Abstract
Protein kinase C θ (PKC θ) is unique among PKC isozymes in its translocation to the center of the immune synapse in T cells and its unique downstream signaling. Here we show that the hematopoietic protein tyrosine phosphatase (HePTP) also accumulates in the immune synapse in a PKC θ-dependent manner upon antigen recognition by T cells and is phosphorylated by PKC θ at Ser-225, which is required for lipid raft translocation. Immune synapse translocation was completely absent in antigen-specific T cells from PKC θ−/− mice. In intact T cells, HePTP-S225A enhanced T-cell receptor (TCR)-induced NFAT/AP-1 transactivation, while the acidic substitution mutant was as efficient as wild-type HePTP. We conclude that HePTP is phosphorylated in the immune synapse by PKC θ and thereby targeted to lipid rafts to temper TCR signaling. This represents a novel mechanism for the active immune synapse recruitment and activation of a phosphatase in TCR signaling.
Isoenzymes of the serine/threonine-specific protein kinase C (PKC) family are involved in several signal transduction pathways downstream of antigen receptors in immune cells (26). Among the PKCs, PKC θ (5, 19) is particularly important in T-lymphocyte activation and survival (26), where it exerts a number of unique functions not seen for other PKC family members. PKC θ activates the NF-κB signaling pathway (9) and the N-terminal c-Jun protein kinase (JNK) pathway (10), and it synergizes with calcineurin for the activation of NFAT and subsequent interleukin-2 (IL-2) production (6, 20). PKC θ also directly phosphorylates and activates the Ser/Thr kinase SPAK, which contributes to AP-1 activation (12). In contrast to other PKCs, PKC θ causes very little activation of the extracellular signal-regulated kinases Erk1 and Erk2 (10).
PKC θ is the only T-cell-expressed PKC that selectively translocates to the center of the immunological synapse in T cells stimulated by antigen-presenting cells (APCs) (7, 15). This translocation depends on the Lck kinase and correlates with the catalytic activation of PKC θ through a Vav- and PI3K-dependent pathway (30). The importance of PKC θ in T-cell physiology was further demonstrated by experiments with the pkcθ−/− mouse, which displays severe defects in antigen receptor/coreceptor-mediated T-cell activation (20, 28).
The hematopoietic protein tyrosine phosphatase (HePTP) is a 38-kDa nonreceptor (intracellular) classical protein tyrosine phosphatase (PTP) (4) expressed in all hematopoietic lineages, including T lymphocytes (1, 32). The physiological function of HePTP is to inactivate the Erk and p38 mitogen-activated protein (MAP) kinases (16, 24). To accomplish this task, HePTP physically associates via a 15-amino-acid kinase interaction motif in its N terminus with the Erk1, Erk2, and p38 kinases (18, 23). HePTP then dephosphorylates the phosphotyrosine (but not phosphothreonine) residue in the activation loops of the bound kinases. Other MAP kinases (JNK, Erk3, Erk5, and Erk7) do not bind and are not substrates for HePTP in intact cells. HePTP−/− mice also display a selective augmentation of their Erk and p38 responses (11).
HePTP is itself regulated by phosphorylation at multiple serine, threonine, and perhaps tyrosine residues. The best-documented phosphorylation site is Ser-23 (17, 25) in the kinase interaction motif; the phosphorylation thereof results in dissociation of the bound Erk or p38. Ser-23 is phosphorylated by cyclic AMP (cAMP)-dependent protein kinase (PKA) in intact T cells in response to agents that elevate cAMP levels (17, 25). Here we report that HePTP is also phosphorylated by PKC θ (and possibly by other PKCs) at Ser-225 in its catalytic domain. While phosphorylation at Ser-23 results in reduced dephosphorylation of Erk and p38, the PKC θ-mediated phosphorylation at Ser-225 results in lipid raft targeting of HePTP, which appears to be crucial for the inhibitory effect of HePTP on T-cell receptor (TCR) signaling. Furthermore, phosphorylation of HePTP at Ser-225 is induced by TCR ligation and appears to take place in the immune synapse, while Ser-23 phosphorylation occurs in discrete submembranous locations distributed throughout the T cell (17). Thus, distinct pools of HePTP are phosphorylated at different residues by two different kinases in different subcellular locations in the cell and thereby integrate input from cAMP and TCR/PKC signaling, respectively, into the fine-tuning of the MAP kinase pathway.
MATERIALS AND METHODS
Antibodies and reagents.
Polyclonal goat or rabbit antibodies against PKC θ and the phosphorylated and unphosphorylated forms of MAP kinases were from Santa Cruz Biotechnology (Santa Cruz, CA). The antiphosphoserine PKC substrate polyclonal antibody was obtained from Cell Signaling. The OKT3 anti-CD3ɛ monoclonal antibody (mAb) was from e-Bioscience, and anti-human CD28 was from Pharmingen. The 12CA5 antihemagglutinin (anti-HA) mAb was from Boehringer Mannheim (Indianapolis, IN). The C305 anti-TCR mAb was from ATCC and was used as the culture supernatant. A series of mAbs were raised against recombinant full-length HePTP (17); mAb B3A/C9 was used in this study.
Plasmids and recombinant proteins.
The expression plasmids for HePTP and its mutants in the pEF/HA vector, which adds an HA tag to the N terminus of the insert, were as described previously (23-25). Recombinant HePTP proteins, with either glutathione S-transferase (GST) or His6 at the N terminus, were produced as described previously (23, 25). The expression plasmids for PKC θ and its mutants (6, 10, 20) were in the in pEF-neo vector. All recombinant PKC proteins were from Calbiochem.
Cells and transfections.
Jurkat T leukemia cells were kept at logarithmic growth in RPMI 1640 medium with 5% fetal calf serum, l-glutamine, and antibiotics. These cells were transiently transfected with a total of 5 μg DNA by electroporation at 950 μF and 240 V. Empty vector was added to control samples to make a constant amount of DNA in each sample. Cells were used for experiments 24 to 48 h after transfection. Primary human T cells were negatively selected directly from whole blood by use of Rosette Sep T-cell depletion cocktails (StemCell Technologies, Vancouver, BC, Canada). The purity of the cell populations was over 90%, as determined by fluorescence-activated cell sorting. CD8+ OT-I T cells and SigOVA257-264MEC/B7.1 cells were prepared as described previously (3).
