Abstract
Elastic fibers provide tissues with elasticity which is critical to the function of arteries, lungs, skin, and other dynamic organs. Loss of elasticity is a major contributing factor in aging and diseases. However, the mechanism of elastic fiber development and assembly is poorly understood. Here, we show that lack of fibulin-4, an extracellular matrix molecule, abolishes elastogenesis. fibulin-4−/− mice generated by gene targeting exhibited severe lung and vascular defects including emphysema, artery tortuosity, irregularity, aneurysm, rupture, and resulting hemorrhages. All the homozygous mice died perinatally. The earliest abnormality noted was a uniformly narrowing of the descending aorta in fibulin-4−/− embryos at embryonic day 12.5 (E12.5). Aorta tortuosity and irregularity became noticeable at E15.5. Histological analysis demonstrated that fibulin-4−/− mice do not develop intact elastic fibers but contain irregular elastin aggregates. Electron microscopy revealed that the elastin aggregates are highly unusual in that they contain evenly distributed rod-like filaments, in contrast to the amorphous appearance of normal elastic fibers. Desmosine analysis indicated that elastin cross-links in fibulin-4−/− tissues were largely diminished. However, expression of tropoelastin or lysyl oxidase mRNA was unaffected in fibulin-4−/− mice. In addition, fibulin-4 strongly interacts with tropoelastin and colocalizes with elastic fibers in culture. These results demonstrate that fibulin-4 plays an irreplaceable role in elastogenesis.
Elastic fibers with morphologically distinct architectures are present in the extracellular matrix (ECM) to accommodate elastic requirements and mechanical stresses imposed on different tissues. They are particularly abundant in elastic tissues such as large blood vessels, lung, and skin. Loss of elasticity is a major contributing factor in aging and a myriad of pathological conditions including emphysema, artery diseases, and cutis laxa (39, 41, 44). Elastic fibers undergo irreversible structural and compositional changes with age and in some pathological conditions (41). Regardless of morphology, all elastic fibers consist of cross-linked elastin, fibrillin-rich microfibrils, and several associated molecules (23, 37, 38, 46). Elastin endows the fiber with the characteristic property of elastic recoil. It is chemically inert, extremely hydrophobic, and insoluble under most conditions. Monomeric elastin, called tropoelastin, is secreted from the cell as a soluble protein. Isolated and purified tropoelastin has been shown to exhibit a great tendency to aggregate (coacervation) in physiological solution and at temperatures in the physiological range, giving rise to supramolecular structures very similar to those found in natural elastic fibers (4, 5, 11). This self-aggregation property of tropoelastin is thought to contribute to elastic fiber assembly in vivo. However, self-aggregation alone is insufficient to explain the efficiency of the assembly process and the variable form of elastic fibers in different tissues.
The formation of elastic fibers has been proposed to require the deposition of tropoelastin on a preexisting scaffold, cross-linking of tropoelastin monomers by lysyl oxidase (LOX) family enzymes, and organization of the resulting insoluble elastin matrix into mature fibers (37). Fibrillin-rich microfibrils are thought to provide the scaffold for the deposition of elastin. Unexpectedly, normal elastic fiber assembly was found to occur in fibrillin-1 or fibrillin-2 mutant mice (2, 9, 42, 43). Therefore, the molecular mechanism of elastic fiber assembly remains elusive.
A significant insight into elastogenesis comes from two recent studies of fibulin-5−/− mice. These mice exhibit disrupted and disorganized elastic fibers throughout the body, indicating that fibulin-5 (also known as DANCE or EVEC) plays an important role in elastic fiber formation (40, 56). fibulin-5−/− mice grow to adulthood without lethality but have loose skin, vascular abnormalities, and emphysematous lungs. Fibulin-5 has an RGD motif and interacts with cell surface integrins and elastin. Thus, it has been proposed to promote elastic fiber formation by linking elastic fibers to cells (40, 56).
Fibulin-5 belongs to the fibulin family of six known ECM proteins that share tandem arrays of calcium-binding epidermal growth factor domains and a characteristic carboxyl-terminal fibulin domain (1, 10, 15, 52). Although little is known about the functions of fibulins, mutations of individual members have been associated with several diseases. A single mutation of an arginine to trypotophan in fibulin-3 (also known as EFEMP1, S1-5, or FBNL) causes an inherited macular degenerative disease termed malattia leventinese or Doyne honeycomb retinal dystrophy (51). Missense variations in other fibulins have been detected in patients with age-related macular degeneration (47, 50), the most common cause of incurable blindness (6). Mutations in fibulin-5 have also been found in some cutis laxa patients (29, 32). So far, fibulin-5 is the only fibulin reported to be necessary for elastogenesis, whereas fibulin-1-null mice are reported to die perinatally as a result of hemorrhages, due to defects associated with capillary endothelial cells (25). Knockout mice for other fibulins have not been reported. Among the fibulins, fibulin-3, fibulin-4 (also known as EFEMP2, MBP1, H411, or UPH1), and fibulin-5 share highest homology with each other. These three fibulins are the smallest members of the family, share >50% amino acid identity, and are nearly identical in their structural organization (1, 10, 15, 52). Despite this homology, fibulin-5 deficiency is not compensated for by fibulin-3 or -4, suggesting that fibulin-3, -4, and -5 are not functionally redundant.
