Abstract
Medulloblastoma, one of the most malignant brain tumors in children, is thought to arise from undifferentiated neural stem/progenitor cells (NSCs) present in the external granule layer of the cerebellum. However, the mechanism of tumorigenesis remains unknown for the majority of medulloblastomas. In this study, we found that many human medulloblastomas express significantly elevated levels of both myc oncogenes, regulators of neural progenitor proliferation, and REST/NRSF, a transcriptional repressor of neuronal differentiation genes. Previous studies have shown that neither c-Myc nor REST/NRSF alone could cause tumor formation. To determine whether c-Myc and REST/NRSF act together to cause medulloblastomas, we used a previously established cell line derived from external granule layer stem cells transduced with activated c-myc (NSC-M). These immortalized NSCs were able to differentiate into neurons in vitro. In contrast, when the cells were engineered to express a doxycycline-regulated REST/NRSF transgene (NSC-M-R), they no longer underwent terminal neuronal differentiation in vitro. When injected into intracranial locations in mice, the NSC-M cells did not form tumors either in the cerebellum or in the cerebral cortex. In contrast, the NSC-M-R cells did produce tumors in the cerebellum, the site of human medulloblastoma formation, but not when injected into the cerebral cortex. Furthermore, the NSC-M-R tumors were blocked from terminal neuronal differentiation. In addition, countering REST/NRSF function blocked the tumorigenic potential of NSC-M-R cells. To our knowledge, this is the first study in which abnormal expression of a sequence-specific DNA-binding transcriptional repressor has been shown to contribute directly to brain tumor formation. Our findings indicate that abnormal expression of REST/NRSF and Myc in NSCs causes cerebellum-specific tumors by blocking neuronal differentiation and thus maintaining the “stemness” of these cells. Furthermore, these results suggest that such a mechanism plays a role in the formation of human medulloblastoma.
Most medulloblastomas are believed to originate from undifferentiated neural stem/progenitor cells (NSCs) present in the external granule layer cells of the cerebellum (15, 18, 22, 41, 50). The primitive “embryonal” appearance of medulloblastoma cells, as well as their capacity for divergent differentiation, has led to the suggestion that they have a neural stem cell-like phenotype (18, 22). In addition to arising from stem cells, human medulloblastomas appear to contain stem-like cells required for tumor propagation (59). Induction of neuronal and glial markers in medulloblastoma cells has been documented as a response to several proposed chemotherapeutic agents (6, 42, 64), supporting the hypothesis that these tumors show lack of terminal differentiation and suggesting regulation of differentiation status as a promising treatment avenue.
Pathways regulating cerebellar development, such as Hedgehog and Wnt, have been found to be activated by genetic alterations during medulloblastoma tumorigenesis (15, 21, 44, 50, 66, 68, 69). Both Hedgehog and Wnt are thought to regulate proliferation and differentiation of neural stem cells and may play a similar role in medulloblastoma. However, mutations activating these pathways have been documented in only a modest percentage of human tumors. The receptor gene PTCH is the member of the Hedgehog pathway most commonly altered in medulloblastoma, but sensitive techniques such as direct sequencing have identified mutations in no more than 10% of cases (14). Similarly, mutations in CTNNB1 and Axin activating the Wnt pathway have been identified in less than 20% of sporadic medulloblastomas (13, 32, 70). Thus, the mechanism of tumorigenesis for the majority of medulloblastomas is still unknown.
The Myc oncoproteins are also important in medulloblastoma pathogenesis (1, 7, 26). c-myc and N-myc are commonly amplified in the biologically aggressive large cell/anaplastic medulloblastoma subtype (17, 40). In addition, overexpression of c-Myc mRNA due to gene amplification or other unidentified mechanisms has been associated with worse clinical outcomes (15, 16, 24, 26). N-Myc also has been implicated in the development of medulloblastoma as a result of Shh pathway activation (30, 39, 51, 54). However, the c-Myc oncoprotein, or its activated form, v-Myc, is insufficient to cause medulloblastoma when acting alone in NSCs (20, 38, 52, 55, 60).
REST/NRSF is a global transcriptional repressor that contains a DNA binding domain and two repressor domains; it silences the transcription of a large number of neuronal differentiation genes by binding to a 23-bp consensus DNA sequence, the RE1 binding site/neuron restrictive silencer (RE1/NRSE), present in these genes' regulatory regions (3, 4, 8, 12). REST/NRSF is mostly expressed in embryonic stem cells and nonneuronal cells and is rarely expressed in neurons in vivo (3, 4). However, REST/NRSF is expressed in certain mature neurons in adults (23, 33, 56), suggesting that it has a complex role that depends on its cellular and physiological environment. Furthermore, there are several isoforms of REST/NRSF, and one such isoform, REST4, functions as a dominant-negative regulator by interfering with REST/NRSF's activity in neurons (56, 58). Both REST/NRSF and REST4 interact with RILP, a LIM domain protein, for nuclear translocation (56, 57). For REST/NRSF-dependent promoter repression to occur, REST/NRSF must interact with several cellular cofactors, including Co-REST, N-CoR, mSin3A, and the histone deacetylase complex, and modulate chromatin structure (2-5, 12, 23, 29). Furthermore, a small, double-stranded RNA has been found to modulate REST/NRSF activity in the NSCs as well (34). Our previous work showed that several medulloblastoma cell lines and many human medulloblastomas overexpress REST/NRSF compared with neuronal progenitor cells and fully differentiated neurons (19, 37). However, neuronal cells constitutively expressing REST/NRSF do not form into tumors and appear to acquire a normal neuronal morphology, except that they manifest axon pathfinding errors (49). Similarly, our transgenic mice expressing REST/NRSF in neuronal cells appeared to develop normally without tumor formation (unpublished data).
To study what happens when the effects of REST/NRSF are opposed, we constructed a recombinant transcription factor, REST-VP16, in which both the repressor domains of REST/NRSF were replaced with the activation domain of the herpes simplex virus protein VP16 (28, 37, 63, 65). We found that REST-VP16 operates through RE1/NRSE, competes with endogenous REST/NRSF for DNA binding, and activates cellular REST/NRSF target genes. Furthermore, the high-efficiency expression of REST-VP16 mediated by the adenovirus construct Ad.REST-VP16 in human medulloblastoma cells countered the endogenous REST/NRSF-mediated repression of neuronal promoters and promoted apoptosis through caspase 3 activation, presumably resulting from the simultaneous opposing action of REST/NRSF and REST-VP16 (19, 37). Because it leads to apoptosis, REST-VP16 also blocks the tumorigenicity of medulloblastoma cells (19, 37).
The experiments described here indicate that abnormal expression of REST and Myc in NSCs cooperate to form cerebellar tumors by blocking neuronal differentiation. Although the role of naturally occurring, DNA-binding transcriptional activators in oncogenesis is well established, the role of transcriptional repressors is not. Currently, there is only indirect evidence linking repressors such as evi-1 and CtBP to oncogenesis; a strong link of these repressors with their in vivo gene targets is lacking (9, 10). The study here also describes such a direct role of a transcriptional repressor in forming medulloblastoma by blocking neuronal differentiation.
MATERIALS AND METHODS
Histological and immunohistochemical assays.
For the histological studies, 21 surgically excised human brain tumor tissue and adjacent normal tissue samples fixed in 10% buffered formalin and embedded in paraffin were obtained from the Brain Tumor Center tissue bank at The University of Texas M. D. Anderson Cancer Center, stained with hematoxylin and eosin (H&E), and examined under a light microscope, as previously described (19, 65). These experiments were done with institutional review board approval. All specimens had been previously determined to be medulloblastomas on the basis of histological findings. Six normal cerebellar tissue samples adjacent to tumors, which were histopathologically distinct from the tumor tissue, were included in the array as negative controls. For the immunohistochemical assays, the brain sections were stained with an anti-REST antibody (a gift of Gail Mandel), an anti-c-Myc antibody (Santa Cruz), and an anti-N-Myc antibody (Santa Cruz), and this was done essentially as previously described (19, 22, 65). The staining intensity was graded as negative (−), weakly positive (+), or strongly positive (++) and was determined on the basis of the staining intensity of 15 to 20 representative fields from each section.
Real-time RT-PCR.
