Abstract
1 Doses of 16, 32, 48 and 64 mg prednisolone were administered intravenously to normal volunteers who also received 100 prednisolone orally. Plasma prednisolone concentrations were estimated by quantitative thin layer chromatography. 2 The bioavailability fraction was 1.063 +/- 0.154 (s.d.) indicating complete availability of prednisolone following oral administration. 3 The mean T 1/2 over all doses were 4.11 +/- 0.97 (s.d.) h and there was no evidence of a dose-related change in its value. 4 The mean systemic clearance over all doses was 0.104 +/- 0.034 (s.d) 1 h-1 kg-1. There was no evidence of a dose-related change in clearance or in the apparent volume of distribution (overall mean 0.588 +/- 0.152 1 kg-1). 5 The area under the plasma concentration-time curve was linearly related to dose. 6 Plasma concentration-time curves normalised for dose were superimposable. 7 It was concluded that over the dose range investigated, non-linear pharmacokinetic behaviour had not been demonstrated in this group of normal volunteers.
Full text
PDF

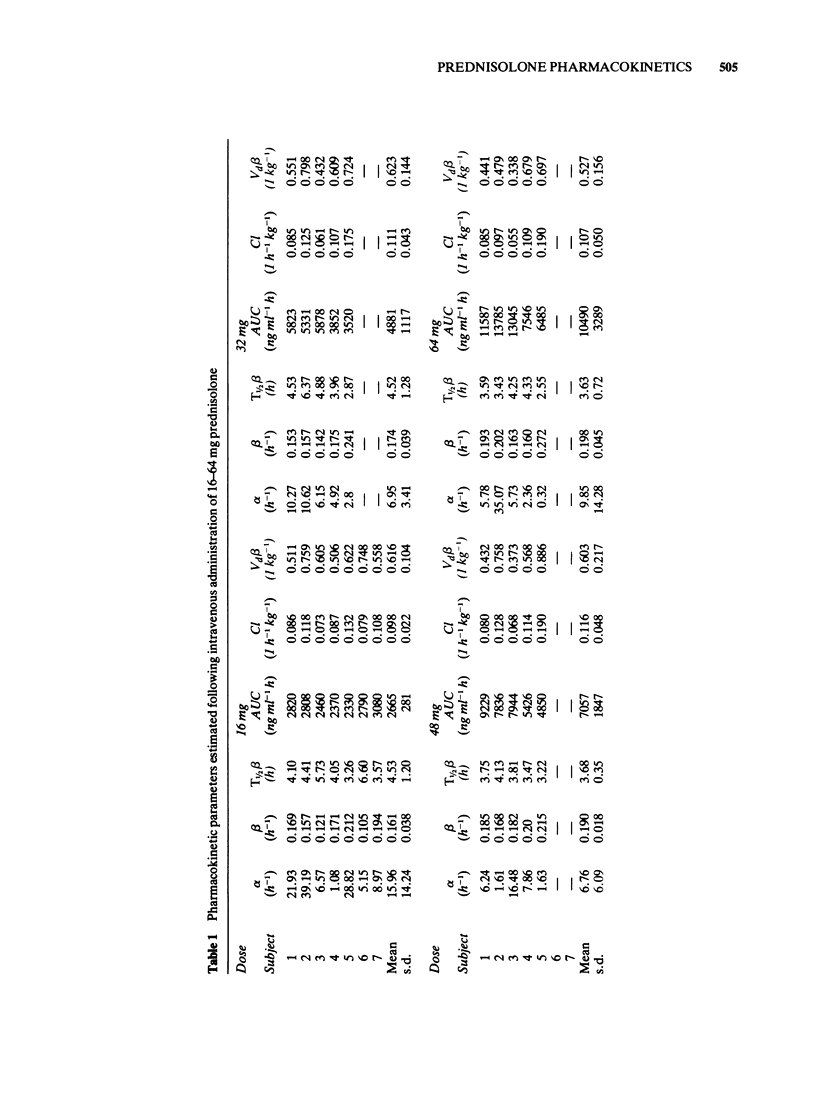
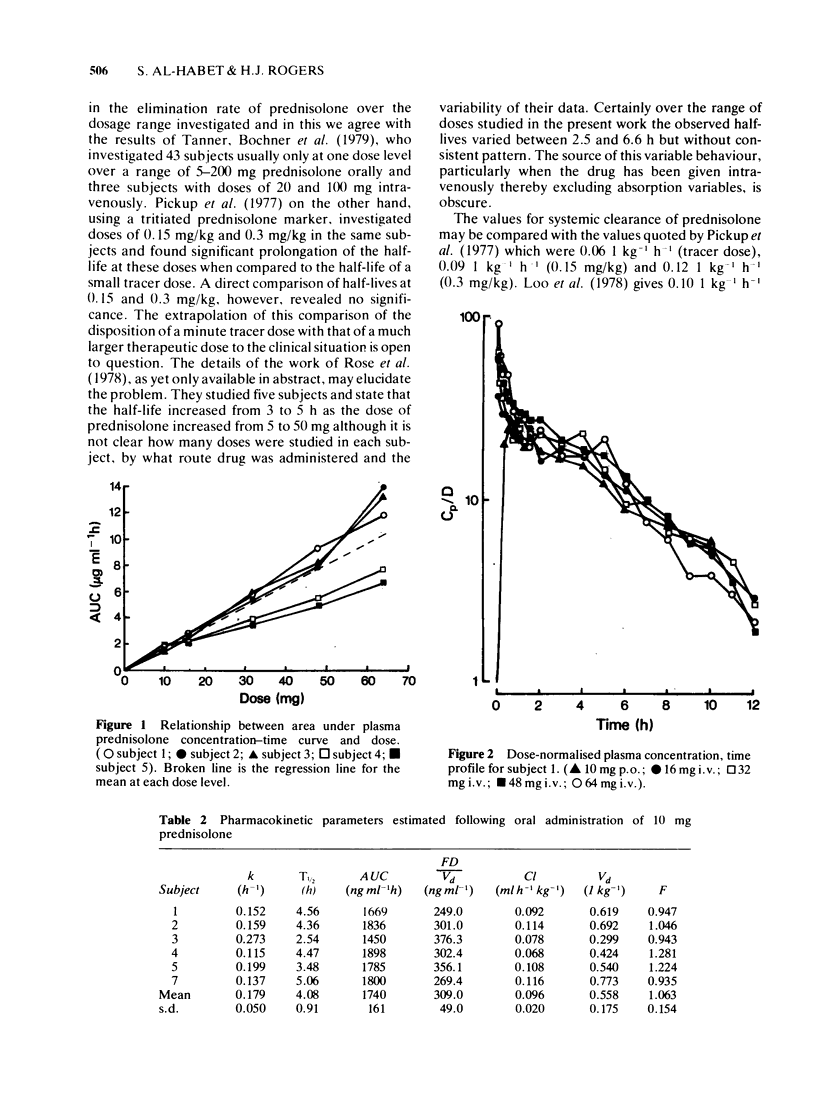


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ağabeyoğlu I. T., Bergstrom R. F., Gillespie W. R., Wagner J. G., Kay D. R. Plasma protein binding of prednisolone in normal volunteers and arthritic patients. Eur J Clin Pharmacol. 1979;16(6):399–404. doi: 10.1007/BF00568200. [DOI] [PubMed] [Google Scholar]
- Dvorchik B. H., Vessell E. S. Significance of error associated with use of the one-compartment formula to calculate clearance of thirty-eight drugs. Clin Pharmacol Ther. 1978 Jun;23(6):617–623. doi: 10.1002/cpt1978236617. [DOI] [PubMed] [Google Scholar]