Isolation of lipid rafts using sucrose gradient centrifugation.
Lipid rafts were isolated from total cell lysates as described previously (33) with a few modifications. Briefly, 10 × 107 to 20 × 107 stimulated or unstimulated Jurkat T cells were lysed on ice in 1 ml TNE buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA containing 1% Triton-X, 1 mM Na3VO4, 5 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 10 μg/ml each of apoprotin-leupeptin and soybean trypsin inhibitor). The cells were left on ice for 15 min, subjected to 10 rounds of Dounce homogenization, and mixed with 1 ml 80% sucrose in TNE buffer. The mixed lysates were transferred to the bottom of Beckman ultracentrifuge tubes and were overlaid with 2 ml 30% sucrose in TNE buffer and then with 1 ml 5% sucrose in TNE buffer. Samples were ultracentrifuged for 20 h at 46,000 rpm in a Beckman SW55Ti rotor. Ten fractions of 0.5 ml were collected from the top of the gradient.
Immunoprecipitation and immunoblotting.
Cells were lysed in 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA containing 1% NP-40, 1 mM Na3VO4, 5 mM NaF, 10 μg/ml aprotinin and leupeptin, 100 μg/ml soybean trypsin inhibitor, and 1 mM phenylmethylsulfonyl fluoride and clarified by centrifugation at 15,000 rpm for 20 min. The clarified lysates were preabsorbed on protein G-Sepharose and then incubated with antibody for 2 h and subsequently with protein G-Sepharose beads. Immune complexes were washed three times in lysis buffer, once in lysis buffer with 0.5 M NaCl, and again in lysis buffer and were suspended in sodium dodecyl sulfate sample buffer.
In vitro phosphorylation, tryptic peptide mapping, and phosphoamino acid analysis.
Four μg GST-HePTP or 0.5 μg GST was incubated with 20 ng PKC for 20 min at 30°C according to the manufacturer's instructions. The reactions were terminated by the addition of sodium dodecyl sulfate sample buffer and boiling, and the products were analyzed by autoradiography and immunoblotting. Tryptic peptide mapping and phosphoamino acid analysis were performed as described by Luo and coworkers (13).
Cell conjugation, confocal microscopy, and three-dimensional reconstructions.
These procedures were performed as described before (21). Three-dimensional reconstructions were obtained with Volocity software (Volocity 2.6.2; Image3, LLC) from 40 serial z sections taken at 0.25-μm increments.
RESULTS
A fraction of HePTP is present in lipid rafts.
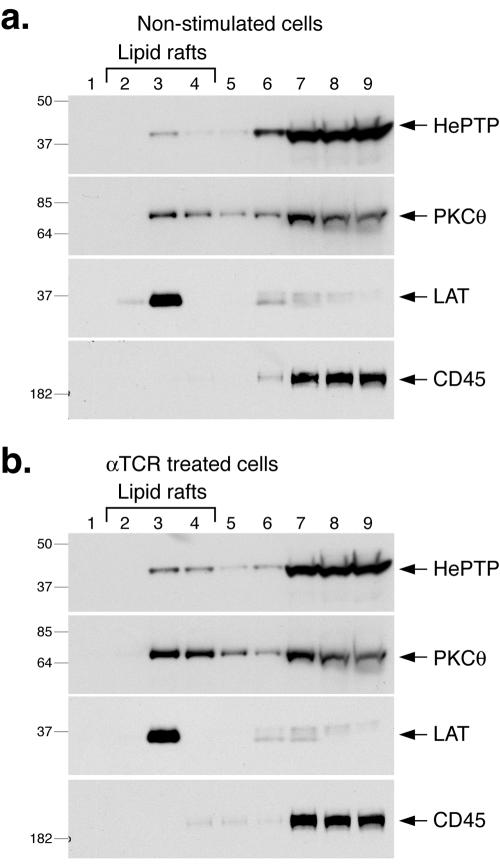
It has been suggested that PTPs are excluded from lipid rafts upon TCR ligation to allow signaling by tyrosine phosphorylation to proceed unopposed. We have begun to test this notion experimentally by immunoblotting the buoyant lipid raft fractions from resting or stimulated T cells by sucrose gradient centrifugation with antibodies against PTPs. Using the specific monoclonal antibody B3A/C9 (17) against HePTP, we consistently detect some HePTP in the lipid rafts of resting T cells (Fig. 1a), and this fraction increased to ∼5% of total HePTP in cells activated by anti-CD3 and anti-CD28 antibodies (Fig. 1b) or 50 nM phorbol ester (not shown). Parallel immunoblots demonstrated that PKC θ had a similar pattern of lipid raft distribution (Fig. 1, second panels from top), while LAT was quantitatively lipid raft located in all cell samples (third panels). In contrast, CD45 was not detected in the lipid rafts (bottom panels).
FIG. 1.
HePTP in lipid rafts. (a) Anti-HePTP (top panel), anti-PKC θ (second panel), anti-LAT (third panel), and anti-CD45 (bottom panel) immunoblots of detergent extracts of resting Jurkat T cells fractionated on a sucrose gradient. Lipid rafts are in fractions 2 to 4, mostly in fraction 3. (b) Similar experiment with Jurkat T cells stimulated with anti-TCR mAbs. Mrs (in thousands) are shown to the left of the gels.
Detection of Erk and phospho-Erk in lipid rafts.