Real-time reverse transcription-PCR (RT-PCR) was performed as follows. Medulloblastoma samples were obtained from The Johns Hopkins Hospital and were snap frozen at the time of resection. Diagnoses were confirmed by a neuropathologist (C.G.E.). These studies were approved by the local institutional review board. Total RNA was prepared using QIAGEN RNA-Easy tissue kits according to the manufacturer's instructions (QIAGEN Corp. Valencia, CA). An RNase-free DNase (QIAGEN) was used to reduce genomic DNA contamination. Quantitation of mRNA levels was performed using a two-step real-time PCR method on a Bio-Rad Icycler (Bio-Rad). Approximately 1,000 ng of total RNA was reverse transcribed to cDNA using random hexamers and Moloney murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA) and then used to determine mRNA levels of β-actin, c-myc, N-myc, and REST in separate reactions. Each unknown was run at least three times, and expression values were generated with Icycler software from a standard curve created using 0.1 to 50 ng of cDNA from the medulloblastoma cell line D283 (American Type Culture Collection). All values were then normalized to β-actin content in the cDNA sample and are shown as means with standard error. β-Actin and c-myc levels were determined using commercially available Taqman primers and probes (Applied Biosystems). The sequences of the forward and reverse REST primers used for SYBR green quantitative PCR are 5′-GAGGAGGAGGGCTGTTTACC-3′ and 5′-TCACAGCAGCTGCCATTTAC-3′, respectively. For N-myc, the forward and reverse primers were 5′-TGAAGAGGAAGATGAAGAGGAAGA-3′ and 5′-GTGACAGCCTTGGTGTTGGA-3′, respectively. Single melt peaks were obtained from both REST and N-myc SYBR green primer pairs, and sequence analysis confirmed the identity of the products. Statistical analysis of the correlation (Spearman test) and categorical association (two-sided Fisher's exact test) between c-Myc and REST was performed using GraphPAD PRISM4 (GraphPAD Software, San Diego, CA).
Plasmids and stable transfection.
The NheI/XhoI fragment of pcDNA3.1-REST (28) was subcloned into the NheI/XhoI-digested plasmid pBig2r (62). The clone obtained was confirmed by sequencing the junction region. The mouse multipotent C17.2 (NSC-M) cells were derived from neonatal mouse cerebellum transduced with v-Myc. Because v-Myc represents the activated form of the c-Myc oncogene (38), these cells provide an excellent system for our experiments (60). Construction and characterization of stable clonal cell lines NSC-M-V and NSC-M-R were performed as described previously (63).
Cell culture conditions.
NSC-M cells were cultured at 5% CO2, at 37°C, in either proliferation medium (Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 5% horse serum, and 2 mM glutamine, and antibiotics/antimycotics [all from Gibco]) or differentiation medium (Neurobasal medium plus B27, antibiotics/antimycotics, and 2 mM glutamine [all from Gibco], plus nerve growth factor [Chemicon], at a final concentration of 200 ng/ml). For NSC-M-V or NSC-M-R stable clonal cells, the media were supplemented with 200 μg/ml hygromycin (Roche) to maintain the presence of the transgene and, when needed, 20 μg/ml doxycycline (Sigma) to repress the human REST transgene expression. NSC-M, NSC-M-V, and NSC-M-R cells were propagated in proliferation medium on uncoated tissue culture dishes. For the assay of cell proliferation or differentiation, the cells were seeded on polylysine (Sigma)- and laminin (Invitrogen)-coated dishes in the presence of proliferation medium. Once the cells attached to the dish (approximately 2 h), they were processed either immediately (proliferation condition, day 0) or at different time points after the medium was replaced by differentiation medium (differentiation conditions, day 2, 4, or 6).
RT-PCR assay.
The RNeasy kit from QIAGEN was used to prepare total RNA from the cells. RNAs were quantitated spectrophotometrically, and 200 ng of total RNA was used for each sample to detect REST/NRSF in the RT-PCR analysis. Primers used in these reactions had the following sequences: mActin F 57-76, 5′-GTCCACACCCGCCACCAGTT-3′; mActin R 816-841, 5′-CGCTCGTTGCCAATAGTGATGACCTG-3′; hREST F 3311-3333, 5′-TATCTTGAAGAAGCAGCTCAAGG-3′; hREST (bGH PolyA), 5′-CAACTAGAAGGCACAGTCGAGG-3′; mREST 2436-2455F, 5′-GAGACAGCAAGCTTCTGAAG-3′; and mREST 2817-2792R, 5′-CAGAGTATCTGTCTTCTGCTCAGTG-3′.
An RT-PCR kit (QIAGEN) was used per the manufacturer's instructions to perform the initial reverse transcription. All three primer sets for actin, hREST, and mREST were used in the same tube at the time of reaction. The cDNA was amplified for 20 or 25 cycles under the following conditions: melting at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min. The PCR products were separated by electrophoresis on a 2.0% agarose gel at 80 V for 1.5 h.
Reporter gene assays.
The REST-dependent promoter repression was determined in NSC-M, NSC-M-V, and NSC-M-R cells growing either in proliferation medium (day 0) or in differentiation medium for 4 days (day 4) by transfection with reporter plasmids pNaCh (+RE) and pNaCh (−RE). Transfected cells were further incubated for 24 h in the same medium, and the assay was performed as described previously (28, 37). The plasmid pSTluc was used as an internal control (37, 53). The average chloramphenicol acetyltransferase (CAT) activities from three experiments, which were normalized to luciferase activity, were calculated as the percentage of REST activity: 100 − [100 × pNaCh (+RE)/pNaCh (−RE)], where the REST activity for NSC-M at day 0 was taken as 100%.
Immunofluorescence assay.
Immunofluorescence assay experiments were performed as described previously (63, 65). We used the following antibodies: anti-VP16 (1:100) (Clontech), anti-unique β-tubulin (1:500) (Tuj1; Covance Research Products), anti-MAP2 (1:1,000, HM-2; Sigma), anti-synaptophysin I (1:200, mab368; Chemicon), anti-glial fibrillary acidic protein (anti-GFAP; 1:500; DAKO), horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G (IgG; H+L, 1:20,000; Amersham), and Cy3-labeled anti-mouse or anti-rabbit IgG (H+L, 1:1,000; Amersham). A Leica epifluorescence microscope was used to examine the staining of cells. Further analysis was carried out using Metamorph software.
Cell proliferation assay.
The cells growing either in proliferation medium (day 0) or in differentiation medium for various time points (day 2 or 4) were incubated with 10 μM bromodeoxyuridine (BrdU; Sigma) and were then washed twice with 1× PBS and fixed with 10% buffered formalin for 20 min at room temperature. The immunofluorescence of BrdU-labeled cells was elicited by first blocking the cells with blocking buffer (1× PBS containing 10% normal goat serum, 10% powdered dry milk, and 0.2% Triton X-100) for 1 h at room temperature. The blocking buffer was removed, and the cells were incubated with 1× PBS containing mouse anti-BrdU antibody (containing nucleases) (Roche) at a dilution of 1:15 for 1 h at 37°C. The cells were washed three times for 5 min each with 1× PBS with gentle agitation. This was followed by incubation with Cy3-conjugated goat anti-mouse antibodies (Amersham) at a dilution of 1:1,000 for 1 h at 37°C. The cells were washed three times with 1× PBS with gentle agitation and allowed to air dry. The stained cells were covered with slow-fade antifade (Molecular Probes) containing 1 μg/ml 4′,6′-diamidino-2-phenylindole (DAPI; Molecular Probes). The cells were analyzed by immunofluorescence assay as described above. The average values from five different fields were used for each data point. Each experiment was repeated four times.
Western blotting assay.
Cells were seeded at a density of 5 × 105 cells per 15-cm petri dish coated with 0.1 mg/ml polylysine and 0.1 mg/ml of laminin and grown in 5 ml Neurobasal medium (GIBCO, Bethesda, MD) supplemented with B27 and nerve growth factor (Chemicon) at a final concentration of 200 ng/ml and incubated at 37°C and 5% CO2 tension. In some experiments, replication-incompetent adenovirus expressing either green fluorescent protein (GFP) or REST-VP16 (Ad.GFP and Ad.REST-VP16, respectively) was used to infect these cells at a multiplicity of infection of 100 for 4 h. Cells were either immediately harvested (day 0), or the volume of medium was then raised to 15 ml and cells were incubated in the differentiation medium further for 3 days postinfection. Infection efficiency was determined by counting the number of GFP-positive cells and was found to be >50%. Cells, adenovirus infected or uninfected, were harvested and lysed by addition of Laemmli buffer, and cell extracts were subjected to Western blot analysis using mouse anti-PARP antibody (BD Pharmingen) at a dilution of 1:500. The antibody recognizes an 85-kDa product from mouse cells. In some experiments, anti-neuronal β-tubulin (Tuj1, 1:500 dilution; Covance) or anti-α-tubulin (1:500 dilution; Covance) was also used.