The function of fibulin-4 is poorly understood. Several studies have consistently found that fibulin-4 promotes cell growth, exhibits oncogenic properties, and is upregulated in tumor tissues (14, 16, 19). fibulin-4 mRNA has been shown to be widely expressed in various tissues throughout the body (14, 16, 18). High protein levels are present in blood vessel walls (18). During development, fibulin-4 mRNA is expressed in mouse embryos as early as embryonic day 7 (E7) (14). In this study, we investigated the biological role of fibulin-4 through targeted gene inactivation in mice. Remarkably, mice lacking fibulin-4 do not form elastic fibers, with resulting severe vascular and lung defects; they die perinatally. These results demonstrate that fibulin-4 plays an irreplaceable role in elastic fiber formation.
MATERIALS AND METHODS
fibulin-4−/− mouse generation, Southern blot analysis, and RT-PCR.
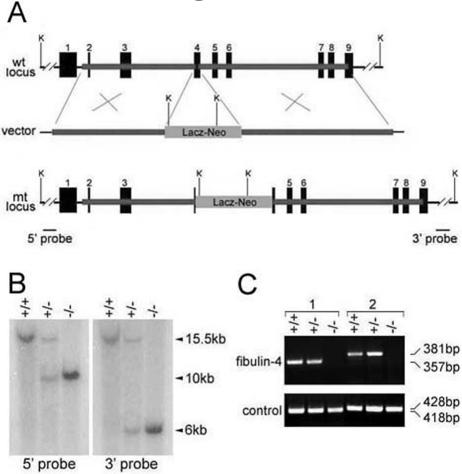
The targeting vector was constructed using 2.5-kb (5′) and 3-kb (3′) mouse fibulin-4 genomic DNA fragments as homology arms. The two arms flanked a promoterless lacZ and a neomycin-resistant gene cassette (lacZ-neo). Homologous recombination in mouse embryonic stem cells resulted in the insertion of the lacZ-neo cassette into exon 4 of the mouse fibulin-4 locus. Germ line-transmitting chimeric mice generated from the targeted embryonic stem cells were bred with C57BL/6 mice to produce F1 fibulin-4+/− mice (Deltagen). Intercrossing of heterozygous F1 mice generated F2 fibulin-4−/− mice. Southern blot analysis was performed for identification of homologous recombinants. Genomic DNA was extracted using a DNA purification kit (Gentra) from mouse tail biopsy samples, digested with KpnI, separated on a 0.5% SeaKem Gold agarose (Cambrex) gel, and transferred to a Hybond-N+ nylon membrane (Amersham) by capillary blotting. The membrane was hybridized with a 5′ or 3′ external probe that is outside of the homologous arm regions. The probes were labeled with 32P through random priming using the Megaprime DNA Labeling system (Amersham). The labeled probes were purified using ProbeQuant G-50 Micro Columns (Amersham). Hybridization was performed using the MiracleHyb hybridization solution (Stratagene) according to the manufacturer's instructions. Reverse transcription-PCR (RT-PCR) was performed as described previously (33) to confirm the absence of fibulin-4 expression in homozygous mice. Total RNA was isolated from wild-type, fibulin-4+/−, and fibulin-4−/− mice at postnatal day 1 (P1). Either a 5′ or 3′ primer set corresponding to mouse fibulin-4 cDNA sequence was used in the PCRs. The 5′ primer set (5′-GGCCAGATCTATGCTCCCTTTTGCCTCCTG-3′ and 5′-ACATCCACACAGCTCTCCTG-3′) generates a 381-bp fragment, and the 3′ primer set (5′-TGTCGAGAGCAGCCTTCATC-3′ and 5′-GGCCGTCGACTCAGAAGGTATAGGCTCCCAC-3′) generates a 357-bp fragment. fibulin-3 primers were used in the positive control.
Morphological and histological analyses.
Timed pregnant mice were sacrificed by CO2 asphyxiation for collection of embryos at E9.5 to E18.5. P1 pups were sacrificed by cervical dislocation. Sacrificed embryos or P1 mice were immobilized on agarose plates on their back. The ventral side of the embryos was gently dissected away to expose the aorta and other large arteries. Blood vessels were photographed with a dissecting microscope equipped with a charge-coupled device camera.
For histology, whole embryos and tissues dissected from newborn pups were fixed in Bouin's fixative or 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2), dehydrated, and embedded in paraffin. Ten-micrometer sections were stained with hematoxylin and eosin or for elastin using elastin van Gieson stain (Sigma).
Transmission electron microscopy.
Dissected tissues were fixed overnight in 3% glutaraldehyde in 0.144 M cacodylate buffer, pH 7.2. One-micrometer sections of plastic-embedded samples were cut and stained with methylene blue to examine the integrity of the tissue. Thin sections were cut on a Reichert Ultracut microtome, counterstained with uranyl acetate, and examined and photographed with a Philips CM-12S electron microscope.
Tissue desmosine assay.
Tissue samples from P1 mice were collected from the thoracic aorta and lung. The tissues were hydrolyzed in 6 N HCl at 100°C for 24 h, evaporated to dryness, and redissolved in water. Desmosine was quantified by radioimmunoassay as previously described (49), and hydroxyproline was determined by amino acid analysis.
