Abstract
The hsp90 chaperoning pathway is a multiprotein system that is required for the production or activation of many cell regulatory proteins, including the progesterone receptor (PR). We report here the identity of GCUNC-45 as a novel modulator of PR chaperoning by hsp90. GCUNC-45, previously implicated in the activities of myosins, can interact in vivo and in vitro with both PR-A and PR-B and with hsp90. Overexpression and knockdown experiments show GCUNC-45 to be a positive factor in promoting PR function in the cell. GCUNC-45 binds to the ATP-binding domain of hsp90 to prevent the activation of its ATPase activity by the cochaperone Aha1. This effect limits PR chaperoning by hsp90, but this can be reversed by FKBP52, a cochaperone that is thought to act later in the pathway. These findings reveal a new cochaperone binding site near the N terminus of hsp90, add insight on the role of FKBP52, and identify GCUNC-45 as a novel regulator of the PR signaling pathway.
The activities of the progesterone receptor (PR) are intimately linked to its associations with other proteins that are essential for normal progesterone action. Unliganded PR is associated with a multifunctional complex of proteins which includes the heat shock proteins hsp70 and hsp90 plus several cochaperone proteins. This heterocomplex is responsible for correct assembly and folding of the PR as well as preventing its degradation (36). Recent studies indicate additional roles for molecular chaperones in receptor trafficking and in the maintenance of receptor function in the nucleus (11, 12). Hormone binding promotes conformational changes in the PR and its release from the chaperone complex. The resulting PR dimer is then able to associate with specific coactivators and general transcription factors as well as progestin response elements in the promoters of target genes (13, 19, 27, 44, 50).
Cell-free systems using the purified proteins have been of crucial importance in dissecting the ordered pathway that leads to the hormone-responsive state of the receptor (36, 39, 52). This hsp90-dependent chaperoning pathway is initiated by hsp40 and hsp70 binding to PR, followed by the binding of Hop-hsp90 to hsp70 (16). This intermediate complex is then modified by loss of hsp70 and Hop and recruitment of p23, resulting in a receptor able to bind hormone. In the cell, this last step also incorporates the cochaperone FKBP51, FKBP52, or Cyp40, but this step has eluded dissection in vitro.
Although the overall mechanism might be as described above, details and dynamics of this process are still unclear. Furthermore, several additional proteins have been discovered recently that interact with hsp90 or hsp70, such as Chip, Bag1, TPR2, and Aha1 (23, 31, 36, 39, 52). These are all likely to have important roles in some aspect of hsp70/hsp90 chaperoning to provide a more intricate and versatile process than that described in the current model. There are also a number of proteins that interact with the PR as transcriptional coregulators or to relate PR functions with other cell signaling pathways (25).
In a search for additional PR binding factors, we used yeast two-hybrid analysis and the PR hormone-binding domain as a target. This revealed the protein GCUNC-45 that is thought to act in the folding and functioning of myosins (37). Here we show that GCUNC-45 can bind directly and simultaneously to either PR isoform and to hsp90. The latter involves a tetratricopeptide repeat (TPR) domain of GCUNC-45 and has revealed a new site for TPR protein regulation in the N-terminal domain of hsp90. GCUNC-45 can inhibit the activation of hsp90's ATPase activity by Aha1 and progression of PR chaperoning in vitro. However, this effect is relieved by FKBP52, and GCUNC-45 clearly acts as a positive effector of PR activity in the cell.
MATERIALS AND METHODS
Yeast two-hybrid screen.
The hinge and hormone-binding domains of human PR were used as bait in a protein interaction screen with a HeLa cell expression library to identify binding partners that interact with PR in the presence of RU486. The approach has been described in detail previously (21). Interacting clones were detected by growth on histidine drop-out plates and were confirmed by β-galactosidase assay. Candidate cDNAs were purified and used to screen for full-length clones from a λgt-11 cDNA library.
GST pull-down.
The GCUNC-45 cDNA was subcloned into pGEX-4T-1 (Promega) and expressed in BL21-CodonPlus Escherichia coli (Stratagene, LaJolla, CA) as a C-terminal fusion to glutathione S-transferase (GST). The expressed GCUNC-45 GST fusion or GST alone bound to glutathione-Sepharose 4B (Amersham-Pharmacia Biotech) was incubated with HeLa whole-cell extracts containing transfected PR-A, PR-B, or glucocorticoid receptor (GR) for 2 h at 4°C. Where indicated, samples were incubated in the presence of 10 nM R5020, 100 nM RU486, 10 nM dexamethasone, or ethanol vehicle. In this instance, the PR- or GR-transfected HeLa cells had also been exposed to the same treatment for 1 h prior to harvesting and preparation of whole-cell protein extracts. Samples were washed twice with binding buffer (20 mM HEPES, pH 7.9, 60 mM NaCl, 0.1 mM EDTA, 6 mM MgCl2, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, 10% glycerol, complete protease inhibitor) containing 0.05% NP-40 and twice with binding buffer alone. Bound proteins were separated on denaturing 7.5% polyacrylamide gels and visualized by immunoblotting.
Cell culture and transcription assays.
The T47Dco and T47D-Y breast cancer cell lines have been described previously (18, 41). HeLa cells (American Type Culture Collection, Manassas, VA) were maintained as continuous monolayer cultures in modified Eagle's medium (MEM) supplemented with 6 ng/ml insulin (Invitrogen-Gibco BRL) and 5% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT) at 37°C and 5% CO2. Transient transfections were carried out in phenol red-free MEM containing 5% charcoal-stripped FBS, using a standard calcium phosphate precipitate-mediated method followed by 24 h of hormone treatment. The cells, 2 × 105/10-cm dish, were harvested and lysed, and β-galactosidase and luciferase activities were measured. The pA3-PRE2LUC construct was used to measure PR activation. This contained two copies of the PRE from the tyrosine aminotransferase gene promoter (22) cloned upstream of a firefly luciferase gene (51). The pCH110 (Amersham-Pharmacia) construct, constitutively expressing β-galactosidase, was used to normalize for transfection efficiency.
siRNA knockdown techniques.
HeLa cells were used containing a chloramphenicol acetyltransferase (CAT) reporter gene composed of two copies of the PRE (22) upstream of the CAT gene (42). Cells at 30 to 40% confluence in 6-well plates were transfected with 100 nM small interfering RNA (siRNA) duplexes to GCUNC-45, SRC-1, SRC-2, FKBP51, or FKBP52 using DharmaFect 1 reagent according to the manufacturer's suggestions (Dharmacon). The siRNA sequences for FKBP51 and FKBP52 were generously supplied by Michael Chinkers. The nonspecific control VIII was used to assess the non-sequence-specific effects. This control was chosen because it has the same GC content (52%) as that of the functional GCUNC-45 siRNA used and it lacks homology with known gene targets. Cells were harvested at 90 h after transfection and were treated with R5020 (0 to 250 nM; Perkin Elmer) for the last 18 h. Protein expression in the cell lysates was assessed by Western blotting from 25 μg protein, and the level of CAT protein was measured by enzyme-linked immunosorbent assay (Roche) using 50 μg protein, as instructed by the supplier (Roche).
GCUNC-45 expression and purification.