Intracranial inoculation of cells into mice and assay for tumor formation.
The intracranial inoculation of cells into nude mice was performed following “M. D. Anderson Institutional Animal Care and Use Guidelines.” Cells (2 × 105 cells in 5 μl of cell growth medium) were inoculated into groups of mice by using an implantable guide-screw system we have described elsewhere (19, 35, 65). The mice were sacrificed by CO2 inhalation 6 weeks later, and their brains were fixed with formalin and embedded in paraffin; 4- to 5-μm brain sections were examined in the same way as the human medulloblastoma brain specimens.
RESULTS
Many primary human medulloblastomas express both REST/NRSF and c-Myc proteins.
As described above, many human medulloblastoma tumor specimens and cell lines have been found to express c-Myc or REST/NRSF. However, because neither protein by itself can cause tumor formation, we wondered whether human medulloblastoma tumor samples expressed both Myc and REST/NRSF proteins. We used immunohistochemical analysis to examine 21 surgically excised, formalin-fixed, paraffin-embedded tumor tissue specimens organized in a tissue array with anti-REST, anti-c-Myc, and anti-N-Myc antibodies. The results are shown in Fig. 1 and summarized in Table 1. As expected, normal cerebellar cortex tissue was composed of granular cell neurons with uniform, round nuclei and interspersed pale neuropil islands (glomeruli), whereas medulloblastoma tissue was composed of densely packed cytologically atypical tumor cells with enlarged, irregular, hyperchromatic nuclei (Fig. 1). All six normal control cerebellar tissues stained negatively for anti-REST/NRSF, anti-c-Myc, and anti-N-Myc antibodies. However, 17 of 21 medulloblastoma tumor samples stained positively for REST/NRSF, with strong signals (++) in 6 and weak signals (+) in 11. The remaining four samples showed no REST/NRSF signal. In contrast, 14 of the 21 tumor samples stained positively for c-Myc, with strong signals (++) in 9 and weak signals (+) in 5. The remaining five samples showed no c-Myc positivity. Similarly, 16 of the 21 tumor samples stained positively for N-Myc, with strong signals (++) in 6 and weak signals (+) in 10. The remaining seven samples showed no c-Myc positivity. High-magnification (×1,000) photomicrographs showed the nuclear staining for REST, c-Myc, and N-Myc (Fig. 1). Thus, whereas 52% samples showed coexpression of both N-Myc and REST/NRSF proteins at various levels (11 of 21), strong signals for both c-Myc and REST/NRSF were observed in only 14% (3 of 21) of samples. Likewise, 48% of samples (10 of 21) showed coexpression of REST/NRSF and N-Myc, with strong signals in 14% (3 of 21) of samples. Finally, 48% of the samples expressed REST/NRSF, c-Myc, and N-Myc proteins. It is worth noting that in two cases on Table 1, REST and N-myc are coexpressed in the absence of detectable c-Myc protein. This suggested the possibility that either c- or N-Myc is capable of cooperating with REST and was supported by our data shown below (Fig. 2). Although double-labeling studies were not performed, the fact that the majority of cells in a given tumor were immunopositive for both REST and Myc suggests that the proteins were being coexpressed in individual nuclei.
FIG. 1.
Primary human medulloblastoma specimens coexpress REST/NRSF and c-Myc proteins. Paraffin-embedded medulloblastoma tissue and normal brain tissue adjacent to the tumor were processed; stained with H&E, anti-REST/NRSF antibody (α-REST), anti-c-Myc antibody (α-c-Myc), or anti-N-Myc antibody (α-N-Myc); analyzed as described in Materials and Methods; and photographed under a light microscope. Magnifications of ×400 and ×1,000 are shown.
TABLE 1.
Status of immunoreactivity in medulloblastoma patient samples
| Age (yr) | Result fora:
|
||
|---|---|---|---|
| REST/NRSF | c-Myc | N-Myc | |
| 22 | ++ | − | + |
| 2 | − | + | + |
| 9 | + | ++ | ++ |
| 25 | − | + | + |
| 34 | + | + | + |
| 8 | − | ++ | + |
| 4 | + | ++ | + |
| 20 | + | + | ++ |
| 15 | ++ | − | − |
| 2 | + | − | − |
| 12 | − | − | + |
| 26 | + | ++ | ++ |
| 11 | + | − | − |
| 2 | + | − | + |
| 2 | ++ | ++ | ++ |
| 26 | ++ | ++ | ++ |
| 20 | ++ | − | − |
| 3 | + | ++ | + |
| 10 | + | + | − |
| 11 | ++ | ++ | ++ |
| 7 | + | ++ | + |
−, negative; +, moderately positive; ++, strongly positive.
FIG. 2.
Coexpression of REST/NRSF and c-Myc mRNA. Quantitative RT-PCR was used to analyze mRNA levels of REST/NRSF and c-Myc in 40 snap-frozen primary human medulloblastomas. Each tumor is represented by a bar, and they are ordered on the x axis from highest to lowest level of c-Myc mRNA expression after normalization to actin. This highlights the elevated REST/NRSF levels in many of the cases with high c-Myc. Interestingly, several of the tumors with elevated REST/NRSF but low c-Myc levels (arrows) had elevated (above median) N-Myc mRNA levels. The inset shows a scatter plot of N-Myc levels for all 40 cases, with the 4 cases marked with arrows circled.
To obtain more quantitative data, we used real-time RT-PCR to investigate the association between REST and Myc transcript levels. Expression was analyzed in mRNA extracted from 40 snap-frozen primary medulloblastomas for which we had previously measured c-Myc and N-Myc expression levels (D. Stearns and C. G. Eberhart, unpublished data). REST was detected in all samples, and many of the tumors with high REST levels were also among those with elevated c-Myc expression (Fig. 2). REST and c-Myc mRNA levels showed a statistically significant positive correlation overall (Spearman r = 0.36; P = 0.02). However, several cases with high REST levels contained relatively little c-Myc. We were curious whether N-myc might substitute for c-Myc in these cases, as N-myc gene amplification has also been associated with aggressive medulloblastoma biology (16). Interestingly, four cases with high REST and low c-Myc had above-median levels of N-Myc (Fig. 2, inset). Thus, high-level REST expression appears to be commonly associated with elevated expression of one of these two myc oncogenes. As a final measure of the association between c-Myc and REST, we used Fisher's exact test to determine whether tumors with very high (top third) REST expression also had very high (top third) c-Myc expression. The combined high-REST/high-c-Myc category (33%) was significantly more common than would be predicted by chance association (P = 0.04), supporting the concept that these genes might act together in medulloblastoma pathogenesis.
Construction of NSC clones stably expressing Myc and doxycycline-regulated human REST/NRSF transgene.
We used cerebellum-derived, neonatal mouse brain NSCs stably expressing an activated form of c-Myc (NSC-M cells) to further study the functional roles of Myc and REST in medulloblastoma tumorigenesis (60, 61). We generated stable clonal cells expressing either the vector (NSC-M-V cells) or human REST transgene (NSC-M-R cells) by using the bidirectional doxycycline-regulated pBig2r vector system by a method we described previously (63, 65). Selected clones were assayed using PCR to verify the appropriate clonal cells (data not shown). The pBig2r construct efficiently expressed the encoded transgene in the absence of doxycycline (−Dox), and the cells of the clonal cell line NSC-M-R stably expressed high levels of the human REST transgene mRNA when grown without doxycycline (−Dox) (data not shown). As expected for normal NSCs (2, 3), NSC-M cells and NSC-M-V cells expressed the endogenous mouse REST (mREST) transcripts only under proliferation conditions (d0), and the expression ceased upon neuronal differentiation (d2, d4, and d6) as assayed by RT-PCR (Fig. 3A). NSC-M-R cells followed the same expression pattern for mREST but, in contrast, expressed the human REST transgene (hREST) under both proliferation and differentiation conditions (Fig. 3A).