Northern blot analysis.
Eight micrograms of total RNA isolated from various tissues of wild-type, fibulin-4+/−, and fibulin-4−/− mice at P1 was separated on a 1% formaldehyde-agarose gel and transferred to a Hybond-XL nylon membrane (Amersham). Prehybridization was performed by incubating the membrane for 4 h at 65°C in the hybridization buffer without the probe. The membrane was hybridized with a 32P-labeled probe using the Megaprime DNA Labeling system (Amersham). The fibulin-4 probe is a 357-bp mouse fibulin-4 cDNA fragment amplified by RT-PCR, the tropoelastin probe is a 3.6-kb mouse tropoelastin cDNA fragment containing the entire coding sequence released by SalI/NotI digestion from vector pCMV-SPORT6-mELN (Open Biosystems), and the LOX probe is a 4-kb mouse Lox cDNA fragment released by SalI-NotI digestion from vector pCMV-SPORT6-mLOX (Open Biosystems). After the fibulin-4 probe was used, the same membranes were stripped by being boiled for 30 min in 0.1% sodium dodecyl sulfate (SDS), washed, and hybridized with other probes. A mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was used as a control. A 1.2-kb GAPDH fragment was generated by RT-PCR using the primers 5′-CCGCATCTTCTTGTGCAGTGCCA-3′ and 5′-CGAACTTTATTGATGGTATTCAAGAGA.
Production of MAbs against human fibulin-4.
cDNA encoding human fibulin-4 (OriGene Technologies) without signal peptide was cloned into vector pGEX-4T-2 (Pharmacia) to generate fibulin-4 fused with glutathione S-transferase (GST). GST-fibulin-4 or GST was purified as described previously (36). Four mice were immunized with GST-fibulin-4. Sera (polyclonal antibodies) from all the mice were characterized with both GST fusion proteins and lysates of transfected 293T cells expressing recombinant fibulin-4. Hybridomas were derived from one of these mice. Several different clones producing monoclonal antibodies (MAbs) against fibulin-4 were identified by enzyme-linked immunosorbent assay. Clones 7B9 (immunoglobulin G2a [IgG2a]) and 11E2 (IgG2b) are of high specificity and titer.
Transfection, immunoprecipitation, and immunoblotting.
293T cells were transfected with a control plasmid, pCMV6-XL4-fb4 (human fibulin-4 cDNA), pCMV6-XL6-heln (human tropoelastin cDNA obtained from Origene), or both pCMV6-XL4-fb4 and pCMV6-XL6-heln with Lipofectamine (Invitrogen). At 48 h after transfection, cells were lysed. Immunoprecipitation and immunoblotting using antibodies against fibulin-4 and human elastin (Elastin Products Company) were performed as previously described (34) with modifications. To avoid the interference of the IgG heavy chain in interpreting results of immunoblotting following immunoprecipitation by the same or similar antibodies, immunoprecipitation was performed with antibodies covalently coupled to protein A-Sepharose. Anti-fibulin-4 MAb 7B9 or a rabbit anti-human tropoelastin polyclonal antibody (Elastin Products Company) was cross-linked to protein A-Sepharose CL-4B (Pharmacia) using dimethylpimelimidate (Sigma) as previously described (35). For each immunoprecipitation, cell lysate containing 200 μg of total protein was diluted to 1 ml with lysis buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 10% glycerol, 0.5% NP-40, 0.5% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and a 1:100 dilution of protease inhibitor mixture III (Calbiochem). A total of 25 μl of antibody-protein A beads was incubated with the cell lysate for 4 h at 4°C. The immunoprecipitates were washed and resuspended in 30 μl of SDS-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer; 10 μl of the suspension was loaded to each well, resolved by SDS-PAGE on a 10% gel, and transferred onto a polyvinylidene difluoride membrane (Millipore). Anti-fibulin-4 MAb 11E2 or the anti-human tropoelastin antibody was used in immunoblotting. Alkaline phosphatase-conjugated anti-mouse IgG2b or anti-rabbit IgG (Jackson ImmunoResearch Laboratories) was used as a secondary antibody.
Production of recombinant fibulin-4 and solid-phase binding assay.
Plasmid pEF6/V5 (Invitrogen) was modified to place a preprotrypsin signal sequence, a FLAG tag, and a His6 tag to the N terminus of its inserted protein. Mouse fibulin-4 cDNA without the signal sequence was subcloned to the modified vector. 293T cells were transfected with the resulting vector with Lipofectamine Plus (Invitrogen), according to the manufacturer's protocol. Stably transfected cells were selected with Blasticidin (Invitrogen). Recombinant fibulin-4 was purified from the serum-free conditioned medium of stable lines with TALON His-Tag Purification resins (Clontech) according to the manufacturer's instructions. The purity of the protein was confirmed by Coomassie blue staining of a SDS-PAGE gel, and the protein concentration was determined with Coomassie Plus reagent (Pierce). Various concentrations of purified fibulin-4 in Tris-buffered saline containing 2% skim milk with 2 mM CaCl2 or 5 mM EDTA were used as ligands for a solid-phase binding assay. Recombinant bovine tropoelastin was prepared as previously described (26). Solid-phase binding assays using purified tropoelastin were performed as previously described (54) with the modification of 2 mM CaCl2 or 10 mM EDTA added in the buffer. Anti-FLAG M2 antibody (Sigma) (1:2,000) was used as a primary antibody; horseradish peroxidase-conjugated anti-mouse IgG antibody (Santa Cruz) (1:3,000) was used as a secondary antibody. A color reaction assay was performed with the R&D Substrate Reagent Pack, followed by optic density measurement at 450 nm.