GCUNC-45 with a thrombin cleavage site followed by a 6× His tag at its C terminus was cloned in pET-23a vector using NdeI and NotI restriction sites. The cDNA was transformed into BL21-CodonPlus (DE3)-RIL cells that were induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside for 3 h at 30°C. Cells were suspended in 20 ml Buffer A (10 mM Tris, 50 mM KCl, 2.5% glycerol) supplemented with protease inhibitors [0.1 mM leupeptin, 0.1 mg/ml bacitracin, 77 μg/ml aprotinin, 1.5 μM of pepstatin, 1 mM 4-(2-aminoethyl) benzensulfonyl fluoride]. After sonication, the cell lysate was supplemented with 5 mM imidazole, 400 mM KCl and clarified by ultracentrifugation (100,000 × g) for 45 min. The supernatant was loaded onto a TALON metal affinity column (BD Bioscience) equilibrated with Buffer A supplemented with 5 mM imidazole and 400 mM KCl. Unbound protein was removed, and GCUNC-45 was eluted with Buffer A containing 100 mM imidazole. GCUNC-45 was further purified using a UnoQ column (Bio-Rad), a heparin-Sepharose column where GCUNC-45 was collected in the unbound protein fraction, and finally a Superdex-200 column (Pharmacia).
Protein purification.
Human hsp90 beta was overexpressed in SF9 cells and purified as described previously (48). Hsp70, Ydj1, Hop, and p23 were expressed and purified as described previously (24).
Monoclonal antibody production.
Purified GCUNC-45 was used to produce a mouse monoclonal immunoglobulin G antibody named AbS1, using conventional methods (47) with the assistance of Thomas Beito in the Mayo Antibody Core Facility.
Progesterone receptor complex assembly with purified proteins.
PR from chick oviduct was adsorbed onto PR22 antibody-protein A-Sepharose and was assembled into complexes as described by Kosano et al. (24). The incubation contained ∼0.05 μM PR plus 1.4 μM hsp70, 0.8 μM hsp90 dimer, 0.2 μM Ydj-1, and 0.08 μM Hop plus 2.6 μM p23. The samples also contained 20 mM Tris, pH 7.5, 5 mM MgCl2, 2 mM dithiothreitol, 0.01% NP-40, 50 mM KCl, and 5 mM ATP. After incubation for 7 min at 37°C, 0.1 μM [3H]progesterone (American Radiolabeled Chemicals, Inc., St. Louis, MO) was added for incubation on ice for 3 h. The complexes were then isolated on antibody resin and assessed for bound progesterone and for protein composition.
Site-directed mutagenesis.
The pET23a-hGCUNC-45 plasmid was used as a template for the generation of double-amino-acid substitution mutants in the TPR and NR regions using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). In the TPR1 motif, lysine 13 was changed to glutamic acid and alanine 20 was changed to aspartic acid. In TPR2, both equivalent residues were changed to glutamic acid. In the four NR motifs, residues 1 and 4 were changed from leucines to serines. All mutant clones were sequenced to confirm the mutations and the authenticity of the remaining GCUNC-45 sequence.
Cytosol preparation and immunoprecipitation.
Cells were lysed in Buffer A with protease inhibitors. After centrifugation (100,000 × g, 45 min), lysates were used for immunoprecipitations of PR complexes, hsp90 complexes, or GCUNC-45 using antibodies AB52 for PR, H9010 for human hsp90, and AbS1 for GCUNC-45. Immunoprecipitation reactions were carried out by incubation of immunoresins with the cytosol for 90 min in ice. After five 1-ml washes with binding buffer, bound proteins were eluted with sodium dodecyl sulfate (SDS) sample buffer, resolved by SDS-10% polyacrylamide gel electrophoresis (PAGE), and transferred to polyvinylidene difluoride membranes. Proteins were then detected by Western blotting with the appropriate antibody.
GCUNC-45 binding assay.
GCUNC-45 was covalently linked to Sepharose 4B as described by Cornillot et al. (8). Bovine serum albumin (BSA) was also immobilized and used as a control. To test the binding of hsp90 and its fragments to GCUNC-45 resin, we used 25 μl resin and 20 μg protein in a 200-μl final reaction volume of incubation buffer. Samples were incubated for 30 min at 37°C and washed four times with 1 ml buffer. Bound proteins were extracted with sample buffer and resolved by SDS-PAGE.
ATPase assay.
The ATPase activity of hsp90 was measured using [α-32P]ATP as described by Owen et al. (32).
Quantification of protein levels.
Arbitrary densitometric values (AU) for the indicated electrophoretic bands of protein were obtained by using IP Lab Gel 1.5e software (Signal Analytics). For evaluation of the binding of GCUNC-45 to hsp90, the mean AU values were converted to micromolar equivalents by normalizing them against 1-μg samples of purified GCUNC-45 run on the same gel. To obtain the values of free GCUNC-45, the amount of bound GCUNC-45 was subtracted from the initial amount added to the sample. The Scatchard plot was linear, with an equation of Y = −22.9 × 0.5951 corresponding to a Kd of 43 nm with the scatter illustrated by R2 = 0.9914.
RESULTS
Cloning and identification of a novel PR-binding protein.
A construct containing the hinge and hormone-binding domains of human PR was used as bait in a yeast two-hybrid screen in the presence of the mixed progestin antagonist RU486 (21). This revealed a number of antagonist-specific PR-interacting proteins, including a 1.2-kb clone with an open reading frame (ORF) encoding 394 amino acids of a human protein that interacted with the PR bait only when RU486 was present. This ORF contained two LXXLL motifs, referred to as NR boxes, in transcriptional coregulators and were shown to be responsible for binding to nuclear receptors (7, 15). A combination of cDNA library screening and 5′ rapid amplification of cDNA ends extended the clone to an N-terminal methionine codon that was downstream of an in-frame termination codon. This extension contained an additional two LXXLL motifs and three putative TPR (tetratricopeptide repeat) motifs shown previously to be involved in protein-protein interactions in a diverse range of molecules (2, 40, 43). The complete ORF predicts a protein of 944 amino acids with a molecular mass of 103 kDa (Fig. 1A). GenBank searches demonstrated that this PR-interacting protein was identical to a recently cloned protein, human GCUNC-45 (SMAP-1), accession number BC037992 (37). GCUNC-45 is a member of the UCS family of proteins that contains a common UCS (UNC-45/Cro1/She4p) domain and interacts with myosin (1, 20, 37, 54). Consistent with a previous report (37), Northern and Western blot analysis showed that the GCUNC-45 transcript and protein were ubiquitously expressed in numerous human tissues and in a variety of cultured cells (results not shown).
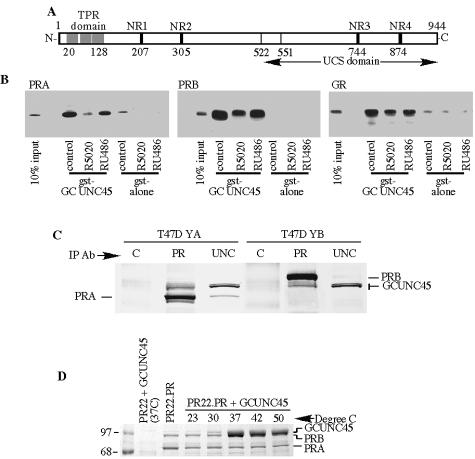
FIG. 1.
Identification of a novel PR-interacting protein, GCUNC-45. A. A schematic of the GCUNC-45 protein. The locations of three TPR sequences and four putative NR box motifs are indicated, as well as the UCS domain that is conserved in all UCS proteins (residues 522 to 944). In the protein sequence, the fragment identified in the yeast two-hybrid screen is residues 551 to 944. B. Interaction between GST-GCUNC-45 and PR-A, PR-B, and GR. HeLa cell lysates containing transfected PR-A, PR-B, or GR were incubated for 1 h at 4°C with glutathione-Sepharose containing bound GST-GCUNC-45 or GST alone in the presence of R5020, RU486, or dexamethasone, as indicated. Protein-bound Sepharose samples were pelleted and washed as indicated in Materials and Methods. Samples were resolved by SDS-PAGE, and PR and GR were visualized by immunoblotting. C. GCUNC-45 binds to PR in cell lysates and to purified PR. Endogenous GCUNC-45, PR-A, and PR-B were immunoprecipitated (IP) from T47D-YA or -YB cytosol using monoclonal antibodies to GCUNC-45 (UNC) and PR (PR). Mouse immunoglobulin G was used as a control (C). Proteins were detected by Western blotting. D. In vitro binding of GCUNC-45 to PR. Avian oviduct cytosol was incubated first with antibody (Ab) PR22-protein A-Sepharose resin to isolate PR. The stripped PR was then incubated with 20 μg of GCUNC-45 at the indicated temperatures. Resin-bound complexes were isolated by SDS-PAGE and stained with Coomassie blue.