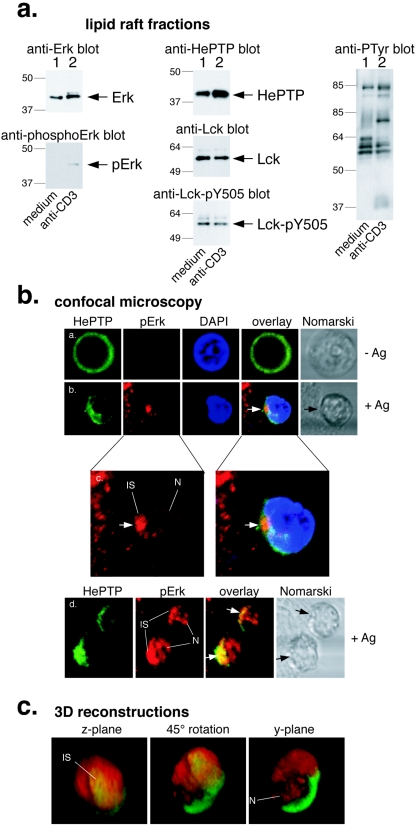
To determine if translocation of HePTP to lipid rafts might serve to bring it to the vicinity of its physiological substrate, the Erk MAP kinase, we immunoblotted raft fractions with antibodies against this kinase and found that a readily detectable amount of unphosphorylated Erk was present in lipid rafts in untreated T cells (Fig. 2a). Upon TCR cross-linking, the amount of Erk increased somewhat and a band of higher Mr became visible. This band was also seen with antibodies against phosphorylated Erk (Fig. 2a, lower left panel). Further immunoblots of the same lipid raft samples showed that HePTP was present and increased upon TCR stimulation (top middle panel), while Lck levels were unchanged (second middle panel) but decreased somewhat in phosphorylation at Y505 (bottom middle panel). As an additional control that TCR stimulation was robust in this experiment, an anti-PTyr blot showed that proteins of 36 to 38, 70 to 76, and 95 kDa became tyrosine phosphorylated in lipid rafts. Erk and phospho-Erk were seen in lipid rafts in several independent experiments.
FIG. 2.
HePTP and Erk activation in the immune synapse. (a) Immunoblots of lipid raft fractions from resting (lane 1) or activated (lane 2) Jurkat T cells with antibodies against total Erk (upper left panel), phospho-Erk (lower left panel), HePTP (top middle panel), Lck (second middle panel), phospho-Y505 Lck (bottom middle panel), or antiphosphotyrosine (right panel). Mrs (in thousands) are shown to the left of the gels. (b) Confocal microscopy of CD8+ antigen-specific, TCR-transgenic OT-I T cells in contact with APCs for 0 min (subpanels a) or 20 min (subpanels b to d) and stained for HePTP (green), phospho-Erk (pErk; red), and DNA (blue). Subpanels c are enlargements of the indicated subpanels b to show phospho-Erk location in the immune synapse (IS) and nucleus (N). DAPI, 4′,6′-diamidino-2-phenylindole. (c) Three-dimensional reconstruction of a representative OT-I T cell stained for HePTP (green) and phospho-Erk (red) in contact with an APC. The reconstructed cell is shown in the z plane, i.e., the direction from which the APC would see the T cell (left panel); 45° between the z and y planes (middle panel); and in the y plane (right panel). The center of the immune synapse and the location of staining in the nucleus are indicated.
HePTP and Erk activation in the immune synapse.
To study the location of HePTP and Erk activation in a more physiological model of T-cell activation, we used naïve CD8+ antigen-specific OT-I TCR-transgenic T cells, which were mixed with antigen-presenting cells for various times at 37°C, fixed, and stained with the anti-HePTP mAb and antibodies against phospho-Erk. These experiments showed that antigen recognition changed the diffuse cytoplasmic distribution of HePTP into a more polarized, APC-oriented location (Fig. 2b). Much of HePTP became concentrated in the vicinity of the contact between the T cell and the APC. HePTP was also seen in deeper portions of the cytoplasm (Fig. 2b) but not in the nucleus. Staining of the cells with antibodies that recognize only the phosphorylated form of Erk showed that Erk activation also occurred in the T cell-APC contact zone (Fig. 2b). A portion of phospho-Erk was also detected in the nucleus of many cells (Fig. 2c and d). In contrast, HePTP always stayed in the cytosol and was never seen in the nucleus. Three-dimensional reconstructions of serial z sections also showed that HePTP and phosphorylated Erk coexist in the immune synapse, particularly its center, and that proportionately more phospho-Erk is located more peripherally and in the cell nucleus. We conclude from all these experiments that HePTP translocates to lipid rafts and the immune synapse, where Erk is also found, and we suggest that Erk activation is initiated in this location but that the phosphorylated and activated Erk can leave. The confocal images show that much of the phosphorylated Erk escapes into the nucleus, where HePTP cannot follow. We suggest that HePTP is a gatekeeper that acts early in the TCR-induced MAP kinase activation cascade rather than a late-acting terminator of the response, and our current data suggest that at least part of this task is carried out in lipid rafts within the immune synapse. Consistent with this model, Erk activation is two- to threefold higher in HePTP−/− T cells but occurs with an essentially unchanged time course (11). Other MAP kinase phosphatases (e.g., PAC1) presumably act to terminate Erk activation in the nucleus.
PKC θ is required for HePTP translocation to the immune synapse.