Cell culture and immunostaining.
Normal human skin fibroblasts were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum at a density of 8 × 104 cells per 1 well of 24-well plate (day 0). On day 3, the medium was changed to Dulbecco's modified Eagle's medium-F12 with 10% fetal bovine serum and recombinant mouse fibulin-4 at a concentration of 5 μg/ml. On day 12, cells were fixed with 100% methanol and stained with rabbit antielastin antibody PR533 (1/100; Elastin Products Company) and mouse anti-FLAG M2 antibody (1/100; Sigma). Alexa Fluor 546-conjugated anti-rabbit IgG (1/100; Invitrogen) and Alexa Fluor 488-conjugated anti-mouse IgG (1/100; Invitrogen) were used as secondary antibodies. Stained cells were mounted with Vectashield with 4,6-diamino-2-phenylindole (DAPI) (Vector) and examined with a confocal microscope (Carl Zeiss).
RESULTS
Targeted inactivation of the fibulin-4 gene results in perinatal lethality.
The mouse fibulin-4 gene was inactivated by insertion of a promoterless lacZ and a neomycin-resistant gene cassette into exon 4 (Fig. 1A). Homologous recombinants were identified by Southern blot analysis (Fig. 1B). The absence of fibulin-4 mRNA was confirmed by RT-PCR (Fig. 1C) and Northern blot analysis (see Fig. 7). We found no adult homozygous offspring of fibulin-4+/− crossings, raising the possibility that ablation of fibulin-4 causes early lethality. Genotype analysis of offspring at several developmental stages indicated that the number of wild-type, heterozygous, and homozygous animals were distributed in a normal Mendelian pattern at embryonic stages E11.5 and E18.5 (Table 1). However, most fibulin-4−/− mice died during birth, only 10% survived to P1, and all the homozygous mice died by P2 (Table 1). The frequency of heterozygous animals was about twice that of the wild type and thus was not affected by the disruption of one fibulin-4 allele. These results demonstrate that lack of fibulin-4 causes perinatal lethality in mice.
FIG. 1.
Targeted disruption of the mouse fibulin-4 gene. (A) Targeting strategy. Thick lines represent the homology arms used for constructing the targeting vector. Numbered solid boxes depict fibulin-4 exons. The external 5′ and 3′ probes (B) are indicated as two bars beneath the mutant (mt) locus. wt, wild-type; K, KpnI. (B) Southern blot analysis of tail genomic DNA from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice. The 15.5-kb wild-type band was detected by both 5′ and 3′ probes, the 10-kb mutant band was detected by the 5′ probe, and the 6-kb mutant band was detected by the 3′ probe. (C) RT-PCR analysis of mouse RNA. No PCR product was detected for homozygous mice with either a 3′ (1) or 5′ (2) primer set, indicating the absence of fibulin-4 mRNA in these mice. In the positive control, PCR products were detected for all mice when a 3′ or 5′ primer set was used for fibulin-3.
FIG. 7.
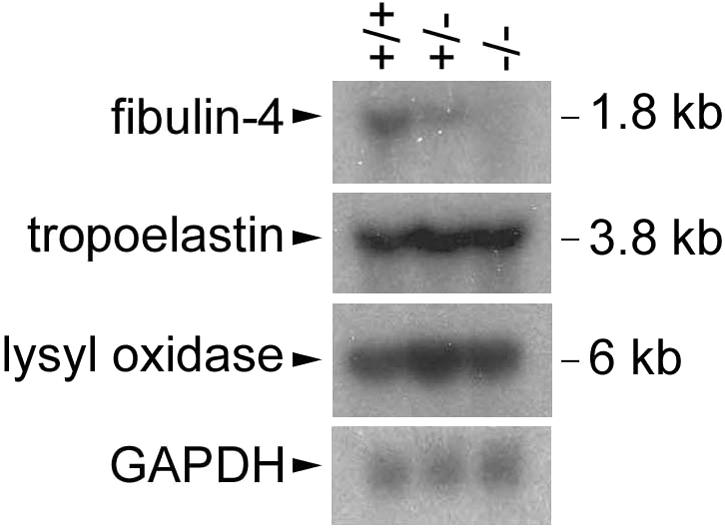
Unaffected tropoelastin and lysyl oxidase expression in fibulin-4−/− mice. Northern blot analysis of equal amount of mouse lung total RNA shows a fibulin-4 mRNA transcript of 1.8 kb detectable in wild-type (+/+) and heterozygous (+/−) but not homozygous (−/−) mice (top). The intensity of the signal is reduced in heterozygous mice. The same blot stripped and reblotted with a tropoelastin probe shows a 3.8-kb tropoelastin transcript with similar intensity in all the mice (second panel from the top). Probing with a Lox probe indicates a 6-kb Lox transcript with similar levels in all mice (third panel). A GAPDH probe was used as a control (bottom).