FIG. 3.
Endogenous REST/NRSF is expressed in proliferating NSCs and the expression is repressed as the NSCs enter neuronal differentiation. (A) Endogenous mouse REST/NRSF (mREST) and transgenic human REST/NRSF (hREST) transcription was assessed by RT-PCR in NSC-M-R, NSC-M-V, and NSC-M cells growing in proliferation medium (day 0) or in differentiation medium in vitro for 2, 4, and 6 days. Actin transcripts were measured as an internal control. (B) The percentage of REST transcriptional repression activity was determined in NSC-M, NSC-M-V, and NSC-M-R cells growing in proliferation medium (day 0) or differentiation medium for 4 days (day 4). The cells were cultured either with (+D) or without (−D) doxycycline, transfected with the reporter plasmid pNaCh (+RE) or pNaCh (−RE), and analyzed as described in the text. The data represent percent REST activity.
To confirm that the expression pattern of REST/NRSF transcript levels during neuronal differentiation shown in Fig. 3A was also reflected in its transcriptional repression activity, we used a reporter gene system that is well established to detect REST/NRSF-specific activity in the cell (11, 28, 37, 65). The system involves comparison of reporter gene activities from two plasmids. The first, pNaCh (+RE), contains the bacterial CAT reporter gene under the control of sodium channel type II promoter/enhancer (NaCh) elements. NaCh elements are mammalian native neuronal promoter/enhancer sequences that contain the REST/NRSF binding site (+RE) and are acted on by functional REST/NRSF. The second reporter plasmid, pNaCh (−RE), contains the CAT reporter gene under the control of the “minimal” NaCh elements without the RE1 sequence (−RE). Functional REST/NRSF does not repress the promoter activity of this plasmid. Thus, when functional REST/NRSF is present in the cell, the CAT activity generated from pNaCh (+RE) is lower than that from pNaCh (−RE) because REST/NRSF causes promoter repression from pNaCh (+RE), and not pNaCh (−RE).
The NSC-M and NSC-M-V cells, grown under either cycling conditions (day 0) or differentiation conditions (day 4), were transiently cotransfected with the reporter plasmid pNaCh (+RE) or pNaCh (−RE) and an internal control plasmid, pSTluc, which contains the luciferase reporter gene under the control of an Sp1-containing basic promoter (36, 43). The average CAT activities from three experiments, which were normalized to luciferase activity, were rendered as the percentage of REST activity, as described in Materials and Methods. As shown in Fig. 3B, whereas undifferentiated cycling NSC-M and NSC-M-V cells grown with or without doxycycline showed high levels of REST/NRSF activity (day 0), the differentiation conditions (day 4) resulted in severely reduced REST/NRSF activity, indicating that REST/NRSF activity is lost during the neuronal differentiation of NSCs.
When NSC-M-R cells were cultured with doxycycline (Fig. 3B), they showed a pattern of REST/NRSF activity similar to that of NSC-M and NSC-M-V cells; that is, activity was high in cycling cells (day 0) and diminished in differentiating cells (day 4). In contrast, when NSC-M-R cells were cultured without doxycycline, they showed higher REST/NRSF activity than did NSC-M cells and NSC-M-Vcells under cycling conditions (day 0) and continued showing high REST activity under differentiation conditions (day 4), suggesting that the human REST transgene was responsible for the continued REST activity in these cells (Fig. 3B). Thus, whereas all cells expressed REST/NRSF activity under proliferation conditions, only the NSC-M-R cells (−Dox) expressed the REST/NRSF activity under differentiation conditions.
Forced expression of REST/NRSF in NSCs blocks neuronal differentiation.
To determine the effect of continued REST/NRSF expression in NSC-M-R cells during neuronal differentiation (day 4), we examined whether the terminal neuronal differentiation markers neuronal β-tubulin and MAP2 were expressed in clonal cells growing under differentiation conditions with and without doxycycline. As shown in Fig. 4, the NSC-M-V cells, like the NSC-M cells, expressed both markers irrespective of whether they were cultured with or without doxycycline. NSC-M-R cells cultured with doxycycline also expressed these markers. In contrast, NSC-M-R cells cultured without doxycycline did not express either marker (Fig. 4). The pattern of expression of other neuronal differentiation genes, synapsin and secretogranin II, was similar (data not shown). Another REST/NRSF clone, NSC-M-R′, behaved very similarly to the NSC-M-R cells in all our experiments (data not shown), indicating that the effect of REST/NRSF was independent of its genomic integration site. Taken together, these results indicate that proliferating NSCs normally express endogenous REST/NRSF, but its activity is rapidly blocked as the cells enter neuronal differentiation. These cells then become mature neurons. In contrast, NSC-M-R cells continue to express REST/NRSF as they enter neuronal differentiation and, as a result, cannot become terminally differentiated neurons.
FIG. 4.
Forced expression of REST/NRSF in NSCs blocks neuronal differentiation. NSC-M, NSC-M-V, and NSC-M-R cells were cultured with (+Dox) or without (−Dox) doxycycline under neuronal differentiation conditions for 4 days, and their level of neuronal differentiation was determined in an immunofluorescence analysis in which anti-neuronal β-tubulin (Tuj1) and anti-MAP2 antibodies were used.
REST/NRSF-mediated blockade of neuronal differentiation confers a proliferation advantage in vitro.
Because forced expression of REST/NRSF in NSCs blocked neuronal differentiation, to determine whether expression of REST/NRSF in NSC-M-R cells also confers a proliferative advantage, we measured BrdU incorporation into NSC-M cells and NSC-M-R cells under in vitro conditions during cycling (day 0) and differentiation (days 2 and 4). By culturing the cells either with or without doxycycline in the medium, we also performed these experiments in the presence and absence of exogenously expressed REST/NRSF. Figure 5A shows the results of the immunocytochemical assay for BrdU uptake, and Fig. 5B shows the percentages of BrdU-positive cells. As expected, the rate of BrdU incorporation into NSC-M cells, cultured either with or without doxycycline, decreased drastically as they differentiated. The rate at which BrdU was incorporated into NSC-M-R cells assumed the same pattern when the expression of the exogenous REST/NRSF was prevented by culturing the cells with doxycycline. In contrast, the rate at which BrdU was incorporated into NSC-M-R cells remained high when the expression of the exogenous REST/NRSF was kept steady by culturing the cells without doxycycline. Thus, although c-Myc is known to cause neuronal progenitor cell proliferation, these experiments indicated that, by blocking differentiation, the additional REST/NRSF expression conferred an enormous proliferative advantage on NSC-M-R cells compared with NSC-M and NSC-M-V cells, but only when they traveled down the neuronal differentiation pathway.
FIG. 5.
REST/NRSF-mediated neuronal differentiation block confers a proliferation advantage in vitro. The amount of BrdU incorporation was determined by immunocytochemical analysis (A) and was represented as the percentage of cells (NSC-M or NSC-M-R) showing BrdU labeling (B) growing in proliferation medium (d0) or differentiation medium for day 2 (d2) or day 4 (d4) with (+Dox) or without (−Dox), as described in the text.
REST/NRSF-mediated blockade of neuronal differentiation in vivo causes tumors in the cerebellum, but not in the cerebral cortex.
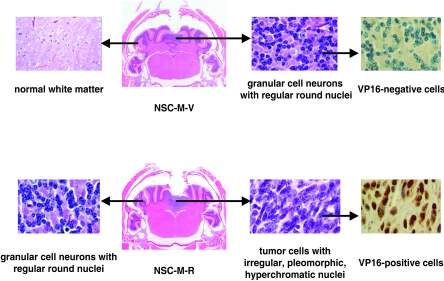
Because the cerebellum is the major site of medulloblastoma tumorigenesis (31, 52), we wanted to determine the properties of NSC-M-R cells in mouse cerebellum. We injected NSC-M, NSC-M-V, or NSC-M-R cells into the cerebellums of nude mice (10 mice per group). We sacrificed the mice 6 weeks later and performed histological analysis of their paraffin-embedded brains. As shown in Fig. 6 and 7, the NSC-M and NSC-M-V cells injected into the cerebellum integrated into the cerebellum within about 4 weeks in a nontumorigenic, cytoarchitecturally appropriate manner (10 out of 10 mice for both NSC-M and NSC-M-V cells). Histological analysis of the brains of these mice showed that they had a normal cerebellar architecture with an appropriate vermian histological morphology, including normal molecular, Purkinje cell, and granule cell layers, with granule cell neurons showing the characteristic monotonous, regular, round nuclei. In contrast, large cerebellar tumors with irregular, pleomorphic, and hyperchromatic nuclei developed in mice inoculated with NSC-M-R cells (8 out of 10 mice), and these tumors were morphologically similar to human medulloblastomas (see Fig. 1). In most cases, the vermian cerebellar cortex, including the granule cell layer, was compressed as a result of tumor expansion. Thus, NSCs that overexpressed activated c-Myc alone (NSC-M and NSC-M-V cells) did not produce cerebellar tumors in mice but did produce tumors when they also overexpressed REST/NRSF, indicating that the overexpression of both activated c-Myc and REST/NRSF in NSCs produces cerebellar tumors.
