Abstract
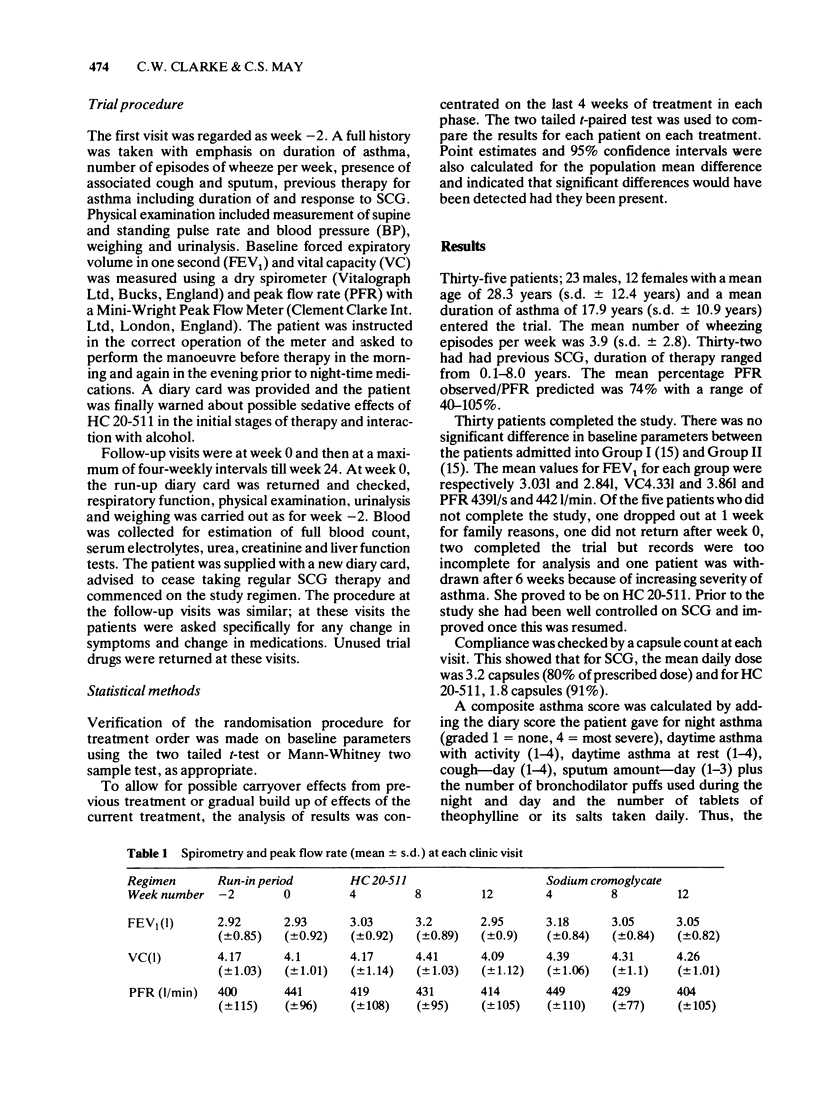
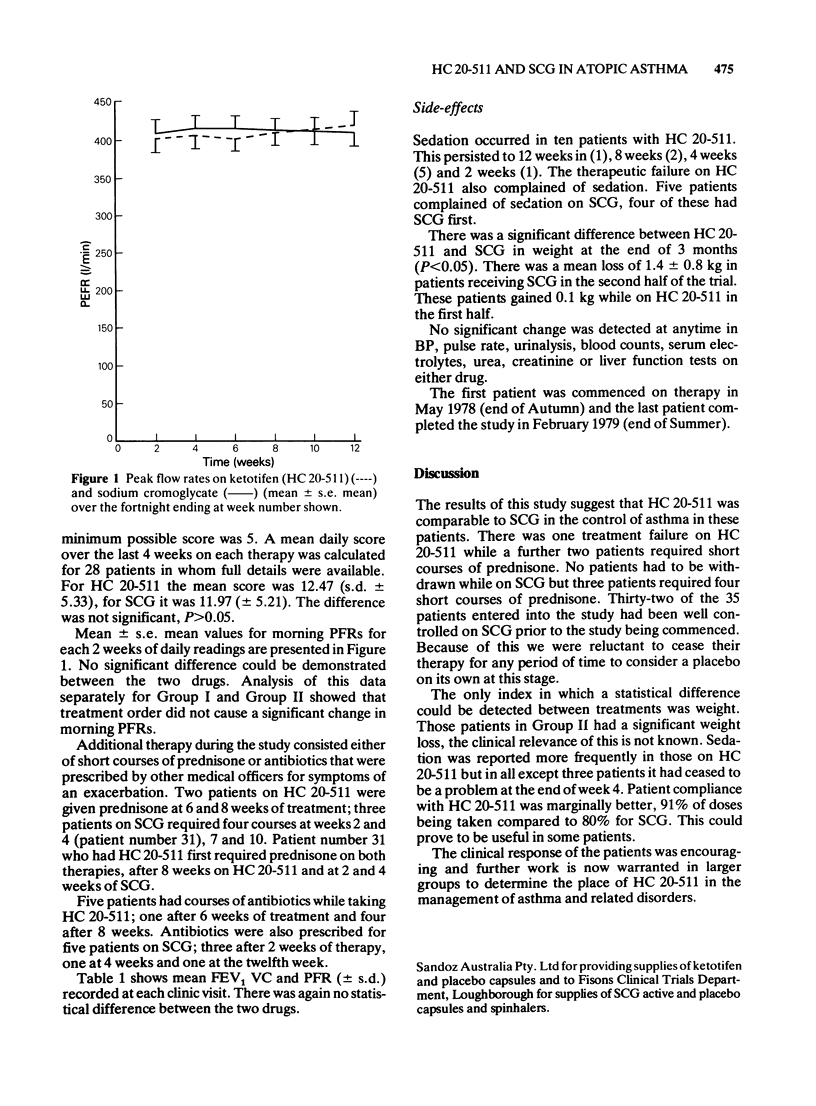
1 Ketotifen (HC 20-511 Sandoz) 1 mg twice daily for 12 weeks was found to be equivalent to sodium cromoglycate (SCG) 20 mg four times daily for 12 weeks in 35 skin test positive asthmatic patients in a randomised double-blind cross-over study. 2 No statistically significant difference between the two drugs in mean values for daily peak flow rates, diary card scores and spirometry at monthly visits was demonstrated. 3 Treatment failures as judged by severe asthma requiring withdrawal from the trial or addition of short courses of prednisone occurred in three patients on each drug. 4 Sedation was noted by 10 patients onHC 20-511 and 5 on SCG. 5 Weight loss was noted in those patients on SCG, but not those on HC 20-511.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craps L., Greenwood C., Radielovic P. Clinical investigation of agents with prophylactic anti-allergic effects in bronchial asthma. Clin Allergy. 1978 Jul;8(4):373–382. doi: 10.1111/j.1365-2222.1978.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Martin U., Römer D. The pharmacological properties of a new, orally active antianaphylactic compound: ketotifen, a benzocycloheptathiophene. Arzneimittelforschung. 1978;28(5):770–782. [PubMed] [Google Scholar]
- Pauwels R., Lamont H., Van Der Straeten M. Comparison between ketotifen and DSCG in bronchial challenge. Clin Allergy. 1978 May;8(3):289–293. doi: 10.1111/j.1365-2222.1978.tb03226.x. [DOI] [PubMed] [Google Scholar]
- Wells A., Taylor B. A placebo-controlled trial of ketotifen (HC 20-511, Sandoz) in allergen induced asthma and comparison with disodium cromoglycate. Clin Allergy. 1979 May;9(3):237–240. doi: 10.1111/j.1365-2222.1979.tb01548.x. [DOI] [PubMed] [Google Scholar]