TABLE 1.
Genotype frequency of offspring from fibulin-4+/− mouse breedings
| Stage | No. of animals (%) of genotype:
|
Total no. | ||
|---|---|---|---|---|
| +/+ | +/− | −/− | ||
| E11.5 | 16 (31) | 23 (45) | 12 (24) | 51 |
| E18.5 | 18 (21) | 45 (52) | 23 (27) | 86 |
| P1 | 33 (32) | 61 (59) | 10 (10) | 104 |
| P2 | 46 (32) | 97 (67) | 1 (<1) | 144 |
| P3 | 53 (31) | 118 (69) | 0 | 171 |
Severe vascular and lung defects in fibulin-4−/− mice.
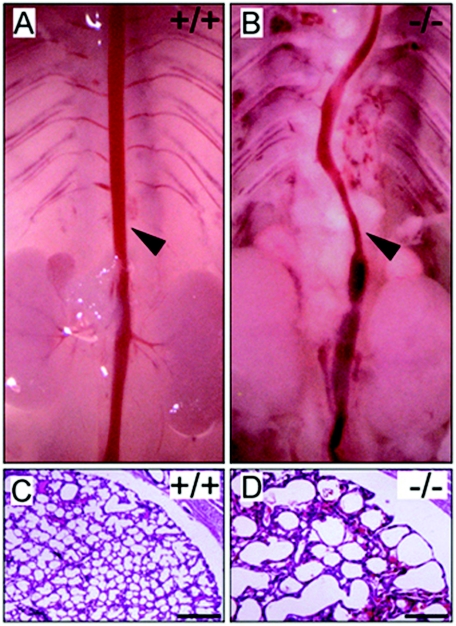
The gross appearances of wild-type, fibulin-4+/−, and fibulin-4−/− mice at P1 were similar. However, on dissection, fibulin-4−/− mice exhibited severe vascular and lung defects. The arteries were tortuous, with irregularities including narrowing, dilatation, aneurysms, rupture, and resulting hemorrhages. The abnormalities were most severe in the aorta (Fig. 2B) and large arteries but occurred in other arteries as well. In addition, all live-born fibulin-4−/− mice were found to have expanded lungs. Histological examinations showed markedly enlarged distal airspaces, similar to those of emphysematous lungs (Fig. 2D). No obvious difference was observed in other organs. Despite the severe phenotype exhibited by homozygous animals, heterozygous (fibulin-4+/−) mice are fertile, have a normal life span, and appear to be indistinguishable from the wild-type littermates.
FIG. 2.
Aorta and lung defects in fibulin-4−/− mice. (A and B) Aorta (arrowheads) of fibulin-4+/+ and fibulin-4−/− mice at P1. (C and D) Hematoxylin and eosin staining of lung sections from mice at P1. The airspaces are significantly enlarged in fibulin-4−/− mice. Scale bars, 400 μm.
The earliest abnormality appears in fibulin-4−/− embryos at E12.5.
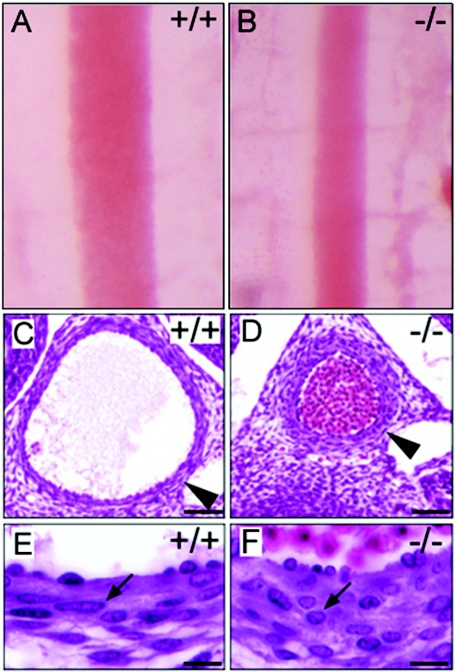
To determine the onset time of defects in fibulin-4−/− mice, we studied different developmental stages of the mice. The earliest abnormality noted was a uniformly narrowing of the descending aorta in fibulin-4−/− embryos at E12.5 (Fig. 3B). The outer diameter of the aorta in fibulin-4−/− mice was only one-half to two-thirds that of wild-type littermates. Histological analyses of cross sections showed that the aortic walls of fibulin-4−/− mice were nearly twice as thick as those of wild-type mice (Fig. 3D). However, the thickening of the fibulin-4−/− aortic wall did not appear to be the result of subendothelial overproliferation of cells, as the number of cells was similar in both fibulin-4−/− and wild-type aortic wall cross sections. There were 193 ± 3 cells for the wild type (data represent means and standard deviations for results with four mice) and 188 ± 7 cells for the homozygote (four mice) in a cross section of the aortic wall at a similar level of the thoracic aorta as shown in Fig. 3C and D. In contrast to the elongated and spindle-shaped cells in wild-type mice (Fig. 3E), the fibulin-4−/− aortic smooth muscle cells were round and appeared to be less stretched (Fig. 3F). The narrowing and the cell shape difference of the fibulin-4−/− aorta became less obvious at E13.5 and at older embryonic ages, likely due to passive expansion of the aorta caused by the dramatic increase in systemic blood pressure during these stages (22). Aortic tortuosity and irregularity were noticeable at E15.5 and became more pronounced with age in homozygous animals. These aorta abnormalities were not observed in fibulin-4+/− embryos.