FIG. 6.
REST/NRSF-mediated neuronal differentiation block causes tumors in the cerebellums but not cerebral cortex of mice. NSC-M, NSC-M-R, and NSC-M-R′ (a second clone of NSC-M-R) cells were injected into the cerebellums and cerebral cortex of mice. The mice were sacrificed 6 weeks later, and their paraffin-embedded brain sections were histologically analyzed. Only the NSC-M-R and NSC-M-R′ cells produced cerebellar tumors (arrows).
FIG. 7.
Morphological details of cerebellar tumors. Detailed histological analysis of paraffin-embedded brain sections of mice injected with VP16-tagged NSC-M-R cells show tumor cells with irregular, pleomorphic, and hyperchromatic nuclei. Immunohistochemistry studies show these cells are also positive for VP16. The paraffin-embedded brain sections of mice injected with control VP16-tagged NSC-M-V cells show normal granule cell neurons with regular, round nuclei. VP16-positive cells cannot be detected in these sections.
To determine whether tumor formation is location specific, we injected NSC-M, NSC-M-V, or NSC-M-R cells into the cerebral cortex of nude mice as described above. As shown in Fig. 6, none of the cells formed tumors, suggesting that the cerebellar environment facilitates c-Myc-REST/NRSF-mediated tumor formation.
REST/NRSF-mediated cerebellar tumors are blocked from terminal neuronal differentiation.
To confirm that the cells that made up the cerebellar tumors produced by NSC-M-R cells were also blocked from terminal neuronal differentiation, we examined the immunohistochemical expression of the differentiation marker MAP2 in paraffin-embedded sections. As shown in Fig. 8, NSC-M-V specimens produced MAP2 at levels indistinguishable from those of background brain structures. In contrast, NSC-M-R tumors did not produce any MAP2. These results indicate that the abnormal expression of REST/NRSF in these tumors prevented the cells from undergoing terminal neuronal differentiation.
FIG. 8.
REST/NRSF-mediated cerebellar tumors are blocked in neuronal differentiation. The paraffin-embedded brain sections of mice injected with NSC-M-R and control NSC-M-V cells were analyzed in immunohistochemistry studies to determine the expression of the neuronal differentiation markers MAP2 and synaptophysin (SYP) and glial differentiation marker GFAP. NSC-M-R cells were negative for synaptophysin, MAP2, and GFAP.
The gene encoding synaptophysin, another marker of terminal neuronal differentiation, is also a direct target of REST/NRSF (63, 65). To further confirm that the tumor-producing cells are blocked from terminal neuronal differentiation and do not express REST/NRSF target genes, we examined paraffin-embedded sections for immunohistochemical expression of synaptophysin (SYP). NSC-M-V cells produced synaptophysin at levels indistinguishable from those of background brain structures, similar to MAP2 staining (data not shown). In contrast, whereas the surrounding granular cell neurons with regular round nuclei expressed synaptophysin, the tumor cells with irregular, pleomorphic nuclei did not, indicating that these cells do not express REST/NRSF target genes and did not undergo terminal neuronal differentiation.
To confirm that the tumor-producing cells did not differentiate in the glial pathway, we examined the expression of GFAP in paraffin-embedded sections with anti-GFAP antibodies. NSC-M-V cells did not show any difference in GFAP staining from those of background brain structures (data not shown). As shown in Fig. 8, strong cytoplasmic reactivity for GFAP was seen in astrocytes but the tumor cells did not express GFAP, suggesting that NSC-M-R cells did not undergo glial differentiation.
Countering REST/NRSF function blocks tumorigenicity.
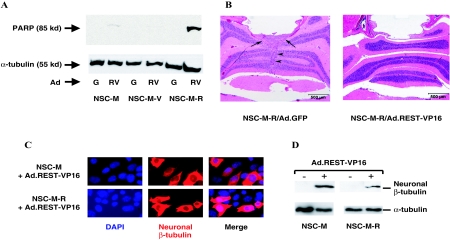
Previously we found that REST-VP16 counters the effects of endogenous REST/NRSF in human medulloblastoma cells overexpressing REST/NRSF, induces apoptosis, and counteracts the cells' tumorigenicity (19, 37). To determine whether the NSC-M-R cells respond to REST-VP16 in the same fashion, we infected NSC-M-R cells grown in the absence of doxycycline in vitro with Ad.GFP (control) and Ad.REST-VP16 at a multiplicity of infection of 100. We then looked for the active apoptosis of these cells on the basis of the detection of the anti-PARP antibody, which recognizes only the cleaved form of PARP. As shown in Fig. 9A, REST-VP16 specifically caused high levels of apoptosis in NSC-M-R cells but not in NSC-M or NSC-M-V cells. Thus, these experiments showed that NSC-M-R cells followed the same fate in vitro in response to REST-VP16 as human medulloblastoma cells do.
FIG. 9.
Countering REST/NRSF function blocks cerebellar tumorigenicity. (A) Cell extracts prepared from Ad.GFP (G)- or Ad.REST-VP16 (RV)-infected NSC-M, NSC-M-V, and NSC-M-R cells were subjected to Western blot analysis in which antibodies were used that detect cleaved PARP (85 kDa) and α-tubulin (55 kDa). (B) NSC-M-R cells infected with Ad.GFP or Ad.REST-VP16 were inoculated into the mouse cerebellum. The mice were sacrificed 6 weeks later, and their paraffin-embedded brain sections were histologically analyzed. The tumor produced by NSC-M-R cells infected with Ad.GFP is indicated by arrows. (C) NSC-M and NSC-M-R cells infected with Ad.REST-VP16 were examined by immunofluorescence using anti-neuronal β-tubulin (red). The cell nuclei were also labeled with DAPI (blue). (D) NSC-M and NSC-M-R cells, uninfected (−) or infected (+) with Ad.REST-VP16, were examined by Western blotting analysis using anti-neuronal β-tubulin. Anti-α-tubulin was used as an internal control.
We then inoculated REST-VP16-infected NSC-M-R cells into the cerebellums of mice, sacrificed the mice 6 weeks later, and examined paraffin-embedded sections of their brains. As shown in Fig. 9B, Ad.REST-VP16, but not Ad.GFP, blocked the tumorigenicity of NSC-M-R cells, indicating that, similar to human REST/NRSF-containing human medulloblastoma cells, countering REST/NRSF in NSC-M-R cells can eliminate their cerebellar tumorigenic potential.
The mechanism of REST-VP16-mediated induction of apoptosis in human medulloblastoma cells (19, 37) and NSC-M-R cells is unknown. One possible mechanism is the persistent presence of the opposing REST/NRSF and REST-VP16 activities in these cells, leading to the generation of conflicting signals of stem cell properties and differentiation properties, respectively. To confirm that REST-VP16-mediated apoptosis in NSC-M-R cells is caused by REST-VP16 activity and not by abnormal production of toxic levels of the protein, we examined the expression of the REST-VP16 target gene neuronal β-tubulin in these cells by performing an immunofluorescence assay. Although neither NSC-M nor NSC-M-R cells produced neuronal β-tubulin when infected with Ad.GFP (data not shown), they did produce it when infected with Ad.REST-VP16 (Fig. 9C). To further confirm this observation, we investigated the expression of neuronal β-tubulin in NSC-M and NSC-M-R cells, uninfected or infected with Ad.REST-VP16, by performing the Western blotting assay. As shown in Fig. 9D, only the Ad.REST-VP16-infected cells showed the expression of neuronal β-tubulin. These results indicate that REST-VP16 expressed in NSC-M and NSC-M-R cells as a result of adenoviral infection produced functionally active protein.