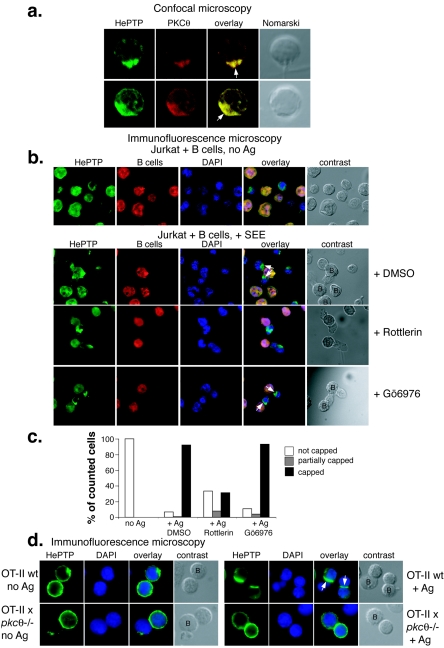
We also stained the naïve CD8+ antigen-specific OT-I T cells with antibodies against PKC θ and found that it colocalized extensively with HePTP upon antigen recognition (Fig. 3a). To determine if HePTP translocation to the immune synapse is indeed functionally connected to PKC θ, we first tested if rottlerin, an inhibitor of atypical PKCs (predominantly PKC θ in T cells) would prevent HePTP accumulation in the immune synapse. Figure 3b shows that endogenous HePTP accumulated in the contact area between Jurkat T cells and B cells in the presence of the superantigen staphylococcal enterotoxin E. This relocation of HePTP was completely abrogated in T cells treated with 10 μM rottlerin for 20 min (Fig. 3b, third panels) but not in cells treated with 1 μM Gö6976, an inhibitor of conventional PKCs (bottom panels). Quantitation of these results is shown in Fig. 3c. Very similar results were obtained with transfected epitope-tagged HePTP (not shown). Next, we used CD4+ T cells from OT-II mice crossed with pkcθ−/− mice and found that while HePTP translocated in T cells from control OT-II mice to the contact site with B cells presenting the OVA peptide, there was no accumulation of HePTP at all in contacts between APCs and PKC θ-negative T cells (Fig. 3d). Thus, HePTP translocation to the immune synapse can be observed in both murine and human T cells, in CD4+ and CD8+ T cells, and with different models of antigen recognition. This translocation depends on functional PKC θ.
FIG. 3.
PKC θ is required for HePTP translocation to the immune synapse. (a) Confocal microscopy of CD8+ antigen-specific, TCR-transgenic OT-I T cells in contact with APCs for 20 min and stained for HePTP (green) and PKC θ (red). (b) Immunofluorescence microscopy of Jurkat T cells in contact with Raji B cells without (top row) or with (all other rows) staphylococcal enterotoxin E and stained for HePTP (green), B cells (red), and DNA (blue). The centers of the contacts between the T cell and the APC are indicated with arrows; B cells are labeled “B” in contrast panels. (c) Quantitation of formed immune synapses in numerous fields of view. (d) Immunofluorescence microscopy of CD4+ antigen-specific, TCR-transgenic OT-II T cells in contact with APCs in the absence (left panels) or presence (right panels) of OVA peptide and stained for HePTP (green) and DNA (blue). The upper panels show control OT-II T cells; the lower panels show T cells from OT-II × pkcθ−/− mice, as indicated. B cells are labeled “B” in contrast panels. wt, wild type; DAPI, 4′,6′-diamidino-2-phenylindole; DMSO, dimethyl sulfoxide.
Together, these results show that HePTP translocates to the immune synapse upon antigen recognition in three different systems: CD8+ OT-I T cells, CD4+ OT-II T cells, and Jurkat cells. The results also show that PKC θ is absolutely necessary for this translocation.
HePTP is phosphorylated by PKC isozymes at Ser-225 in vitro.
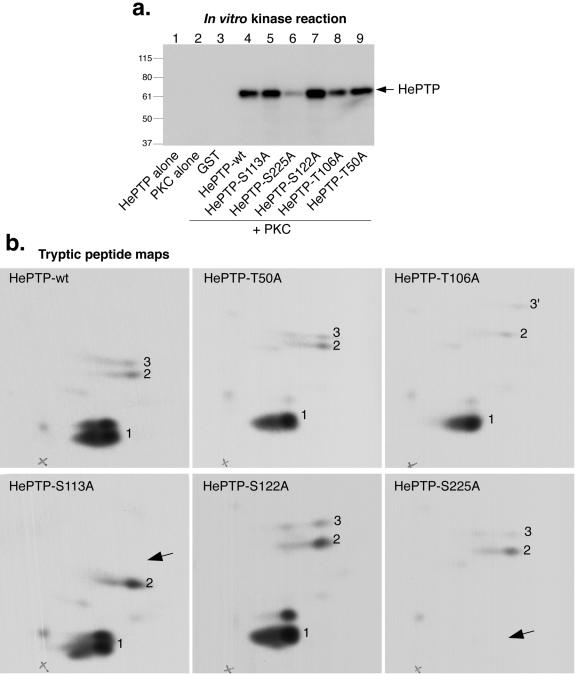
We previously reported that tryptic peptide maps of HePTP from metabolically 32P-labeled T cells contain several spots (25), one of which corresponds to an unidentified phosphoserine (PSer). Since the amino acid sequence of HePTP contains several potential PKC phosphorylation sites, we asked if HePTP is a substrate for PKC.
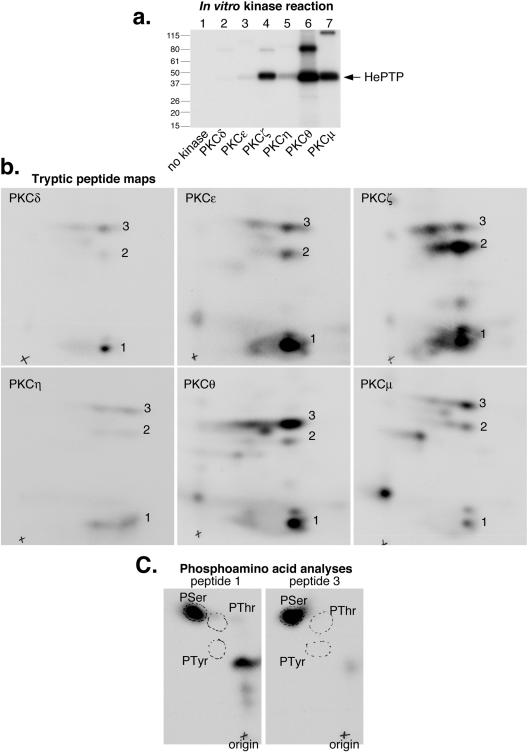
First, we used a catalytically active PKC-M preparation consisting predominantly of the α, βI, βII, and γ isoenzymes and asked if it could phosphorylate recombinant HePTP in vitro in the presence of [γ-32P]ATP. Indeed, 32P-labeled HePTP was readily obtained (Fig. 4a). Tryptic peptide maps (Fig. 4b) showed that most of the phosphate was located in a major spot (peptide 1) that migrated only a short distance in the second dimension (ascending chromatography) on the thin-layer plates. This site was identified as Ser-225 by repeating the in vitro phosphorylation experiments with a series of Ser-to-Ala and Thr-to-Ala mutants of HePTP (Fig. 4a and b). While the maps of HePTP mutants T50A and S122A were indistinguishable from that of wild-type HePTP, the map of HePTP-S225A completely lacked peptide 1. Additionally, the maps of HePTP-T106A and HePTP-S113A were different: in the former, peptide 3 migrated higher up, indicating that it was more hydrophobic, while in the map of HePTP-S113A, peptide 3 was completely missing. Thus, Ser-113 is a minor phosphorylation site. Since Thr-106 is located in the same tryptic peptide as Ser-113, the T106A mutation made this peptide only a bit more hydrophobic; it did not affect the phosphorylation at Ser-113 by PKC. The minor phosphorylation site presenting itself as peptide 2 remains unidentified. The in vivo significance of this site, as well as that of Ser-113, is questionable.