FIG. 3.
The earliest abnormality in fibulin-4−/− mice at E12.5. (A and B) Aortas of fibulin-4+/+ and fibulin-4−/− embryos at E12.5. (C to F) Hematoxylin and eosin staining of cross sections of descending aorta from E12.5 embryos. Note the smaller diameter and thicker wall of the fibulin-4−/− aorta (D). In contrast to the elongated, spindle-shaped cells (E, arrow) in wild-type mice, aortic wall cells were round in the fibulin-4−/− mice (F, arrow). Scale bars, 50 μm (C and D) and 20 μm (E and F).
fibulin-4−/− mice do not form elastic fibers.
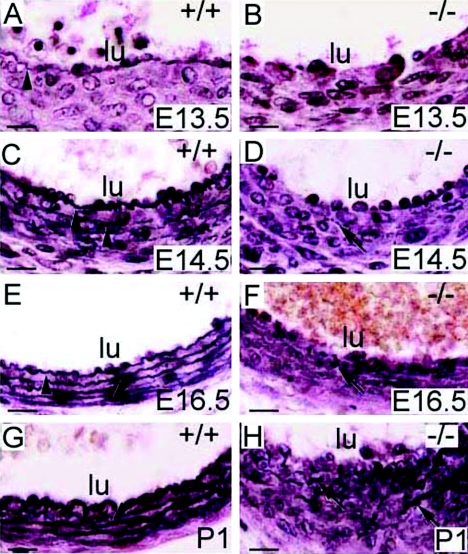
The vascular and lung abnormalities of fibulin-4−/− mice were suggestive of elastic fiber defects. The appearance of abnormalities, coincident with the onset of elastogenesis in mice, suggests that genesis of elastic fibers may be impaired in fibulin-4−/− mice. Thus, we stained tissue sections from E12.5 to P1 with elastin van Gieson staining, which specifically stains elastic fibers. In the wild type, the innermost elastic lamina of the aorta was identifiable at E13.5 under a light microscope (Fig. 4A). By E14.5, four elastic laminae could be distinguished (Fig. 4C). All five elastic laminae were present at E16.5 or older ages (Fig. 4E and G). In contrast, no continuous elastic lamina was observed in the fibulin-4−/− aorta at any stage (Fig. 4B, D, F, and H). Instead, irregular elastin aggregates were visible at E14.5 (Fig. 4D), and more and larger aggregates accumulated with age (Fig. 4F and H). In the lung and skin, elastic fibers were not distinguishable by light microscopy at any embryonic stages. At P1, fine elastic fibers could be observed in lung and the hypodermal connective tissue of the skin of wild-type mice (Fig. 5A and C) but not in fibulin-4−/− mice (Fig. 5B and D). Despite this, the skin of fibulin-4−/− mice did not show obvious gross differences at this young age. Elastic fibers of fibulin-4+/− mice were similar to those of wild-type littermates. These results indicate that fibulin-4 is required for general elastogenesis.
FIG. 4.
Lack of elastic lamina in the fibulin-4−/− aorta. Elastin van Gieson staining of cross sections of descending aortae at different developmental stages is shown. The innermost elastic lamina (arrowheads) was identifiable in the wild-type aorta at E13.5 (A) and became more numerous and thicker at subsequent developing stages (C, E, and G). Only irregular elastin aggregates (arrows) were observed in the fibulin-4−/− aorta (B, D, F, and H). lu, lumen. Scale bars, 20 μm.
FIG. 5.
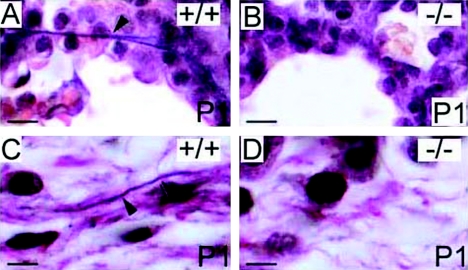
Lack of elastic fibers in the skin and lung of fibulin-4−/− mice. (A and B) Elastin van Gieson staining of lung sections from P1 mice. Fine branched elastic fibers (arrowhead) were present in the wild-type (A) but not fibulin-4−/− (B) lung. (C and D) Elastin staining of skin sections from P1 mice. Elastic fibers were observed in the hypodermal connective tissue area of the wild-type skin (C, arrowhead) but not in the fibulin-4−/− skin (D). Scale bars, 10 μm (A and B) and 5 μm (C and D).
Elastin aggregates containing electron dense rod-like filaments in fibulin-4−/− mice.