- Loo J. C., McGilveray I. J., Jordan N., Moffat J., Brien R. Dose-dependent pharmacokinetics of prednisone and prednisolone in man. J Pharm Pharmacol. 1978 Nov;30(11):736–736. doi: 10.1111/j.2042-7158.1978.tb13381.x. [DOI] [PubMed] [Google Scholar]
- Morrison P. J., Bradbrook I. D., Rogers H. J. Plasma prednisolone levels from enteric and non-enteric coated tablets estimated by an original technique. Br J Clin Pharmacol. 1977 Oct;4(5):597–603. doi: 10.1111/j.1365-2125.1977.tb00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak E., Gilbertson T. J., Seckman C. E., Stewart R. D., DiSanto A. R., Stubbs S. S. Anorectal pruritus after intravenous hydrocortisone sodium succinate and sodium phosphate. Clin Pharmacol Ther. 1976 Jul;20(1):109–112. doi: 10.1002/cpt1976201109. [DOI] [PubMed] [Google Scholar]
- Pickup M. E., Lowe J. R., Leatham P. A., Rhind V. M., Wright V., Downie W. W. Dose dependent pharmacokinetics of prednisolone. Eur J Clin Pharmacol. 1977 Nov 14;12(3):213–219. doi: 10.1007/BF00609864. [DOI] [PubMed] [Google Scholar]
- Tanner A. R., Caffin J. A., Halliday J. W., Powell L. W. Concurrent administration of antacids and prednisone: effect on serum levels of prednisolone. Br J Clin Pharmacol. 1979 Apr;7(4):397–400. doi: 10.1111/j.1365-2125.1979.tb00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A., Bochner F., Caffin J., Halliday J., Powell L. Dose-dependent prednisolone kinetics. Clin Pharmacol Ther. 1979 May;25(5 Pt 1):571–578. doi: 10.1002/cpt1979255part1571. [DOI] [PubMed] [Google Scholar]
- Tse F. L., Welling P. G. Relative bioavailability of prednisone and prednisolone in man. J Pharm Pharmacol. 1979 Jul;31(7):492–493. doi: 10.1111/j.2042-7158.1979.tb13568.x. [DOI] [PubMed] [Google Scholar]