FIG. 4.
Phosphorylation of HePTP by PKC at S225. (a) Autoradiogram of in vitro kinase assay containing HePTP alone (lane 1), PKC-M alone (lane 2), GST plus PKC-M (lane 3), GST-HePTP plus PKC (lane 4), or the indicated S/T-to-A mutants of GST-HePTP plus PKC-M (lanes 5 to 9) in the presence of [γ-32P]ATP. Mrs (in thousands) are shown to the left of the gel. (b) Tryptic peptide maps (peptides labeled 1, 2, and 3) of the phosphorylated HePTP proteins from panel a. Exposure time for all maps was 21 h. Arrows indicate missing peptides. wt, wild type.
PKC θ is the most potent PKC isozyme.
Next, we compared the isozymes in a series of PKC isozymes for the ability of each to phosphorylate recombinant HePTP in vitro. These recombinant proteins were used at identical specific activities. While all isozymes phosphorylated Ser-225 predominantly and Ser-113 to a lesser extent (Fig. 5), they differed strikingly in how much 32P they incorporated into HePTP during the 30-min assay. PKC θ was the most efficient, while PKC ζ and PKC μ were clearly less potent; PKC δ, ɛ, and η were quite inefficient. Interestingly, the different PKCs also showed somewhat different relative preferences for the serine residues (Fig. 5b). Phosphoamino acid analysis of the two peptides phosphorylated by PKC θ confirmed that both sites were indeed serines (Fig. 5c). Maps of HePTP-S225A phosphorylated by PKC θ lacked the S225 spot (not shown).
FIG. 5.
Phosphorylation of HePTP by different PKC isoenzymes. (a) Autoradiogram of GST-HePTP incubated with the indicated PKC isoenzymes in the presence of [γ-32P]ATP for 30 min. Mrs (in thousands) are shown to the left of the gel. (b) Tryptic peptide maps (peptides labeled 1, 2, and 3) of the phosphorylated HePTP proteins from panel a. Exposure times were as follows: 5 days for PKC δ, PKC ɛ, and PKC η; 17 h for PKC θ; and 20 h for PKC ζ and PKC μ. (c) Phosphoamino acid analysis of peptides 1 and 3 from the PKC θ map, showing that both contain only phosphoserine.
HePTP is phosphorylated at Ser-225 by PKC θ in intact T cells.
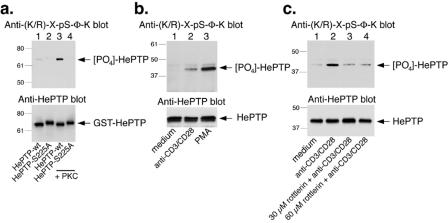
To verify that HePTP is indeed phosphorylated at S225 in intact T cells, we used a phospho-specific antibody that recognizes PKC substrates with the sequence R/K-X-pS-Φ-K (X, any amino acid; Φ, hydrophobic residue), a motif that fits the sequence R-R-pS-V-K (amino acid residues 223 to 227) containing phospho-S225 but no other sites in HePTP. First, we verified that this antibody reacts with HePTP phosphorylated by PKC θ (Fig. 6a) in contrast to recombinant HePTP-S225A incubated with PKC θ, which did not react with the phospho-specific antibody (lane 4). Next, we immunoprecipitated HePTP from resting primary human T cells or cells treated with anti-CD3 or phorbol ester and then immunoblotted the precipitates with the phospho-specific antibody. As shown in Fig. 6b, HePTP in resting cells reacted weakly with the phospho-specific antibody (lane 1), while HePTP from activated cells reacted quite well. Furthermore, this increased phosphorylation of HePTP at S225 in primary T cells was blocked by addition of the PKC θ inhibitor rottlerin (Fig. 6c). These experiments show that HePTP is indeed phosphorylated at S225 in intact normal T cells and that PKC θ is involved.
FIG. 6.
PKC θ phosphorylates HePTP at S225 in primary T cells. (a) Upper panel, anti-(K/R)-X-pS-Φ-K immunoblot of GST-HePTP (lanes 1 and 3) and GST-HePTP-S225A (lanes 2 and 4) incubated with kinase buffer alone (lanes 1 and 2) or with PKC (lanes 3 and 4). Lower panel, anti-HePTP blot of the same filter. (b) Upper panel, anti-(K/R)-X-pS-Φ-K immunoblot of anti-HePTP immunoprecipitates from primary human T cells left untreated (lane 1) or stimulated with anti-CD3 and anti-CD28 Dynabeads (lane 2) or 50 nM phorbol ester (lane 3) for 10 min before lysis. Lower panel, anti-HePTP immunoblot of the same immunoprecipitates. (c) Upper panel, anti-(K/R)-X-pS-Φ-K immunoblot of anti-HePTP immunoprecipitates from primary human T cells left untreated (lane 1), stimulated with anti-CD3 (lane 2), or treated with 30 μM rottlerin plus anti-CD3/CD28 beads (lane 3) or with 60 μM rottlerin plus anti-CD3/CD28 beads (lane 4). Lower panel, anti-HePTP immunoblot of the same immunoprecipitates. Mrs (in thousands) are shown to the left of the gels.