DISCUSSION
Our results indicate that many human medulloblastomas coexpress abnormally high levels of Myc and REST/NRSF. We also observed that NSCs overexpressing activated Myc and REST/NRSF proteins are blocked in neuronal differentiation and give rise to cerebellar tumors morphologically similar to human medulloblastoma. These cells did not produce tumors in the cortex, indicating a role of the local brain environment in formation of the tumors. Although the environmental difference between the cerebellum and the cortex very likely is because of the difference in trophic factors, the exact nature of such factors is not yet known. We further found that countering the effects of REST/NRSF in NSC-M-R cells caused apoptosis and counteracted the tumorigenic potential of the cells. On the basis of these findings, we propose a model in which efficient medulloblastoma tumorigenesis occurs when two conditions occur in NSCs: overall increased proliferation as a result of Myc expression and lack of differentiation (maintenance of “stemness”) as a result of REST/NRSF expression (Fig. 10).
FIG. 10.
A model for medulloblastoma tumorigensis triggered by the transcriptional repressor REST/NRSF and Myc. Please see the text for details.
Normally, when NSCs are signaled to travel down the neuronal differentiation pathway, they rapidly stop transcribing the REST/NRSF gene. Because the REST/NRSF protein is no longer present to block its target terminal differentiation genes, the cells can successfully differentiate into neurons. In medulloblastoma, the Myc and REST/NRSF proteins are abnormally overexpressed. When NSCs are induced to proliferate rapidly by Myc, then travel down the neuronal differentiation pathway, the deregulated REST/NRSF expression cannot be extinguished, leading to an ongoing block of neuronal differentiation. These still-dividing cells abnormally accumulate, thereby initiating medulloblastoma formation.
Our hypothesis is not at odds with the possibility that tumor maintenance and progression then result from the deregulation of other genes, caused by the abnormal physiology of the tumor cells. This hypothesis of tumor formation resulting from the capture of dividing cells at a stage before full differentiation may be a new paradigm explaining the initiation of tumors believed to originate from and resemble undifferentiated or partially differentiated cells, such as medulloblastomas, neuroblastomas, and rhabdomyosarcomas (48). Indeed, such tumors usually occur in children and adolescents, in whom differentiation pathways are probably more active than they are in adults.
Our model of efficient medulloblastoma formation from NSCs as the result of the cooperation of both proliferation advantage and differentiation-block is not at odds with the less-efficient medulloblastoma formation that occurs in the presence of only one of these steps. For example, activation of the Shh pathway as the result of a PTCH gene mutation, which increases NSC proliferation, is known to occur in approximately 10% of medulloblastoma patients (50, 66). The severity of this disease can be enhanced by addition of c-Myc, another factor promoting NSC proliferation (52). Thus, it is conceivable that medulloblastomas develop as a result of a factor or factors favoring either the proliferation step or the differentiation-block step but that additional factors reinforcing the same step and/or the other step augment tumorigenesis.
Previously, we found that many human medulloblastoma cell lines and human medulloblastoma patient samples abnormally overexpress REST/NRSF as compared with neuronal cells or normal brain cells (19, 37). As expected, the REST/NRSF-positive tumor cells do not express the REST/NRSF target genes, such as synapsin, which, in turn, can be expressed by REST-VP16 (19, 37). That the overexpression of REST/NRSF plays such an oncogenic role in neuronal cells is further supported by studies from other laboratories indicating overexpression of REST/NRSF in several neuroblastoma cells with concomitant repression of neuronal differentiation genes (27, 46). Furthermore, when the neuroblastoma cells were forced to differentiate, they showed a decrease in REST/NRSF expression and an increase in neuronal markers. These studies suggested that the abnormal overexpression of REST/NRSF in neuronal cells, in which REST/NRSF is normally not present, blocks these cells from terminal neuronal differentiation and produces the cancerous phenotype, perhaps by forcing the cells to persist in a stem/progenitor state.
In contrast, several studies also showed that whereas normal bronchial epithelium expressed REST/NRSF activity, several established small cell lung cancer cell lines as well as primary samples showed reduced REST/NRSF activity with concomitant abnormal expression of REST/NRSF target genes, such as the glycine receptor α1 subunit or vasopressin (12, 25, 45). Such a tumor-suppressor function of REST/NRSF was also seen in colorectal cancers (47, 67). These studies suggested that the abnormal lack of REST/NRSF activity in lung and colon epithelial cells, which normally express REST/NRSF and repress expression of neuronal genes, leads to oncogenic conversion of these cells, perhaps by aberrant expression of REST/NRSF target genes resulting in the abnormal neuroendocrine phenotype sometimes observed in these tumors. Thus, REST may have both tumorigenic and tumor-suppressor effects, depending on the cell type, which would indicate that REST is a major biological regulator of normal and abnormal development.
Acknowledgments
We are extremely grateful to Gail Mandel for the pREST-Express, p73, pBS.REST, and pSDK7 and to Craig Strathdee for pBig2r. We are also grateful to the anonymous reviewers, whose comments made the paper better. We thank Mary Majumder and Beth Notzon for their comments on the manuscript and Belinda Rivera, Helen Yang, and Lynda Corley for their help with the mouse experiments and immunohistochemical assays.
This work was supported by grants from the National Cancer Institute (CA 81255), the Goodwin Family Funds and Katie's Kids for the Cure (S.M.), as well as K08NS43279 (C.G.E.). DNA sequencing and veterinary resources were supported by NIH Cancer Center Support (Core) grant CA16672.
REFERENCES
- 1.Aldosari, N., S. H. Bigner, P. C. Burger, L. Becker, J. L. Kepner, H. S. Friedman, and R. E. McLendon. 2002. MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children's Oncology Group. Arch. Pathol. Lab. Med. 126:540-544. [DOI] [PubMed] [Google Scholar]
- 2.Ballas, N., E. Battaglioli, F. Atouf, M. E. Andres, J. Chenoweth, M. E. Anderson, C. Burger, M. Moniwa, J. R. Davie, W. J. Bowers, H. J. Federoff, D. W. Rose, M. G. Rosenfeld, P. Brehm, and G. Mandel. 2001. Regulation of neuronal traits by a novel transcriptional complex. Neuron 31:353-365. [DOI] [PubMed] [Google Scholar]
- 3.Ballas, N., C. Grunseich, D. D. Lu, J. C. Speh, and G. Mandel. 2005. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121:645-657. [DOI] [PubMed] [Google Scholar]
- 4.Ballas, N., and G. Mandel. 2005. The many faces of REST oversee epigenetic programming of neuronal genes. Curr. Opin. Neurobiol. 15:500-506. [DOI] [PubMed] [Google Scholar]
- 5.Battaglioli, E., M. E. Andres, D. W. Rose, J. G. Chenoweth, M. G. Rosenfeld, M. E. Anderson, and G. Mandel. 2002. REST repression of neuronal genes requires components of the hSWI.SNF complex. J. Biol. Chem. 277:41038-41045. [DOI] [PubMed] [Google Scholar]