To confirm the receptor-binding activity, full-length GCUNC-45 was expressed as a fusion protein with glutathione S-transferase (GST) and used in GST pull-down experiments with extracts from cells expressing PR-A, PR-B, or glucocorticoid receptor (GR). The GST-GCUNC-45 fusion protein interacted with both PR-A and PR-B as well as with GR in the absence of ligand (Fig. 1B). However, unlike the yeast two-hybrid screen, a decrease in receptor binding was seen when the mixed antagonist RU486 was present, and even weaker binding was detected in the presence of the receptor agonist R5020. To verify this interaction, we also tested a mammalian two-hybrid system in HeLa cells containing PR-B, GCUNC-45, and a luciferase end point and observed an interaction between PR-B and GCUNC-45 that was diminished by the addition of RU486 or R5020 (results not shown). The ligand-induced loss of interaction is inconsistent with the original yeast two-hybrid screen where interaction depended upon RU486. Apparently, the PR fragment used in the screen and the full-length PR used in Fig. 1 respond differently to ligand binding. In general, ligand binding by steroid receptors reduces their interaction with chaperones and cochaperones, and our results suggest a role for GCUNC-45 at the level of PR chaperoning.
GCUNC-45 binds directly to PR.
Immunoprecipitation was used to further study the binding of GCUNC-45 to both receptor forms A and B. Two variants of the human breast cancer cell line T47D were analyzed; one, YA, expressed exclusively the PR-A isoform, and the other, YB, expressed exclusively PR-B. Antibodies were used to immunoprecipitate PR-A, PR-B, or GCUNC-45 from cell lysates, and the proteins were resolved by SDS-PAGE and analyzed by Western blotting. As shown in Fig. 1C, GCUNC-45 was detected in complexes with each PR isoform. When an antibody to GCUNC-45 was used for immunoprecipitation, coprecipitation of PR-A was clearly observed, but that for PR-B was barely detectable with this technique. Thus, both receptor isoforms can be observed in complexes with GCUNC-45, although their abundance in these complexes may differ.
The results in Fig. 1C could not distinguish GCUNC-45-PR binding from indirect interactions of GCUNC-45 with other PR-associated proteins. To test for direct interaction, PR-A and PR-B were immunoprecipitated from chicken oviduct cytosol, stripped of associated proteins, and incubated with purified GCUNC-45. Chicken PR was used because preliminary studies showed that human and chicken PR interact in a similar manner with GCUNC-45 (data not shown), and methods for chicken PR isolation and analysis in vitro are much more clearly defined than those for human PR. Binding of GCUNC-45 to PR was observed by antibody pull-down and was very temperature dependent, with optimal binding at 37°C (Fig. 1D). The interaction was relatively inefficient at temperatures below 30°C, indicating that the binding may be accompanied by significant conformational changes in either GCUNC-45 or PR. In the experiment of Fig. 1B, GCUNC-45 binding was observed at 4°C, and higher temperatures were not feasible because of PR instability in the lysate. It is possible that the binding is facilitated by other associated factors in cell lysates. Note that the binding of GCUNC-45 is seen as an increased intensity of the bands in the PR-B region, since the two proteins have similar mobilities.
GCUNC-45 binds directly to hsp90 through its TPR domain.
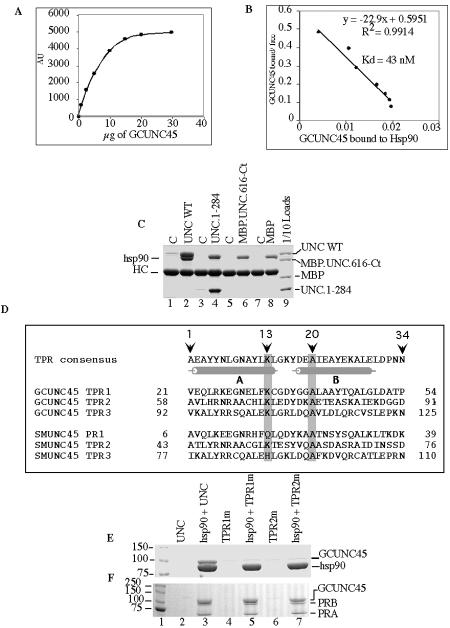
Previous studies have shown that the UNC-45 from Caenorhabditis elegans, which has 50% sequence identity with human GCUNC-45, binds to hsp90 through its TPR domain (1). To test the direct binding of GCUNC-45 to hsp90 in vitro, we used antibody pull-down experiments with purified hsp90 and GCUNC-45. As shown in Fig. 2A and B, GCUNC-45 binds to hsp90, and this is saturable and of high affinity (Kd = 43 nM). We next tested N-terminal and C-terminal fragments of GCUNC-45 for their binding to hsp90 (Fig. 2C). The binding of residues 1 to 284 (designated fragment 1-284), which contains the TPR domain, (lane 4) was similar to that of the full-length protein (lane 2), but no binding of fragment 616-944 to hsp90 was observed (lane 6). Attempts to test fragments containing only the TPR motifs failed because these were poorly expressed and unstable.
FIG. 2.
TPR motifs of GCUNC-45 are essential for hsp90 binding. A. Increasing amounts of GCUNC-45, in duplicate, were added to samples of immobilized hsp90 (2 μg) and incubated for 40 min at (30°C). The protein complexes were isolated and resolved by SDS-PAGE, and Coomassie-stained bands were quantified by densitometry. The plot of arbitrary units (AU) of bound GCUNC-45 is shown and represents the mean values for each concentration. B. Scatchard analysis of the results in panel A. C. The binding of 20 μg of wild-type GCUNC-45 (lane 2) or fragment 1-284 (lane 4) or 616-944 (lane 6) to 3 μg of hsp90 was tested. Fragment 616-944 was fused with maltose-binding protein (MBP), and 20 μg of MBP was used as a control (lanes 7 and 8). Complexes were isolated using antibody resin to hsp90 and antibody resin without hsp90 was a control (C). Proteins were resolved by SDS-PAGE and stained with Coomassie blue. Lane 9 shows 1 μg of each protein. D. Comparison of the TPR motifs of human GCUNC-45 and SMUNC-45. Mutated amino acids are shaded. The positions of helices A and B are indicated according to Das et al. (9). E. Coimmunoprecipitation of 2 μg of wild-type GCUNC-45 (lane 3) and TPR1 (lane 5) and TPR2 (lane 7) mutants with 5 μg of hsp90 bound to H9010 antibody. Lanes 2, 4, and 6 represent GCUNC-45 resin controls without hsp90. F. Coimmunoprecipitation of wild-type GCUNC-45 (lane 3) and TPR1 (lane 5) and TPR2 (lane 7) mutants with avian PR-A/PR-B bound to antibody resin. Lanes 2, 4, and 6 show the background without PR. WT, wild type; C, control.