Phosphorylation of HePTP at S225 is required for lipid raft targeting.
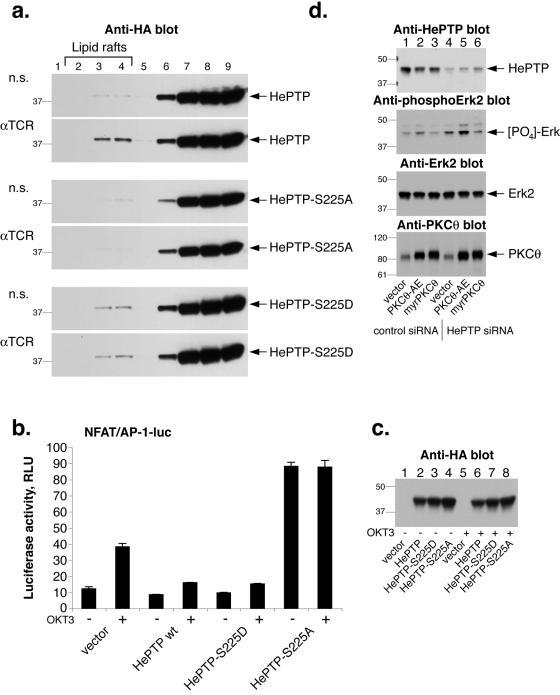
To begin to explore the physiological role of HePTP phosphorylation at S225, we analyzed the buoyant lipid raft fractions from cells transfected with HePTP, HePTP-S225A, or HePTP-S225D and found that while HePTP translocated to lipid rafts upon TCR stimulation, the S225D mutant was present in these fractions both before and after TCR triggering; however, the HePTP-S225A mutant was not present at all in fractions 1 through 5 (Fig. 7a). Thus, it seems that HePTP phosphorylation at S225 is required for lipid raft partition. In agreement with this notion, the stoichiometry of HePTP phosphorylation is very similar to the percent of HePTP in lipid rafts. We also found that an HePTP-S113D mutant partitions more into lipid rafts (data not shown), supporting the notion that PKC θ-mediated phosphorylation promotes this targeting.
FIG. 7.
Role of HePTP phosphorylation at S225. (a) Anti-HA immunoblots of detergent extracts of Jurkat T cells transfected with HePTP (top panel), HePTP-S225D (middle panel), or HePTP-S225A (bottom panel) fractionated on a sucrose gradient. Lipid rafts are in fractions 2 to 4, mostly in fraction 3. n.s., nonstimulated. (b) Luciferase assay (relative light units [RLU]) of Jurkat T cells transfected with an NFAT/AP-1 reporter construct and empty vector (HePTP, HePTP-S225D, or HePTP-S225A, as indicated) and either left unstimulated or treated with OKT3 anti-CD3ɛ mAb for 7 h. The luciferase activity was normalized using a cotransfected Renilla luciferase. The data represent the means, and the error bars standard deviations, from triplicate determinations; this experiment is representative of three independent experiments. wt, wild type. (c) Anti-HA immunoblot to show the expressed HePTP in the same cell samples. (d) Elimination of HePTP by RNA interference augments PKC θ-induced Erk activation. Jurkat T cells were transfected with a nonspecific RNA oligonucleotide (lanes 1 to 3) or with an HePTP-specific siRNA (lanes 4 to 6) and cotransfected with expression plasmids for the constitutively active PKC θ-AE (lanes 2 and 5) or myristylated PKC θ (lanes 3 and 6). The four panels represent blots with different antibodies, as indicated. Mrs (in thousands) are shown to the left of the gels.
HePTP needs to be phosphorylated to inhibit TCR signaling.
Expression of HePTP results in decreased transactivation of the IL-2 gene in response to TCR ligation (23, 24). Since this effect requires the catalytic activity of HePTP and the inactive mutant HePTP-C270S augments these responses, we decided to express the phosphorylation-mimicking HePTP-S225D and the unphosphorylatable HePTP-S225A in T cells and measure their effects on TCR-induced gene activation. While wild-type and S225D mutant HePTP reduced the TCR-driven transactivation of an NFAT/AP-1 reporter (taken from the IL-2 promoter), HePTP-S225A acted as a dominant negative and strongly augmented the response (Fig. 7b). The HePTP constructs were all well expressed (Fig. 7c). This result suggests that HePTP must be phosphorylated at S225 to inhibit TCR signaling and that prevention of this phosphorylation by changing S225 to an alanine leads to enhanced downstream signals, perhaps due to the diminished phosphorylation of endogenous HePTP. In contrast, the S225D mutant HePTP, which is targeted to lipid rafts (Fig. 7), apparently does not require phosphorylation to carry out the normal duties of HePTP. Thus, it was as active as, or even slightly more active than, wild-type HePTP.
Loss of HePTP augments the ability of PKC θ to activate Erk.
To directly address the question of whether PKC θ is less efficient than other PKCs in activating Erk because it activates HePTP, we designed a small interfering RNA (siRNA) oligonucleotide duplex that efficiently reduced the expression of HePTP in Jurkat T cells by approximately 80% (Fig. 7d). These cells also contained a somewhat increased level of phospho-Erk (lane 4). While expression of constitutively active PKC θ-AE in control siRNA-treated cells had little effect on basal Erk activation, cotransfection of PKC θ-AE into cells with reduced levels of HePTP caused a much larger increase in phospho-Erk levels (lane 5). In contrast, a membrane-targeted PKC θ had little effect on basal phospho-Erk levels (lane 6). Thus, it seems that PKC θ can activate the Erk pathway, as other PKCs do, but also activates HePTP (through S225 phosphorylation), thereby canceling out Erk activation in favor of other pathways. Based on our results, we propose that this occurs in lipid raft fractions within the immune synapse.