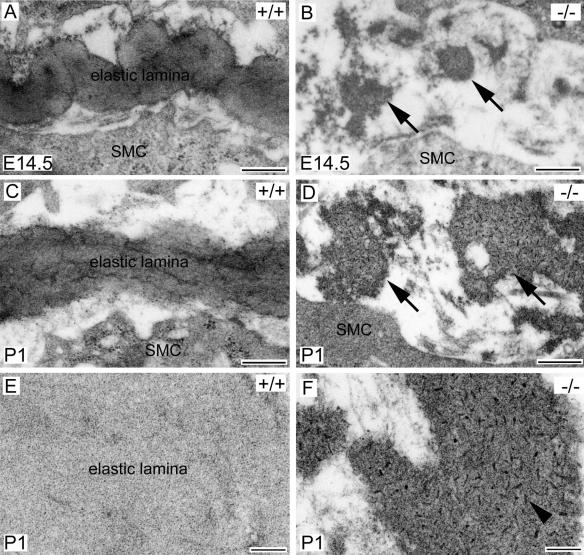
To further understand the elastic fiber defects in fibulin-4−/− mice, we examined the aortae of E14.5 and P1 mice by electron microscopy (Fig. 6). Consistent with the finding by light microscopy, no intact elastic lamina was observed in the fibulin-4−/− aorta at either E14.5 or P1. Continuous elastic lamina was present in the wild-type aorta (Fig. 6A and C), but only irregular elastin aggregates were found in the fibulin-4−/− aorta at both stages (Fig. 6B and D, arrows). Noticeably, the content of the elastin aggregates appeared to be different from that of normal elastic fibers. Instead of an amorphous content, these elastin aggregates contained distinguishable electron-dense substances. Under higher magnification, dark rod-like filaments with similar sizes were seen to be evenly distributed in the elastin aggregates (Fig. 6F). These findings demonstrate that elastin does not properly assemble without fibulin-4.
FIG. 6.
Electron microscopy of elastic laminae in fibulin-4−/− mice. (A to D) Descending aorta cross sections from E14.5 (A and B) and P1 (C and D) mice. The wild-type aorta contained continuous elastic lamina (A and C). In contrast, irregular elastin aggregates (arrows) were randomly distributed in the fibulin-4−/− aorta (B and D). Under higher magnification (E and F), the wild-type elastic lamina appeared to be amorphous (E). But distinct, dark rod-like filaments (F, arrowhead) were evenly distributed in the fibulin-4−/− elastin aggregates. SMC, smooth muscle cell. Scale bars, 400 nm (A to D) and 100 nm (E and F).
Desmosine content is severely reduced in fibulin-4−/− mice.
To determine whether mature elastin content is affected in fibulin-4−/− mice, we assessed the level of desmosine, an elastin cross-link-specific amino acid, in the aorta and lungs of wild-type, heterozygous, and homozygous mutant mice. As shown in Table 2, elastin cross-linking was nearly absent in fibulin-4−/− mice. There was a 94% decrease in the amount of desmosine in the aorta and 88% decrease in lungs of fibulin-4−/− mice compared with wild-type mice. Interestingly, there was a near 20% increase in the amount of desmosine in heterozygous mutants compared with wild-type mice (Table 2), although this difference was not statistically significant (P > 0.05 in a t test). We did not find any difference in the level of hydroxyproline, an indicator of collagen content, between wild-type and fibulin-4−/− mice (data not shown), indicating that the amount of collagen did not differ in fibulin-4−/− mice.
TABLE 2.
Desmosine in picomoles per milligram of protein in aorta and lung at P1
| Genotype (n) | Desmosine ± SD (% of wild type) in:
|
|
|---|---|---|
| Aorta | Lung | |
| +/+ (12) | 419.05 ± 125.94 (100) | 29.7 ± 14.87 (100) |
| +/− (21) | 501.86 ± 133.85 (120)a | 35.63 ± 9.08 (120)b |
| −/− (6) | 25.35 ± 8.69 (6)c | 3.15 ± 1.45 (12)c |
P = 0.09, compared to the wild type by t test.
P = 0.23, compared to the wild type by t test.
P < 0.0001, compared to the wild type by t test.
Tropoelastin and LOX expression is not affected in fibulin-4−/− mice.
Elastin cross-linking is catalyzed by LOX family enzymes. Among the five known members, LOX has been shown to be necessary for elastic fiber development (20, 31). To determine whether lack of fibulin-4 causes elastinopathy by affecting tropoelastin or Lox expression, we examined the mRNA levels of fibulin-4, tropoelastin, and Lox in wild-type, heterozygous, and homozygous littermates at P1. As shown in Fig. 7, while heterozygotes showed reduced fibulin-4 mRNA levels compared to those in wild-type mice and homozygotes had no detectable fibulin-4 mRNA, all of them had similar levels of tropoelastin or Lox expression. Elastin has been shown to have an antiproliferative effect on vascular smooth muscle cells, and mice lacking elastin (ELN−/−) die of vascular occlusion resulting from subendothelial cell proliferation (27). The unaffected tropoelastin expression is consistent with the lack of cell overproliferation and the accumulation of irregular elastin aggregates in fibulin-4−/− mice.
Fibulin-4 interacts with tropoelastin and assembles into elastic fibers.