TPR repeats are degenerate 34-amino-acid sequences with no position characterized by an invariant residue. However, certain amino acids are commonly observed in particular positions (9, 26). Alignment of TPR sequences (Fig. 2D) shows that lysine 13 and alanine 20, located in helixes A and B, respectively, are conserved within the UNC-45 TPR motifs. They are also conserved in most of the TPR motifs of the hsp90 cochaperones Hop, FKBP52, FKBP51, Cyp40, and PP5 (data not shown). Therefore, these two residues were mutated to glutamic and aspartic acids in the TPR1 or TPR2 repeat of GCUNC-45. Mutation of either TPR1 (TPR1m in Fig. 2E, lane 5) or TPR2 (TPR2m, lane 7) abrogated GCUNC-45-hsp90 interaction, indicating that the two motifs are essential for hsp90 binding. Importantly, the three-dimensional structures of both mutants appeared to be stable and similar to the wild type, because they (i) comigrated with wild-type GCUNC-45 in size exclusion chromatography analysis with no signs of aggregation (data not shown) and (ii) retained binding to PR (Fig. 2F, lanes 3, 5, and 7).
GCUNC-45 is a positive modulator of PR activity in vivo.
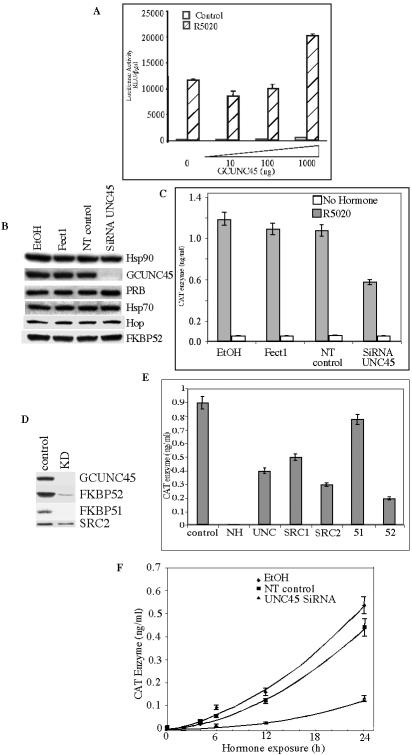
Because NR box-containing proteins have been shown to act as coactivators, we tested GCUNC-45 for its ability to regulate transcriptional activation by PR under conditions of ligand excess using a HeLa cell model. HeLa cells were used because they provided a model where the PR requirement for effects could be readily tested, and, in comparison to T47D cells, they were found to be more sensitive to PR activation and allowed more effective transfection experiments. When transfected into PR-negative HeLa cells with PR-B and a progestin-responsive luciferase reporter, reporter activity was weakly, but consistently, enhanced (∼2-fold) with increasing amounts of GCUNC-45 (Fig. 3A). This suggested that GCUNC-45 slightly increased the availability of PR to activate transcription on its reporter.
FIG. 3.
GCUNC-45 is required for optimal biological activity of PR. A. HeLa cells were transfected with 10 ng hPR1, 1 μg PRE2-TATAtk-LUC, and 250 ng pCH110 in the presence of increasing quantities of GCUNC-45 DNA as indicated. Luciferase and β-galactosidase activities were measured in harvested cell lysates after 16 to 20 h treatment of the cells with 10 nM R5020 (striped bars) or vehicle (open bars). Reporter activity is shown corrected for β-galactosidase activity. B to F. Cells were treated with ethanol vehicle, DharmaFECT1 reagent (FECT1), siRNA nontargeting control (NT Control), or siRNA specific to GCUNC-45 (siRNA UNC-45) SRC-1, SRC-2, FKBP51, or FKBP52. R5020 (250 nM) was added to the cells 72 h after transfection and incubated for an additional 18 h. Cells were harvested, protein levels were assessed by Western blot (B and D), and CAT enzyme production was measured by ELISA (C, E, and F). F. Time course of hormone treatment. R5020 (250 nM) was added 72 h after transfection with siRNA, and the cells were harvested at the indicated times and assessed for CAT enzyme production. EtOH, ethanol; KD, knockdown.
We next tested the effects of a GCUNC-45 knockdown using siRNA technology. The knockdown was very successful, with an almost complete loss of the protein 72 h after treatment of HeLa cells containing human PR-B and a CAT reporter gene (Fig. 3B). Protein levels of major chaperones and cochaperones of PR were not altered by the siRNA treatments. The loss of GCUNC-45 consistently resulted in reduced reporter gene induction by 40 to 70% (Fig. 3C). This effect was observed throughout the 24-h time course of hormone treatment (Fig. 3F) and was also observed with a range of R5020 concentrations, from 1 nM to 250 nM (data not shown). For comparison, Fig. 3D and E also show the effects of siRNA knockdown of the PR coactivators SRC-1 and SRC-2 and the hsp90 cochaperones FKBP51 and FKBP52. Little or no effect was observed with the loss of FKBP51, which is consistent with recent studies using knockout mice (49). However, reduction in PR activity was observed with the loss of the two coactivators and FKBP52, and these effects were comparable to those with GCUNC-45 loss. In all, these results show GCUNC-45 to be a biologically significant positive cofactor for modulating PR activity.
GCUNC-45 is a predominantly cytoplasmic protein.
To measure the cellular localization of GCUNC-45, a fusion protein construct with GFP (green fluorescent protein) linked to the N terminus of GCUNC-45 was expressed in T47D-YB cells expressing a high level of PR-B. The cells were treated with either R5020 or RU486 to see if it would alter localization of GCUNC-45. Figure 4 shows that GCUNC-45 is distributed throughout the cytoplasm and is predominant in the perinuclear region. Little protein is evident in the nucleus, and this distribution is unaffected by R5020 or RU486 treatment. PR was also visualized immunohistochemically and was localized mainly in the nuclei with some weak cytoplasmic staining in the untreated cells (results not shown). Thus, GCUNC-45 does not appear to colocalize with ligand-activated nuclear PR, which is consistent with a role in the chaperoning of PR.
FIG. 4.
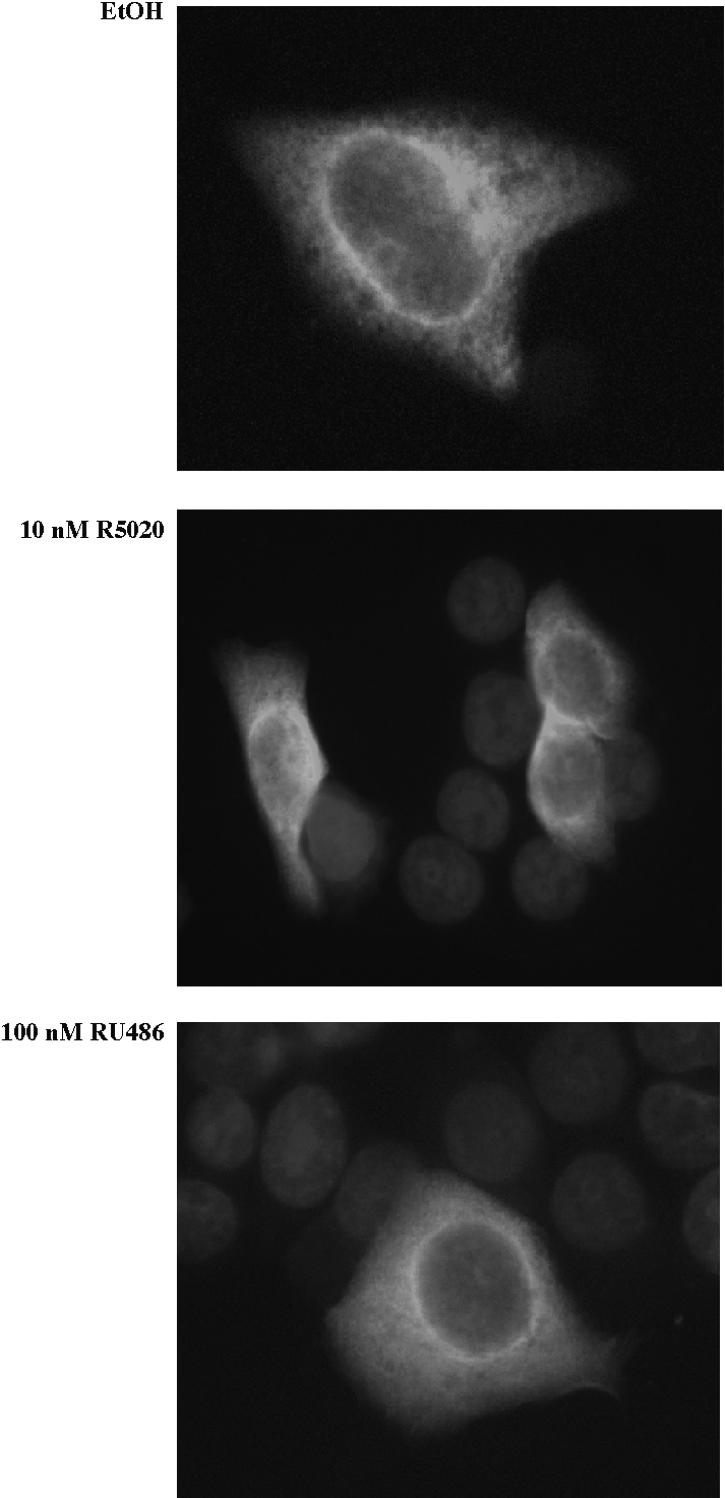
GFP-GCUNC-45 is a cytoplasmic protein. GFP-GCUNC-45 was transiently transfected into T47D-YB cells expressing high levels of PR-B. The cells were treated with R5020, RU486, or vehicle as indicated. Nuclei were stained blue using 4′,6′-diamidino-2-phenylindole. EtOH, ethanol.