DISCUSSION
The active recruitment of a cytosolic PTP to the immune synapse by PKC θ-mediated phosphorylation represents a mechanism for the participation of a PTP in TCR signaling that is novel but reminiscent of the recruitment and activation of the VHR phosphatase by ZAP-70-mediated tyrosine phosphorylation (3). In both cases, it appears that phosphorylation occurs on a small pool of the phosphatase (<5%) but that this pool becomes biologically important. The two kinases responsible, PKC θ and ZAP-70, respectively, are key players of TCR signaling and accumulate in the center of the immune synapse. In HePTP as well as in VHR (3), the phosphorylation site is on the far side of the catalytic domain, opposite from the catalytic pocket, and in both cases a phosphorylation site mutant acts as a much more potent dominant negative than a catalytically inactive form of the enzyme. This suggests that the mutant effectively competes for the kinase and/or other interacting proteins and thereby reduces the phosphorylation and/or function of endogenous phosphatase. Thus, it appears that TCR-induced recruitment, phosphorylation, and activation of phosphatases that act on MAP kinases comprise a more general theme in TCR signaling.
The amino acid sequence surrounding S225, ERRSVKH (underlining indicates the phosphorylated serine), contains the basic residues typically found in PKC substrates. Interestingly, the amino acid residues around the major phosphorylation site for cAMP-dependent kinase in HePTP, S23, is quite similar (RRGSNVA), yet the two kinases maintain a high degree of fidelity even in vitro. We suspect that one of the minor in vitro phosphorylation sites, peptide 2, represents S23. However, using a phospho-S23 specific antibody, we recently showed that phorbol myristate acetate does not cause any change in the phosphorylation of this site in intact T cells (17). Conversely, PKA did not phosphorylate any site other than S23, even in vitro (25). The two phosphorylation sites also have opposite effects on HePTP: phosphorylation at S225 functionally activates HePTP, while phosphorylation at S23 mediates the inhibition of HePTP function by dissociating it from its substrates (25).
One may wonder why TCR signaling would promote both the activation and the inactivation of MAP kinases. Although a wealth of literature supports a vital role for Erk activation in growth signaling, there are also many papers that show that only a transient MAP kinase activation promotes proliferation. In contrast, a prolonged or excessive Erk response often results in differentiation (14), cell cycle arrest (2, 8, 22, 29), or even cell senescence (27, 31). Thus, TCR-induced activation of HePTP may serve to ensure that Erk and p38 kinases are activated only to a certain level and not as much as needed to trigger these growth-inhibitory effects of prolonged MAP kinase activation. In this context, it is interesting to note that PKC θ, which is intimately coupled with TCR signaling and T-cell expansion, differs from other PKC isozymes in that it does not cause the same Erk activation as that caused by other PKC isozymes but instead preferentially activates the JNK and NF-κB pathways (10). The activation of HePTP by PKC θ may explain this difference between PKC θ and other PKCs. This in turn suggests the possibility of fine-tuning the relative activation of different pathways downstream of the TCR and PKC θ. The increased expression of HePTP in T cells stimulated in the presence of interleukin-2 (32) may cause a shift in TCR-triggered signaling cascades by this mechanism. Similarly, the phosphorylation of HePTP by cAMP-dependent kinase (25) may also alter the balance between (i) Erk and p38 activation and (ii) JNK and NF-κB activation triggered by TCR-activated PKC θ.
In conclusion, our data imply that HePTP is not simply an off switch for MAP kinases but is also involved early in the cascade and serves to integrate cross talk between several signaling pathways, such as TCR-mediated PKC θ activation, cAMP-dependent protein kinase, and the regulation of the amplitude and spatial organization of MAP kinase responses.
Acknowledgments
This work was supported by grants AI48032, AI53585, AI55741, AI35603, and CA96949 from the National Institutes of Health.
REFERENCES
- 1.Adachi, M., M. Sekiya, M. Isobe, Y. Kumura, Z. Ogita, Y. Hinoda, K. Imai, and A. Yachi. 1992. Molecular cloning and chromosomal mapping of a human protein-tyrosine phosphatase LC-PTP. Biochem. Biophys. Res. Commun. 186:1607-1615. [DOI] [PubMed] [Google Scholar]
- 2.Alblas, J., R. Slager-Davidov, P. H. Steenbergh, J. S. Sussenbach, and B. van den Burg. 1998. The role of MAP kinase in TPA-mediated cell cycle arrest of human breast cancer cells. Oncogene 16:131-139. [DOI] [PubMed] [Google Scholar]
- 3.Alonso, A., S. Rahmouni, S. Williams, M. van Stipdonk, L. Jaroszewski, A. Godzik, R. T. Abraham, S. P. Schoenberger, and T. Mustelin. 2003. Tyrosine phosphorylation of VHR phosphatase by ZAP-70. Nat. Immunol. 4:44-48. [DOI] [PubMed] [Google Scholar]
- 4.Alonso, A., J. Sasin, N. Bottini, I. Friedberg, I. Friedberg, A. Osterman, A. Godzik, T. Hunter, J. E. Dixon, and T. Mustelin. 2004. Protein tyrosine phosphatases in the human genome. Cell 117:699-711. [DOI] [PubMed] [Google Scholar]
- 5.Baier, G., D. Telford, L. Giampa, K. M. Coggeshall, G. Baier-Bitterlich, N. Isakov, and A. Altman. 1993. Molecular cloning and characterization of PKCθ, a human novel member of the PKC gene family expressed predominately in hematopoietic cells. J. Biol. Chem. 268:4997-5004. [PubMed] [Google Scholar]
- 6.Baier-Bitterlich, G., F. Uberall, B. Bauer, F. Fresser, H. Watcher, H. Grunicke, G. Utermann, A. Altman, and G. Baier. 1996. Protein kinase C-θ isoenzyme selective stimulation of the transcription factor complex AP-1 in T lymphocytes. Mol. Cell. Biol. 16:1842-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi, K., Y. Tanaka, N. Courdonniere, S. Hong, K. Sugie, M. J. B. van Stipdonk, and A. Altman. 2001. Antigen-induced translocation of PKC-θ to membrane rafts is required for T cell activation. Nat. Immunol. 2:556-563. [DOI] [PubMed] [Google Scholar]