- 6.Berman, D. M., S. S. Karhadkar, A. R. Hallahan, J. I. Pritchard, C. G. Eberhart, D. N. Watkins, J. K. Chen, M. K. Cooper, J. Taipale, J. M. Olson, and P. A. Beachy. 2002. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 297:1559-1561. [DOI] [PubMed] [Google Scholar]
- 7.Bruggers, C. S., K. F. Tai, T. Murdock, L. Sivak, K. Le, S. L. Perkins, C. M. Coffin, and W. L. Carroll. 1998. Expression of the c-Myc protein in childhood medulloblastoma. J. Pediatr. Hematol. Oncol. 20:18-25. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Z. F., A. J. Paquette, and D. J. Anderson. 1998. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat. Genet. 20:136-142. [DOI] [PubMed] [Google Scholar]
- 9.Chinnadurai, G. 2003. CtBP family proteins: more than transcriptional corepressors. Bioessays 25:9-12. [DOI] [PubMed] [Google Scholar]
- 10.Chinnadurai, G. 2002. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol. Cell 9:213-224. [DOI] [PubMed] [Google Scholar]
- 11.Chong, J. A., J. Tapia-Ramirez, S. Kim, J. J. Toledo-Aral, Y. Zheng, M. C. Boutros, Y. M. Altshuller, M. A. Frohman, S. D. Kraner, and G. Mandel. 1995. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 80:949-957. [DOI] [PubMed] [Google Scholar]
- 12.Coulson, J. M. 2005. Transcriptional regulation: cancer neurons and the REST. Curr. Biol. 15:R665-R668. [DOI] [PubMed] [Google Scholar]
- 13.Dahmen, R. P., A. Koch, D. Denkhaus, J. C. Tonn, N. Sorensen, F. Berthold, J. Behrens, W. Birchmeier, O. D. Wiestler, and T. Pietsch. 2001. Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res. 61:7039-7043. [PubMed] [Google Scholar]
- 14.Dong, J., M. R. Gailani, S. L. Pomeroy, D. Reardon, and A. E. Bale. 2000. Identification of PATCHED mutations in medulloblastomas by direct sequencing. Hum. Mutat. 16:89-90. [DOI] [PubMed] [Google Scholar]
- 15.Eberhart, C. G., and P. C. Burger. 2003. Anaplasia and grading in medulloblastomas. Brain Pathol. 13:376-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberhart, C. G., J. Kratz, Y. Wang, K. Summers, D. Stearns, K. Cohen, C. V. Dang, and P. C. Burger. 2004. Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J. Neuropathol. Exp. Neurol. 63:441-449. [DOI] [PubMed] [Google Scholar]
- 17.Eberhart, C. G., J. E. Kratz, A. Schuster, P. Goldthwaite, K. J. Cohen, E. J. Perlman, and P. C. Burger. 2002. Comparative genomic hybridization detects an increased number of chromosomal alterations in large cell/anaplastic medulloblastomas. Brain Pathol. 12:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuller, G. N. 1996. Central nervous system tumors in pediatric neoplasia: morphology and biology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 19.Fuller, G. N., X. Su, R. E. Price, Z. R. Cohen, F. F. Lang, R. Sawaya, and S. Majumder. 2005. Many human medulloblastoma tumors overexpress repressor element-1 silencing transcription (REST)/neuron-restrictive silencer factor, which can be functionally countered by REST-VP16. Mol. Cancer Ther. 4:343-349. [DOI] [PubMed] [Google Scholar]
- 20.Fults, D., C. Pedone, C. Dai, and E. C. Holland. 2002. MYC expression promotes the proliferation of neural progenitor cells in culture and in vivo. Neoplasia 4:32-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodrich, L. V., and M. P. Scott. 1998. Hedgehog and patched in neural development and disease. Neuron 21:1243-1257. [DOI] [PubMed] [Google Scholar]
- 22.Goussia, A. C., J. M. Bruner, A. P. Kyritsis, N. J. Agnantis, and G. N. Fuller. 2000. Cytogenetic and molecular genetic abnormalities in primitive neuroectodermal tumors of the central nervous system. Anticancer Res. 20:65-73. [PubMed] [Google Scholar]
- 23.Griffith, E. C., C. W. Cowan, and M. E. Greenberg. 2001. REST acts through multiple deacetylase complexes. Neuron 31:339-340. [DOI] [PubMed] [Google Scholar]
- 24.Grotzer, M. A., M. D. Hogarty, A. J. Janss, X. Liu, H. Zhao, A. Eggert, L. N. Sutton, L. B. Rorke, G. M. Brodeur, and P. C. Phillips. 2001. MYC messenger RNA expression predicts survival outcome in childhood primitive neuroectodermal tumor/medulloblastoma. Clin. Cancer Res. 7:2425-2433. [PubMed] [Google Scholar]
- 25.Gurrola-Diaz, C., J. Lacroix, S. Dihlmann, C. M. Becker, and M. von Knebel Doeberitz. 2003. Reduced expression of the neuron restrictive silencer factor permits transcription of glycine receptor alpha1 subunit in small-cell lung cancer cells. Oncogene 22:5636-5645. [DOI] [PubMed] [Google Scholar]
- 26.Herms, J., I. Neidt, B. Luscher, A. Sommer, P. Schurmann, T. Schroder, M. Bergmann, B. Wilken, S. Probst-Cousin, P. Hernaiz-Driever, J. Behnke, F. Hanefeld, T. Pietsch, and H. A. Kretzschmar. 2000. C-MYC expression in medulloblastoma and its prognostic value. Int. J. Cancer 89:395-402. [PubMed] [Google Scholar]
- 27.Higashino, K., T. Narita, T. Taga, S. Ohta, and Y. Takeuchi. 2003. Malignant rhabdoid tumor shows a unique neural differentiation as distinct from neuroblastoma. Cancer Sci. 94:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Immaneni, A., P. Lawinger, Z. Zhao, W. Lu, L. Rastelli, J. H. Morris, and S. Majumder. 2000. REST-VP16 activates multiple neuronal differentiation genes in human NT2 cells. Nucleic Acids Res. 28:3403-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jepsen, K., O. Hermanson, T. M. Onami, A. S. Gleiberman, V. Lunyak, R. J. McEvilly, R. Kurokawa, V. Kumar, F. Liu, E. Seto, S. M. Hedrick, G. Mandel, C. K. Glass, D. W. Rose, and M. G. Rosenfeld. 2000. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102:753-763. [DOI] [PubMed] [Google Scholar]
- 30.Kenney, A. M., M. D. Cole, and D. H. Rowitch. 2003. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development 130:15-28. [DOI] [PubMed] [Google Scholar]
- 31.Kleihues, P., D. N. Louis, B. W. Scheithauer, L. B. Rorke, G. Reifenberger, P. C. Burger, and W. K. Cavenee. 2002. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol. 61:215-229. [DOI] [PubMed] [Google Scholar]
- 32.Koch, A., A. Waha, J. C. Tonn, N. Sorensen, F. Berthold, M. Wolter, J. Reifenberger, W. Hartmann, W. Friedl, G. Reifenberger, O. D. Wiestler, and T. Pietsch. 2001. Somatic mutations of WNT/wingless signaling pathway components in primitive neuroectodermal tumors. Int. J. Cancer 93:445-449. [DOI] [PubMed] [Google Scholar]
- 33.Koenigsberger, C., J. J. Chicca II, M. C. Amoureux, G. M. Edelman, and F. S. Jones. 2000. Differential regulation by multiple promoters of the gene encoding the neuron-restrictive silencer factor. Proc. Natl. Acad. Sci. USA 97:2291-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwabara, T., J. Hsieh, K. Nakashima, K. Taira, and F. H. Gage. 2004. A small modulatory dsRNA specifies the fate of adult neural stem cells. Cell 116:779-793. [DOI] [PubMed] [Google Scholar]
- 35.Lal, S., M. Lacroix, P. Tofilon, G. N. Fuller, R. Sawaya, and F. F. Lang. 2000. An implantable guide-screw system for brain tumor studies in small animals. J. Neurosurg. 92:326-333. [DOI] [PubMed] [Google Scholar]
- 36.Lawinger, P., L. Rastelli, Z. Zhao, and S. Majumder. 1999. Lack of enhancer function in mammals is unique to oocytes and fertilized eggs. J. Biol. Chem. 274:8002-8011. [DOI] [PubMed] [Google Scholar]
- 37.Lawinger, P., R. Venugopal, Z. S. Guo, A. Immaneni, D. Sengupta, W. Lu, L. Rastelli, A. Marin Dias Carneiro, V. Levin, G. N. Fuller, Y. Echelard, and S. Majumder. 2000. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat. Med. 6:826-831. [DOI] [PubMed] [Google Scholar]
- 38.Lee, C. M., and E. P. Reddy. 1999. The v-myc oncogene. Oncogene 18:2997-3003. [DOI] [PubMed] [Google Scholar]
