doi: 10.1111/j.1749-6632.1968.tb48255.x. [DOI] [PubMed] [Google Scholar]
- FOX W. Self-administration of medicaments. A review of published work and a study of the problems. Bull Int Union Tuberc. 1962 Jul;32:307–331. [PubMed] [Google Scholar]
- Fox W. General considerations in the choice and management of regimens of chemotherapy for pulmonary tuberculosis. Bull Int Union Tuberc. 1972 Feb;47:49–67. [PubMed] [Google Scholar]
- Girling D. J. The hepatic toxicity of antituberculosis regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle. 1978 Mar;59(1):13–32. doi: 10.1016/0041-3879(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Glassroth J. L., White M. C., Snider D. E., Jr An assessment of the possible association of isoniazid with human cancer deaths. Am Rev Respir Dis. 1977 Dec;116(6):1065–1074. doi: 10.1164/arrd.1977.116.6.1065. [DOI] [PubMed] [Google Scholar]
- HUGHES H. B. On the metabolic fate of isoniazid. J Pharmacol Exp Ther. 1953 Dec;109(4):444–452. [PubMed] [Google Scholar]
- La Du B. N. Isoniazid and psuedocholinesterase polymorphisms. Fed Proc. 1972 Jul-Aug;31(4):1276–1285. [PubMed] [Google Scholar]
- MCKENNIS H., Jr, YARD A. S., PAHNELAS E. V. The production of fatty livers in rabbits by isoniazid and other hydrazine derivatives. Am Rev Tuberc. 1956 Jun;73(6):956–959. doi: 10.1164/artpd.1956.73.6.956. [DOI] [PubMed] [Google Scholar]
- Marston M. V. Compliance with medical regimens: a review of the literature. Nurs Res. 1970 Jul-Aug;19(4):312–323. [PubMed] [Google Scholar]
- Mitchell J. R., Thorgeirsson U. P., Black M., Timbrell J. A., Snodgrass W. R., Potter W. Z., Jollow H. R., Keiser H. R. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydranize metabolites. Clin Pharmacol Ther. 1975 Jul;18(1):70–79. doi: 10.1002/cpt197518170. [DOI] [PubMed] [Google Scholar]
- Olson W. A., Dayton P. G., Israili Z. H., Pruitt A. W. Spectrophotofluorometric assay for isoniazid and acetyl isoniazid in plasma adapted to pediatric studies. Clin Chem. 1977;23(4):745–748. [PubMed] [Google Scholar]
- Peters J. H. Genetic factors in relation to drugs. Annu Rev Pharmacol. 1968;8:427–452. doi: 10.1146/annurev.pa.08.040168.002235. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Miller K. S., Brown P. Studies on the metabolic basis for the genetically determined capacities for isoniazid inactivation in man. J Pharmacol Exp Ther. 1965 Nov;150(2):298–304. [PubMed] [Google Scholar]
- Stark J. E., Ellard G. A., Gammon P. T., Fox W. The use of isoniazid as a marker to monitor the self-administration of medicaments. Br J Clin Pharmacol. 1975 Aug;2(4):355–358. doi: 10.1111/j.1365-2125.1975.tb02784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott H., Peto J., Stephens R., Fox W. An assessment of the carcinogenicity of isoniazid in patients with pulmonary tuberculosis. Tubercle. 1976 Mar;57(1):1–15. doi: 10.1016/0041-3879(76)90014-3. [DOI] [PubMed] [Google Scholar]
- YARD A. S., MCKENNIS H., Jr ASPECTS OF THE METABOLISM OF ISONIAZID AND ACETYLISONIAZID IN THE HUMAN AND THE DOG. J Med Pharm Chem. 1962 Jan;5:196–203. doi: 10.1021/jm01236a019. [DOI] [PubMed] [Google Scholar]