GCUNC-45 binds to a novel TPR-binding site on hsp90.
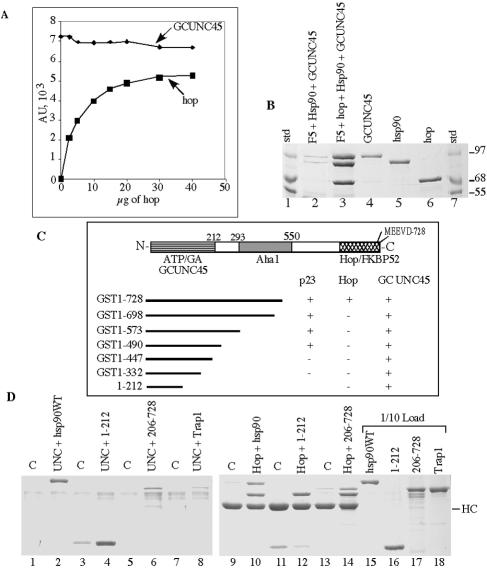
Since the above results suggest that GCUNC-45 functions in the cytoplasmic chaperoning process for PR, we investigated its interaction with hsp90 in greater detail. The binding of the cochaperone Hop to hsp90 involves a TPR domain of Hop and residues near the C terminus of hsp90, including the C-terminal sequence MEEVD (3, 43). Hop has been shown to compete with other TPR-containing proteins, such as FKBP52 and PP5, for binding to hsp90 (4, 33), indicating a common binding site for TPR proteins. However, when hsp90 was saturated with GCUNC-45 and increasing amounts of Hop were added, Hop was incorporated in the hsp90 complex without affecting the binding of GCUNC-45 (Fig. 5A). These unexpected findings show that hsp90 can bind simultaneously to both TPR proteins, as shown by the fact that the hsp90-Hop complex, immunoprecipitated using a Hop-specific antibody, contains bound GCUNC-45 (Fig. 5B, lane 3). Densitometry analysis of GCUNC-45, hsp90, and Hop in this complex showed mass ratios of about 1:1:1, suggesting two monomers of GCUNC-45 and one dimer of Hop bind per hsp90 dimer.
FIG. 5.
hsp90 contains a second TPR-binding site near the N terminus. A. Hsp90 (3 μg) was bound to antibody resin and then saturated with GCUNC-45 (30 μg). Excess GCUNC-45 was removed, and samples were incubated with increasing amounts of Hop. Complexes were isolated and analyzed by SDS-PAGE. Gels were stained with Coomassie blue and scanned. Densitometry values for bound GCUNC-45 and Hop are plotted against the amount of Hop added in the reaction mixture. B. Hop and hsp90 were incubated with Hop antibody resin (F5). Excess protein was removed by centrifugation, and the resin complex was incubated with 10 μg of GCUNC-45 and 5 mM ADP for 30 min at 37°C. The complex was isolated and resolved by SDS-PAGE (lane 3). Background binding of hsp90 and GCUNC-45 to antibody resin without Hop is shown in lane 2. Lanes 4 to 6 show 1 μg of each protein, and lanes 1 and 7 show the protein standards. C. Schematic representation of the hsp90 domain of interaction and deletion fragments used to map the TPR-binding site. The ability to bind p23, Hop, or GCUNC-45 was tested as indicated. D. Immobilized GCUNC-45 (lanes 2, 4, 6, and 8) or Hop antibody complex (lanes 10, 12, and 14) was used to pull down wild-type hsp90, hsp90 fragments (1-222 or 206-728), or TRAP1, respectively. Nonspecific binding was assessed using immobilized BSA (lanes 1, 3, 5, and 7) or antibody without Hop (lanes 9, 11, and 13). The protein loads are also shown in lanes 15 to 18 (1/10 of the total). HC indicates the heavy chain of Hop antibody (F5). C, control; WT, wild type.
The above results indicate that hsp90 contains a second TPR-binding site. However, since hsp90 is a dimer, it is also possible that one hsp90 monomer can bind a Hop dimer while the other binds GCUNC-45. For additional clarification, several C-terminal deletion fragments of hsp90 were used to map the binding site for GCUNC-45. Pull-down experiments of GCUNC-45 with these fragments demonstrated that hsp90's N-terminal fragment 1-332 was sufficient for the binding of GCUNC-45 (summarized in Fig. 5C). A smaller fragment containing only the ATP-binding domain of hsp90 (1-212) was also shown to bind GCUNC-45 (Fig. 5D, lane 4). However, only a very small amount of binding was observed with the hsp90 fragment 206-728 (lane 6) and with the mitochondrial hsp90 homolog TRAP-1 (lane 8). In a comparative experiment, the same fragments were also tested for Hop binding using a Hop antibody. Unlike GCUNC-45 binding, the fragment 1-212 did not bind Hop above the background (lanes 11 and 12); however, binding was observed with fragment 206-728 (lane 14). Further deletions in the ATP-binding domain were unsuccessful because of structural instability. These findings clearly establish a second TPR recognition site within the ATP-binding domain of hsp90 and provide a molecular basis for a model where hsp90 preferentially binds GCUNC-45 through this domain toward the N terminus and binds Hop via the more classic C-terminal TPR recognition motif.
GCUNC-45 regulates the ATPase activity of hsp90.
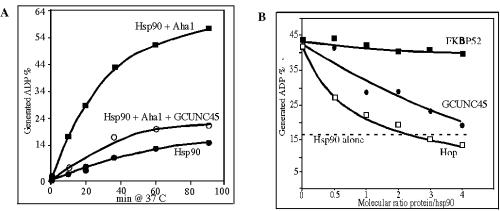
The properties of hsp90 are strongly influenced by factors that regulate its conformation. For example, binding of the cochaperone p23 to hsp90 requires bound ATP, and this is inhibited by ADP or the hsp90 inhibitor geldanamycin (48). However, Hop favors hsp90 that lacks ATP, and its binding blocks the binding and hydrolysis of ATP (17, 38). The weak ATPase activity of hsp90 is important for its biological functions (14, 28, 29). It is stimulated by the hsp90 cochaperone Aha1 (31) and inhibited by Hop (38). Using highly purified proteins, the possible influence of GCUNC-45 on the ATPase activity of hsp90 was tested and compared to that of Aha1 and to that of Hop. GCUNC-45 did not itself have any ATPase activity and had no effect on the ATPase activity of hsp90 alone (data not shown). However, GCUNC-45 blocked the stimulation of ATPase activity by Aha1 (Fig. 6A). This inhibition was dose dependent and nearly as effective as that of Hop (Fig. 6B). However, the immunophilin FKBP52, which binds to the C terminus of hsp90 through its TPR domain (33), showed no inhibitory activity.