- 8.Chau, A. S.-S., and E. K. Shibuya. 1999. Inactivation of p42 mitogen-activated protein kinase is required for exit from M-phase after cyclin destruction. J. Biol. Chem. 274:32085-32090. [DOI] [PubMed] [Google Scholar]
- 9.Coudronniere, N., M. Villaba, N. Englund, and A. Altman. 2000. NF-κB activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-θ. Proc. Natl. Acad. Sci. USA 97:3394-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghaffari-Tabrizi, N., B. Bauer, A. Villunger, G. Baier-Bitterlich, A. Altman, G. Utermann, F. Uberall, and G. Baier. 1999. Protein kinase C θ, a selective upstream regulator of JNK/SAPK and IL-2 promoter activation in Jurkat T cells. Eur. J. Immunol. 29:132-142. [DOI] [PubMed] [Google Scholar]
- 11.Gronda, M., S. Arab, B. Iafrate, H. Suzuki, and B. Zanke. 2001. Hematopoietic protein tyrosine phosphatase suppresses extracellular stimulus-regulated kinase activation. Mol. Cell. Biol. 21:6851-6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, Y., H. Junru, R. Vita, B. Sun, H. Tabata, and A. Altman. 2004. SPAK kinase is a substrate and target of PKCθ in T-cell receptor-induced AP-1 activation pathway. EMBO J. 23:1112-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo, K., T. R. Hurley, and B. M. Sefton. 1990. Transfer of proteins to membranes facilitates both cyanogen bromide cleavage and two-dimensional proteolytic mapping. Oncogene 5:921-923. [PubMed] [Google Scholar]
- 14.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 15.Monks, C. R., H. Kupfer, I. Tamir, A. Barlow, and A. Kupfer. 1997. Selective modulation of protein kinase C-theta during T cell activation. Nature 385:83-86. [DOI] [PubMed] [Google Scholar]
- 16.Mustelin, T., T. Vang, and N. Bottini. 2005. Protein tyrosine phosphatases and the immune response. Nat. Rev. Immunol. 5:43-57. [DOI] [PubMed] [Google Scholar]
- 17.Nika, K., H. Huynh, S. Williams, S. Paul, N. Bottini, K. Taskén, P. J. Lombroso, and T. Mustelin. 2004. Haematopoietic protein tyrosine phosphatase (HePTP) phosphorylation by cAMP-dependent protein kinase in T cells: dynamics and subcellular location. Biochem. J. 378:335-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh-hora, M., M. Ogata, Y. Mori, M. Adachi, K. Imai, A. Kosugi, and T. Hamaoka. 1999. Direct suppression of TCR-mediated activation of extracellular signal-regulated kinase by leukocyte protein tyrosine phosphatase, a tyrosine-specific phosphatase. J. Immunol. 163:1282-1288. [PubMed] [Google Scholar]
- 19.Osada, S., K. Mizuno, T. C. Saido, K. Suzuki, T. Kuroki, and S. Ohno. 1992. A new member of the protein kinase C family, nPKCθ, predominantly expressed in skeletal muscle. Mol. Cell. Biol. 12:3930-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeifhofer, C., K. Kofler, T. Cruber, N. Ghaffari-Tabrizi, C. Lutz, K. Maly, M. Leitges, and G. Baier. 2003. Protein kinase Cθ affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 197:1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahmouni, S., T. Vang, A. Alonso, S. Williams, M. van Stipdonk, C. Soncini, M. Moutschen, S. P. Schoenberger, and T. Mustelin. 2005. Removal of C-terminal Src kinase from the immune synapse by a new binding protein. Mol. Cell. Biol. 25:2227-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sablina, A. A., P. M. Chumakov, A. J. Levine, and B. P. Kopnin. 2001. p53 activation in response to microtubule disruption is mediated by integrin-Erk signaling. Oncogene 20:899-909. [DOI] [PubMed] [Google Scholar]
- 23.Saxena, M., S. Williams, J. Brockdorff, J. Gilman, and T. Mustelin. 1999. Inhibition of T cell signaling by MAP kinase-targeted hematopoietic tyrosine phosphatase (HePTP). J. Biol. Chem. 274:11693-11700. [DOI] [PubMed] [Google Scholar]
- 24.Saxena, M., S. Williams, J. Gilman, and T. Mustelin. 1998. Negative regulation of T cell antigen receptor signaling by hematopoietic tyrosine phosphatase (HePTP). J. Biol. Chem. 273:15340-15344. [DOI] [PubMed] [Google Scholar]
- 25.Saxena, M., S. Williams, K. Taskén, and T. Mustelin. 1999. Crosstalk between cAMP-dependent kinase and MAP kinase through hematopoietic protein tyrosine phosphatase (HePTP). Nat. Cell Biol. 1:305-311. [DOI] [PubMed] [Google Scholar]
- 26.Sedwick, C. E., and A. Altman. 2004. Perspectives on PKCθ in T cell activation. Mol. Immunol. 41:675-686. [DOI] [PubMed] [Google Scholar]
- 27.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 28.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-θ is required for TCR-induced NF-B activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 29.Tang, D., D. Wu, A. Hirao, J. M. Lahti, L. Liu, B. Mazza, V. J. Kidd, T. W. Mak, and A. J. Ingram. 2002. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J. Biol. Chem. 277:12710-12717. [DOI] [PubMed] [Google Scholar]
- 30.Villalba, M., K. Bi, H. Junru, Y. Altman, P. Bushway, E. Reits, J. Neefjes, G. Baier, R. T. Abraham, and A. Altman. 2002. Translocation of PKC-θ in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J. Cell Biol. 157:253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, W., J. X. Chen, R. Liao, Q. Deng, J. J. Zhou, S. Huang, and P. Sun. 2002. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell. Biol. 22:3389-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanke, B., H. Suzuki, K. Kishihara, L. Mizzen, M. Minden, A. Pawson, and T. W. Mak. 1992. Cloning and expression of an inducible lymphoid-specific, protein tyrosine phosphatase (HePTPase). Eur. J. Immunol. 22:235-239. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, W., R. P. Trible, and L. E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9:239-246. [DOI] [PubMed] [Google Scholar]