- 39.Lee, Y., H. L. Miller, P. Jensen, R. Hernan, M. Connelly, C. Wetmore, F. Zindy, M. F. Roussel, T. Curran, R. J. Gilbertson, and P. J. McKinnon. 2003. A molecular fingerprint for medulloblastoma. Cancer Res. 63:5428-5437. [PubMed] [Google Scholar]
- 40.Leonard, J. R., D. X. Cai, D. J. Rivet, B. A. Kaufman, T. S. Park, B. K. Levy, and A. Perry. 2001. Large cell/anaplastic medulloblastomas and medullomyoblastomas: clinicopathological and genetic features. J. Neurosurg. 95:82-88. [DOI] [PubMed] [Google Scholar]
- 41.Leung, C., M. Lingbeek, O. Shakhova, J. Liu, E. Tanger, P. Saremaslani, M. Van Lohuizen, and S. Marino. 2004. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428:337-341. [DOI] [PubMed] [Google Scholar]
- 42.Li, X. N., S. Parikh, Q. Shu, H. L. Jung, C. W. Chow, L. Perlaky, H. C. Leung, J. Su, S. Blaney, and C. C. Lau. 2004. Phenylbutyrate and phenylacetate induce differentiation and inhibit proliferation of human medulloblastoma cells. Clin. Cancer Res. 10:1150-1159. [DOI] [PubMed] [Google Scholar]
- 43.Majumder, S., Z. Zhao, K. Kaneko, and M. L. DePamphilis. 1997. Developmental acquisition of enhancer function requires a unique coactivator activity. EMBO J. 16:1721-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMahon, A. P. 2000. More surprises in the Hedgehog signaling pathway. Cell 100:185-188. [DOI] [PubMed] [Google Scholar]
- 45.Neumann, S. B., R. Seitz, A. Gorzella, A. Heister, M. K. Doeberitz, and C. M. Becker. 2004. Relaxation of glycine receptor and onconeural gene transcription control in NRSF deficient small cell lung cancer cell lines. Brain Res. Mol. Brain Res. 120:173-181. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura, E., K. Sasaki, K. Maruyama, T. Tsukada, and K. Yamaguchi. 1996. Decrease in neuron-restrictive silencer factor (NRSF) mRNA levels during differentiation of cultured neuroblastoma cells. Neurosci. Lett. 211:101-104. [DOI] [PubMed] [Google Scholar]
- 47.Paddison, P. J., J. M. Silva, D. S. Conklin, M. Schlabach, M. Li, S. Aruleba, V. Balija, A. O'Shaughnessy, L. Gnoj, K. Scobie, K. Chang, T. Westbrook, M. Cleary, R. Sachidanandam, W. R. McCombie, S. J. Elledge, and G. J. Hannon. 2004. A resource for large-scale RNA-interference-based screens in mammals. Nature 428:427-431. [DOI] [PubMed] [Google Scholar]
- 48.Pappo, A. S. 1996. Rhabdomyosarcoma and other soft tissue sarcomas in children. Curr. Opin. Oncol. 8:311-316. [DOI] [PubMed] [Google Scholar]
- 49.Paquette, A. J., S. E. Perez, and D. J. Anderson. 2000. Constitutive expression of the neuron-restrictive silencer factor (NRSF)/REST in differentiating neurons disrupts neuronal gene expression and causes axon pathfinding errors in vivo. Proc. Natl. Acad. Sci. USA 97:12318-12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pomeroy, S. L., and L. M. Sturla. 2003. Molecular biology of medulloblastoma therapy. Pediatr. Neurosurg. 39:299-304. [DOI] [PubMed] [Google Scholar]
- 51.Pomeroy, S. L., P. Tamayo, M. Gaasenbeek, L. M. Sturla, M. Angelo, M. E. McLaughlin, J. Y. Kim, L. C. Goumnerova, P. M. Black, C. Lau, J. C. Allen, D. Zagzag, J. M. Olson, T. Curran, C. Wetmore, J. A. Biegel, T. Poggio, S. Mukherjee, R. Rifkin, A. Califano, G. Stolovitzky, D. N. Louis, J. P. Mesirov, E. S. Lander, and T. R. Golub. 2002. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415:436-442. [DOI] [PubMed] [Google Scholar]
- 52.Rao, G., C. A. Pedone, C. M. Coffin, E. C. Holland, and D. W. Fults. 2003. c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia 5:198-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rastelli, L., K. Robinson, Y. Xu, and S. Majumder. 2001. Reconstitution of enhancer function in paternal pronuclei of one-cell mouse embryos. Mol. Cell. Biol. 21:5531-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romer, J. T., H. Kimura, S. Magdaleno, K. Sasai, C. Fuller, H. Baines, M. Connelly, C. F. Stewart, S. Gould, L. L. Rubin, and T. Curran. 2004. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell 6:229-240. [DOI] [PubMed] [Google Scholar]
- 55.Ryder, E. F., E. Y. Snyder, and C. L. Cepko. 1990. Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J. Neurobiol. 21:356-375. [DOI] [PubMed] [Google Scholar]
- 56.Shimojo, M., and L. B. Hersh. 2004. Regulation of the cholinergic gene locus by the repressor element-1 silencing transcription factor/neuron restrictive silencer factor (REST/NRSF). Life Sci. 74:2213-2225. [DOI] [PubMed] [Google Scholar]
- 57.Shimojo, M., and L. B. Hersh. 2003. REST/NRSF-interacting LIM domain protein, a putative nuclear translocation receptor. Mol. Cell. Biol. 23:9025-9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimojo, M., A. J. Paquette, D. J. Anderson, and L. B. Hersh. 1999. Protein kinase A regulates cholinergic gene expression in PC12 cells: REST4 silences the silencing activity of neuron-restrictive silencer factor/REST. Mol. Cell. Biol. 19:6788-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh, S. K., C. Hawkins, I. D. Clarke, J. A. Squire, J. Bayani, T. Hide, R. M. Henkelman, M. D. Cusimano, and P. B. Dirks. 2004. Identification of human brain tumour initiating cells. Nature 432:396-401. [DOI] [PubMed] [Google Scholar]
- 60.Snyder, E. Y., D. L. Deitcher, C. Walsh, S. Arnold-Aldea, E. A. Hartwieg, and C. L. Cepko. 1992. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68:33-51. [DOI] [PubMed] [Google Scholar]
- 61.Snyder, E. Y., K. I. Park, J. D. Flax, S. Liu, C. M. Rosario, B. D. Yandava, and S. Aurora. 1997. Potential of neural “stem-like” cells for gene therapy and repair of the degenerating central nervous system. Adv. Neurol. 72:121-132. [PubMed] [Google Scholar]
- 62.Strathdee, C. A., M. R. McLeod, and J. R. Hall. 1999. Efficient control of tetracycline-responsive gene expression from an autoregulated bi-directional expression vector. Gene 229:21-29. [DOI] [PubMed] [Google Scholar]
- 63.Su, X., S. Kameoka, S. Lentz, and S. Majumder. 2004. Activation of REST/NRSF target genes in neural stem cells is sufficient to cause neuronal differentiation. Mol. Cell. Biol. 24:8018-8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, Q., H. Li, X. W. Wang, D. C. Wu, X. Y. Chen, and J. Liu. 2003. Resveratrol promotes differentiation and induces Fas-independent apoptosis of human medulloblastoma cells. Neurosci. Lett. 351:83-86. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe, Y., S. Kameoka, V. Gopalakrishnan, K. D. Aldape, Z. Z. Pan, F. F. Lang, and S. Majumder. 2004. Conversion of myoblasts to physiologically active neuronal phenotype. Genes Dev. 18:889-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wechsler-Reya, R., and M. P. Scott. 2001. The developmental biology of brain tumors. Annu. Rev. Neurosci. 24:385-428. [DOI] [PubMed] [Google Scholar]
- 67.Westbrook, T. F., E. S. Martin, M. R. Schlabach, Y. Leng, A. C. Liang, B. Feng, J. J. Zhao, T. M. Roberts, G. Mandel, G. J. Hannon, R. A. Depinho, L. Chin, and S. J. Elledge. 2005. A genetic screen for candidate tumor suppressors identifies REST. Cell 121:837-848. [DOI] [PubMed] [Google Scholar]
- 68.Wetmore, C., D. E. Eberhart, and T. Curran. 2000. The normal patched allele is expressed in medulloblastomas from mice with heterozygous germ-line mutation of patched. Cancer Res. 60:2239-2246. [PubMed] [Google Scholar]
- 69.Xie, J., R. L. Johnson, X. Zhang, J. W. Bare, F. M. Waldman, P. H. Cogen, A. G. Menon, R. S. Warren, L. C. Chen, M. P. Scott, and E. H. Epstein, Jr. 1997. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res. 57:2369-2372. [PubMed] [Google Scholar]
- 70.Zurawel, R. H., S. A. Chiappa, C. Allen, and C. Raffel. 1998. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 58:896-899. [PubMed] [Google Scholar]