FIG. 6.
GCUNC-45 inhibits ATP hydrolysis by hsp90. A. ATPase activity was measured with time for 2 μM hsp90 alone (filled circles), hsp90, and 2 μM Aha1 (filled squares) or hsp90, Aha1, and 5.5 μM GCUNC-45 (open circles). B. Comparison of the inhibitory effects of GCUNC-45, Hop, and FKBP52 on hsp90 activated with Aha1. Hsp90 (2 μM) was incubated with 2 μM of Aha1 for 1 h at 37°C in the presence of the indicated ratio of protein. The broken line indicates the level of ATPase activity of hsp90 without Aha1.
GCUNC-45 blocks the progression of hsp90 chaperoning but is counteracted by FKBP52.
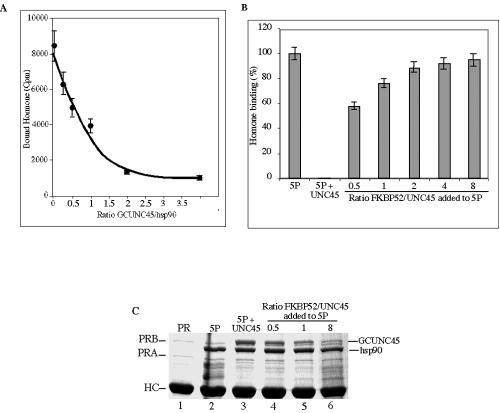
Seeking to understand the function of GCUNC-45 in the chaperoning pathway of PR, we monitored the effect of GCUNC-45 on the hormone-binding activity of PR using a cell-free assembly system (Fig. 7A). In this assay, five proteins (hsp90, hsp70, Hop, hsp40, and p23) function together to efficiently chaperone PR into its hormone-binding conformation (17, 24). GCUNC-45 strongly repressed the hormone-binding activity, and nearly complete inhibition was achieved at a molar ratio of 2:1 GCUNC-45/hsp90. This effect of GCUNC-45 requires its interaction with hsp90, since the TPR domain mutants have no influence on PR chaperoning or hormone binding (data not shown).
FIG. 7.
GCUNC-45 is incorporated into chaperone complexes and inhibits the hormone-binding activity of PR. PR was reconstituted as described in Materials and Methods using the five-protein system (5P). GCUNC-45 (5 to 80 μg) was added to the protein mixtures. The recovered hormone-binding activity of PR is shown in panel A. B and C. FKBP52 relieved the inhibitory effect of GCUNC-45 in a concentration-dependent manner. Shown is the hormone-binding activity (B) and the protein composition of PR complexes analyzed by SDS-PAGE (C). HC indicates the heavy chain of Hop antibody (F5).
The fact that PR complexes containing GCUNC-45 displayed reduced hormone-binding capacity indicates a role for GCUNC-45 in intermediate stages of PR processing and raises the possibility that another cochaperone that binds late in the chaperoning process opposes the effect of GCUNC-45. As TPR proteins, the large immunophilins were obvious candidates. Thus, we tested FKBP52, FKBP51, and Cyp40. FKBP51 and Cyp40 had some activity (data not shown), but FKBP52 efficiently stimulated the progression toward the hormone-binding state, as shown in Fig. 7B. The composition of PR complexes for the samples in this experiment was analyzed by SDS-PAGE (Fig. 7C). The addition of FKBP52 caused a dose-dependent reduction in the binding of GCUNC-45. Under the conditions of this experiment, the mobility of FKBP52 was very close to that of antibody heavy chain, and its binding could not be observed. These findings suggest a role for GCUNC-45 upstream of FKBP52 and shed light on the positive but poorly understood role of FKBP52 in the PR activation pathway.
DISCUSSION
In this study, we show that GCUNC-45 is a component of PR and hsp90 complexes isolated from cell lysates. Direct in vitro interactions of GCUNC-45 with both isoforms of PR and with hsp90 were shown, and a second, novel TPR-binding site on hsp90 was revealed. We also establish that GCUNC-45 is a positive cofactor for the cellular transcriptional activity of PR, and this appears to involve interplay between GCUNC-45 and the cochaperone FKBP52.
GCUNC-45 has only recently been identified as a protein closely related to SMUNC-45, and it appears to be unique to vertebrates (37). Because all other members of the UCS family have been implicated in the processing and functions of myosins, GCUNC-45 was proposed to be a myosin chaperone for non-muscle cells that would be involved in cytoskeletal functions (37). It has been shown to differ from SMUNC-45 in its tissue distribution and pattern of expression during embryogenesis. Antisense experiments indicated that a loss of GCUNC-45 suppressed myotube formation in muscle cells and reduced the rate of proliferation in non-muscle cells (37). Our results show that GCUNC-45 activities are not confined to myosin interaction. One can even consider the possibility that the myosin-binding activity might be important to the PR chaperone complex, perhaps as a means for localization or trafficking of the complex.
The presence of potential NR box motifs initially suggested that GCUNC-45 might be a transcriptional coregulator of PR. We have not excluded this possibility, but its primary localization in the cytoplasm and its association with hsp90 indicates a role in PR chaperoning. We have found that the LXXLL sequences are not essential for binding of GCUNC-45 to PR in vitro, although their mutation reduces binding somewhat (results not shown).
UNC-45 from Caenorhabditis elegans has been reported to interact with hsp90 through its TPR motifs (1). This interaction appeared to involve the C terminus of hsp90, based on competition by a peptide containing the C-terminal sequence MEEVD. Because of these results and the proposed mechanism for the interaction of hsp90 with UNC-45 (1) and other cochaperones (6, 43, 53), it seemed likely that the TPR recognition motif in the C terminus of hsp90 would be involved in the interaction with GCUNC-45. However, instead, a new site within the N-terminal ATP-binding domain of hsp90 was revealed. The existence of a second TPR-binding site on hsp90 opens new possibilities for identifying factors that might bind to this site to regulate hsp90 functions and may stimulate a reassessment of known chaperone-cochaperone interactions. Perhaps some cochaperones can recognize either TPR-binding site depending on the conformational state of hsp90. An intriguing parallel may exist with the complex of Hip-hsp70-Hop. Like hsp90, hsp70 has an EEVD sequence at its C terminus which appears to be part of the Hop-binding domain near the C terminus of hsp70 (5, 43). However, while the cochaperone Hip binds hsp70 through its TPR domain, it binds to a site, yet to be identified, in the N-terminal ATPase domain of hsp70 (10, 34).
GCUNC-45 has a positive role in the hsp90 chaperoning pathway for PR. In the cell, its overexpression caused only a small enhancement of PR transcriptional activity to induce a reporter gene, but the loss of endogenous GCUNC-45 resulted in a marked reduction of PR activity. This reduction of PR activity appears linked to the loss of GCUNC-45, since the protein levels of PR, hsp90, hsp70, Hop, and FKBP52 were not altered by the treatment. However, in simplified cell-free systems, GCUNC-45 inhibits the ATPase activity of hsp90 and blocks progression of PR chaperoning to its hormone-binding state. We have only observed the inhibition of ATPase activity when this activity was enhanced by the presence of Aha1. Aha1 has been shown to bind to the middle domain of hsp90 and is thought to facilitate repositioning of a catalytic loop of this domain that interacts with the γ phosphate of ATP bound in the N-terminal domain (28). Thus, the binding of GCUNC-45 to hsp90 may interfere with the conformational changes needed to bring the catalytic loop of the middle domain into contact with bound ATP, or it may block the binding of Aha1 to hsp90 even though the two proteins do not seem to share a common binding site.
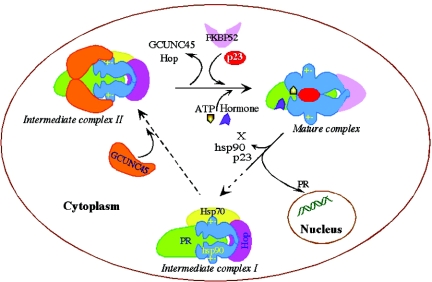
Since ATP binding and hydrolysis are essential to the activity of hsp90 (14, 29, 30), their inhibition explains the loss of proper PR chaperoning in the presence of GCUNC-45 as illustrated by a loss of hormone-binding activity. However, this appears not to be the case in the complete chaperoning system of the cell. The cochaperone Hop is also a potent inhibitor of the hsp90 ATPase activity, but this inhibition is confined to one step in the chaperoning process and is reversed by the association of Hop-hsp90 with hsp70 (17). As a result, Hop has a very positive effect on the chaperoning activity of hsp90. The cochaperone Cdc37 is essential for the chaperoning of many protein kinases, yet it inhibits the ATPase activity of hsp90 in vitro (45). It has been suggested that this inhibited state is more optimal for initial interaction of hsp90 with client proteins. In like manner, we propose that GCUNC-45 enters the chaperone complex at an intermediate stage where its inhibitory action serves a purpose, but that this inhibition is relieved by subsequent events that are missing from the in vitro system (Fig. 8). One such event is the binding of the immunophilin FKBP52 in the PR complex. This protein is observed in PR complexes from cell lysates and appears to interact with both PR and hsp90. Its function is still unclear, but it may be involved in modulating PR conformation or trafficking (35, 46). Future studies may reveal additional interactions between GCUNC-45 and other hsp90 cochaperones and clients.
FIG. 8.
Model for PR chaperoning showing a transient association of GCUNC-45 during the progression from the intermediate to the mature complex. FKBP52, and possibly other cochaperones that bind hsp90 through TPR domains, may displace GCUNC-45 from PR complexes as a response to specific signals.
Acknowledgments
We express our appreciation to Sherry Linander for assistance in the preparation of the manuscript. We also thank Bridget Stensgard, Becky Bruinsma, and Maria Thorson for technical assistance, Hany Abdel Hafiz for the transcription study, Thomas Beito in the Mayo Antibody Core Facility for assistance in antibody production, and Michael Chinkers for providing siRNA sequences for the knockdown of FKBP51 and FKBP52.
This work was supported by NIH grants RO1 DK 59284 and RO1 CA26869 and by the National Foundation for Cancer Research.
REFERENCES
- 1.Barral, J. M., A. H. Hutagalung, A. Brinker, F. U. Hartl, and H. F. Epstein. 2002. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science 295:669-671. [DOI] [PubMed] [Google Scholar]
- 2.Blatch, G. L., and M. Lassle. 1999. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. BioEssays 21:932-939. [DOI] [PubMed] [Google Scholar]
- 3.Brinker, A., C. Scheufler, F. von der Mülbe, B. Fleckenstein, C. Herrmann, G. Jung, I. Moarefi, and F. U. Hartl. 2002. Ligand discrimination by TPR domains. J. Biol. Chem. 277:19265-19275. [DOI] [PubMed] [Google Scholar]
- 4.Carrello, A., E. Ingley, R. F. Minchin, S. Tsai, and T. Ratajczak. 1999. The common tetratricopeptide repeat acceptor site for steroid receptor-associated immunophilins and Hop is located in the dimerization domain of hsp90. J. Biol. Chem. 274:2682-2689. [DOI] [PubMed] [Google Scholar]
- 5.Carrigan, P. E., G. M. Nelson, P. J. Roberts, J. N. Stoffer, D. L. Riggs, and D. F. Smith. 2004. Multiple domains of the co-chaperone Hop are important for Hsp70 binding. J. Biol. Chem. 279:16185-16193. [DOI] [PubMed] [Google Scholar]
- 6.Chen, S., W. P. Sullivan, D. O. Toft, and D. F. Smith. 1998. Differential interactions of p23 and the TPR-containing proteins Hop, Cyp40, FKBP52 and FKBP51 with Hsp90 mutants. Cell Stress Chaperones 3:118-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collingwood, T. N., F. D. Urnov, and A. P. Wolffe. 1999. Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription. J. Mol. Endocrinol. 23:255-275. [DOI] [PubMed] [Google Scholar]
- 8.Cornillot, J. D., M. Caron, R. Joubert-Caron, and D. Bladier. 1992. Use of an immobilized human endogenous lectin for the purification of complementary ligands. Int. J. Biochem. 24:1585-1589. [DOI] [PubMed] [Google Scholar]
- 9.Das, A. K., P. T. W. Cohen, and D. Barford. 1998. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 17:1192-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desmond, J., J. Lüders, and J. Höhfeld. 1998. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol. Cell. Biol. 18:2023-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbi, C., D. A. Walker, G. Romero, W. P. Sullivan, D. O. Toft, H. L. Hager, and D. B. DeFranco. 2004. Molecular chaperones function as steroid receptor nuclear mobility factors. Proc. Natl. Acad. Sci. USA 101:2876-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman, B. C., and K. R. Yamamoto. 2002. Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 296:2232-2235. [DOI] [PubMed] [Google Scholar]
- 13.Glass, C. K., D. W. Rose, and M. G. Rosenfeld. 1997. Nuclear receptor coactivators. Curr. Opin. Cell Biol. 9:222-232. [DOI] [PubMed] [Google Scholar]
- 14.Grenert, J. P., B. D. Johnson, and D. O. Toft. 1999. The importance of ATP binding and hydrolysis by Hsp90 in formation and function of protein heterocomplexes. J. Biol. Chem. 274:17525-17533. [DOI] [PubMed] [Google Scholar]
- 15.Heery, D. M., E. Kalkhoven, S. Hoare, and M. G. Parker. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733-736. [DOI] [PubMed] [Google Scholar]
- 16.Hernández, M. P., A. Chadli, and D. O. Toft. 2002. HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J. Biol. Chem. 277:11873-11881. [DOI] [PubMed] [Google Scholar]
- 17.Hernández, M. P., W. P. Sullivan, and D. O. Toft. 2002. The assembly and intermolecular properties of the hsp70-Hop-hsp90 molecular chaperone complex. J. Biol. Chem. 277:38294-38304. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz, K. B., and G. R. Freidenberg. 1985. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 45:167-173. [PubMed] [Google Scholar]
- 19.Horwitz, K. B., T. A. Jackson, D. L. Bain, J. K. Richer, G. C. Takimoto, and L. Tung. 1996. Nuclear receptor coactivators and corepressors. Mol. Endocrinol. 10:1167-1177. [DOI] [PubMed] [Google Scholar]
- 20.Hutagalung, A. H., M. L. Landsverk, M. G. Price, and H. F. Epstein. 2002. The UCS family of myosin chaperones. J. Cell Sci. 115:3983-3990. [DOI] [PubMed] [Google Scholar]
- 21.Jackson, T. A., J. K. Richer, D. L. Bain, G. S. Takimoto, L. Tung, and K. B. Horwitz. 1997. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol. Endocrinol. 11:693-705. [DOI] [PubMed] [Google Scholar]
- 22.Jantzen, H.-M., U. Strahle, B. Gloss, F. Stewart, W. Schmid, M. Boshart, R. Miksicek, and G. Schutz. 1987. Cooperaativity of glucocorticoid response elements located far upstream of the tyrosine aminotransferase gene. Cell 49:29-38. [DOI] [PubMed] [Google Scholar]
- 23.King, F. W., A. Wasrzynow, J. Höhfeld, and M. Zylicz. 2001. Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J. 20:6297-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosano, H., B. Stensgard, M. C. Charlesworth, N. McMahon, and D. O. Toft. 1998. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J. Biol. Chem. 273:32973-32979. [DOI] [PubMed] [Google Scholar]
- 25.Lange, C. A., J. K. Richer, and K. B. Horwitz. 1999. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol. Endocrinol. 13:829-836. [DOI] [PubMed] [Google Scholar]
- 26.Lapouge, K., S. J. M. Smith, P. A. Walker, S. J. Gamblin, S. J. Smerdon, and K. Rittinger. 2000. Structure of the TPR domain of p67phox in complex with Rac-GTP. Mol. Cell 6:899-907. [DOI] [PubMed] [Google Scholar]
- 27.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 28.Meyer, P., C. Prodromou, C. Liao, B. Hu, S. M. Roe, C. K. Vaughan, I. Vlasic, B. Panaretou, P. W. Piper, and L. H. Pearl. 2004. Structural basis for recruitment of the ATPase activator Aha1 to the hsp90 chaperone machinery. EMBO J. 23:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermann, W. M. J., H. Sondermann, A. A. Russo, N. P. Pavletich, and F. U. Hartl. 1998. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J. Cell Biol. 143:901-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panaretou, B., C. Prodromou, S. M. Roe, R. O'Brien, J. E. Ladbury, P. W. Piper, and L. H. Pearl. 1998. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 17:4829-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panaretou, B., G. Siligardi, P. Meyer, A. Maloney, J. K. Sullivan, S. Singh, S. H. Millson, P. A. Clarke, S. Naaby-Hansen, R. Stein, R., Cramer, M. Mollapour, P. Workman, P. W. Piper, L. H. Pearl, and C. Prodromou. 2002. Activation of the ATPase activity of Hsp90 by the stress-regulated cochaperone Aha1. Mol. Cell 10:307-1318. [DOI] [PubMed] [Google Scholar]
- 32.Owen, B. A. L., W. P. Sullivan, S. J. Felts, and D. O. Toft. 2002. Regulation of heat shock protein 90 ATPase activity by sequences in the carboxyl terminus. J. Biol. Chem. 277:7086-7091. [DOI] [PubMed] [Google Scholar]
- 33.Owens-Grillo, J. K., M. J. Czar, K. A. Huchison, K. Hoffmann, G. H. Perdew, and W. B. Pratt. 1996. A model of protein targeting mediated by immunophilins and other proteins that bind to hsp90 via tetratricopeptide repeat domains. J. Biol. Chem. 271:13468-13475. [DOI] [PubMed] [Google Scholar]
- 34.Prapapanich, V., S. Chen, E. J. Toran, R. A. Rimerman, and D. F. Smith. 1996. Mutational analysis of the hsp70-interacting protein Hip. Mol. Cell. Biol. 16:6200-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pratt, W. P., M. D. Galigniana, J. M. Harrell, and D. B. DeFranco. 2004. Role of hsp90 and the hsp90-binding immunophilins in signalling protein movements. Cell. Signal. 16:857-872. [DOI] [PubMed] [Google Scholar]
- 36.Pratt, W. P., and D. O. Toft. 2003. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 228:111-133. [DOI] [PubMed] [Google Scholar]
- 37.Price, M. G., M. L. Landsverk, J. M. Barral, and H. F. Epstein. 2002. Two mammalian UNC-45 isoforms are related to distinct cytoskeletal and muscle-specific functions. J. Cell Sci. 115:4013-4023. [DOI] [PubMed] [Google Scholar]
- 38.Prodromou, C., G. Siligardi, R. O'Brien, D. N. Woolfson, L. Regan, B. Panaretou, J. E. Ladbury, P. W. Piper, and L. H. Pearl. 1999. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 18:754-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richter, K., and J. Buchner. 2001. Hsp90: chaperoning signal transduction. J. Cell Physiol. 188:281-290. [DOI] [PubMed] [Google Scholar]
- 40.Russell, L. C., S. R. Whitt, M.-S. Chen, and M. Chinkers. 1999. Identification of conserved residues required for the binding of a tetracopeptide repeat domain to heat shock protein 90. J. Biol. Chem. 174:20060-20063. [DOI] [PubMed] [Google Scholar]
- 41.Sartorius, C. A., S. D. Groshong, L. A. Miller, R. L. Powell, L. Tung, G. S. Takimoto, and K. B. Horwitz. 1994. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 54:868-3877. [PubMed] [Google Scholar]
- 42.Sartorius, C. A., M. Y. Melville, A. R. Hovland, L. Tung, G. S. Takimoto, and K. B. Horwitz. 1994. A third transactivation function (AF3) of human progesterone receptors located in the unique N-terminal segment of the B-isoform. Mol. Endocrinol. 8:1347-1360. [DOI] [PubMed] [Google Scholar]
- 43.Scheufler, C., A. Brinker, G. Bourenkov, S. Pegoraro, L. Moroder, H. Bartunik, F. U. Hartl, and I. Moarefi. 2000. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 101:99-210. [DOI] [PubMed] [Google Scholar]
- 44.Shibata, H., T. E. Spencer, S. A. Onate, G. Jenster, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1997. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog. Horm. Res. 52:141-164. [PubMed] [Google Scholar]
- 45.Siligardi, G., B. Panaretou, P. Meyer, S. Singh, D. N. Woolfson, P. W. Piper, L. H. Pearl, and C. Prodromou. 2002. Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37. J. Biol. Chem. 277:20151-20159. [DOI] [PubMed] [Google Scholar]
- 46.Smith, D. F. 2004. Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones 9:109-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan, W. P., T. G. Beito, J. Proper, C. J. Krco, and D. O. Toft. 1986. Preparation of monoclonal antibodies to the avian progesterone receptor. Endocrinology 119:1549-15557. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan, W. P., B. Stensgard, G. Caucutt, B. Bartha, N. McMahon, E. S. Alnemri, G. Litwack, and D. O. Toft. 1997. Nucleotides and two functional states of hsp90. J. Biol. Chem. 272:8007-8012. [DOI] [PubMed] [Google Scholar]
- 49.Tranguch, S., J. Cheung-Flynn, T. Daikoku, V. Prapapanich, M. B. Cox, H. Xie, H. Wang, S. K. Das, D. F. Smith, and S. K. Dey. 2005. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. USA 102:14326-14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai, M.-J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 51.Wood, W. M., M. Y. Kao, D. F. Gordon, and E. C. Ridgway. 1989. Thyroid hormone regulates the mouse thyrotropin beta subunit gene promoter in transfected primary thyrotropes. J. Biol. Chem. 264:14840-14847. [PubMed] [Google Scholar]
- 52.Young, J. C., I. Moarefi, and F. U. Hartl. 2001. Hsp90: a specialized but essential protein-folding tool. J. Cell Biol. 154:267-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Young, J. C., M. J. Obermann, and F. U. Hartl. 1998. Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J. Biol. Chem. 273:18007-18010. [DOI] [PubMed] [Google Scholar]
- 54.Yu, Q., and S. I. Bernstein. 2003. UCS proteins: managing the myosin motor. Curr. Biol. 13:R525-R527. [DOI] [PubMed] [Google Scholar]