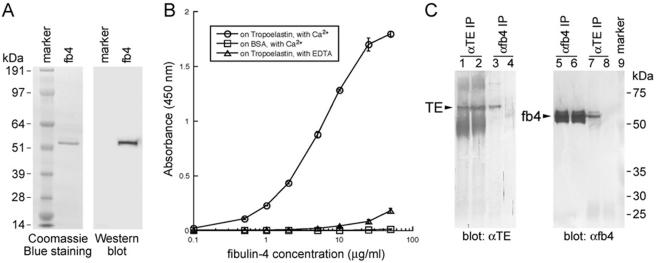
To investigate possible mechanisms by which fibulin-4 affects elastogenesis, we assessed the potential for interaction between fibulin-4 and elastin. Recombinant mouse fibulin-4 was expressed and purified from stably transfected 293T cell medium (Fig. 8A). A FLAG tag and a His6 tag were added at the N terminus of fibulin-4 without its signal peptide to facilitate the purification and characterization of fibulin-4. The tagged protein was secreted from a preprotrypsin signal sequence. Although we found that untagged fibulin-4 was secreted efficiently from its native signal peptide, the C-terminal-tagged fibulin-4 was poorly secreted. It is possible that the C-terminal domain affects protein folding and is sensitive to modification. In a solid-phase binding assay, purified tropoelastin was used as an immobilized protein substrate, and fibulin-4 was used as a soluble ligand. As shown in Fig. 8B, fibulin-4 bound strongly to tropoelastin in the presence of Ca2+ and the binding was inhibited in the presence of EDTA, suggesting that calcium is required for the binding and the calcium-binding epidermal growth factor domains of fibulin-4 may be necessary for this binding. However, it has not been demonstrated experimentally that fibulin-4 binds calcium. No binding was observed between fibulin-4 and a control substrate (bovine serum albumin). These results indicate that fibulin-4 and tropoelastin can interact directly.
FIG. 8.
Fibulin-4 interacts with tropoelastin. (A) Purified recombinant mouse fibulin-4. A total of 1 μg of purified fibulin-4 was used for the Coomassie blue-stained gel, and 10 ng of the protein was used for the Western blot. An anti-FLAG antibody was used for immunodetection. A single band with the correct mass for fibulin-4 was detected by both Coomassie blue staining and immunoblotting. (B) Solid-phase binding assay using recombinant fibulin-4 as a soluble ligand. Note that fibulin-4 binds to tropoelastin in the presence of Ca2+ but that the binding is inhibited in the presence of EDTA. Data were obtained as the results of triplicate experiments, and values shown are means ± standard deviations. (C) Coimmunoprecipitation of fibulin-4 with tropoelastin. Lanes 1 to 4, antitropoelastin blotting of immunoprecipitates from lysates of 293T cells transfected with both tropoelastin and fibulin-4 (lanes 1 and 3) or tropoelastin alone (lanes 2 and 4). Tropoelastin was detected in the immunoprecipitate by anti-fibulin-4 (7B9) (lane 3). Lanes 5 to 8, anti-fibulin-4 (11E2) blotting of immunoprecipitates from lysates of 293T cells transfected with both tropoelastin and fibulin-4 (lanes 5 and 7) or fibulin-4 alone (lanes 6 and 8). Fibulin-4 was detected in the immunoprecipitate by antitropoelastin (lane 7). IP, immunoprecipitation; TE, tropoelastin; fb4, fibulin-4.
To assess whether fibulin-4 and tropoelastin interact in solution, we performed a coimmunoprecipitation assay. Human fibulin-4 cDNA, human tropoelastin cDNA, or both were transfected into 293T cells. Tropoelastin was expressed at a very low level. We could detect tropoelastin in the cell lysate, but it was difficult to detect it in the culture medium, presumably due to its self-coacervation and formation of insoluble elastin in the medium. Fibulin-4 was expressed at a relatively high level and could be readily detected in both lysate and medium. Thus, we chose to use transfected cell lysates for the coimmunoprecipitation assay. As shown in Fig. 8C, tropoelastin was immunoprecipitated from lysates transfected with either tropoelastin alone (lane 2) or both tropoelastin and fibulin-4 (lane 1) by the antitropoelastin antibody. It was also coimmunoprecipitated by an anti-fibulin-4 monoclonal antibody from the lysate cotransfected with tropoelastin and fibulin-4 (lane 3). The coimmunoprecipitation did not appear to be due to a nonspecific association of tropoelastin with the fibulin-4 antibody beads, as no tropoelastin signal was detected from the immunoprecipitate by the same antibody from the lysate transfected only with tropoelastin (lane 4). Reciprocally, fibulin-4 was coimmunoprecipitated by the anti-tropoelastin antibody from the lysate transfected with both tropoelastin and fibulin-4 (lane 7) but not from the lysate transfected with fibulin-4 alone (lane 8), while it was immunoprecipitated by the fibulin-4 antibody from both lysates (lanes 5 and 6). These results demonstrate that fibulin-4 binds specifically with tropoelastin.
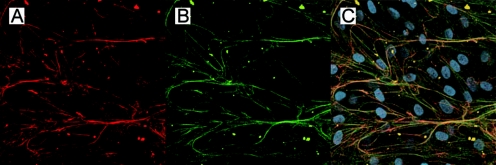
To further determine whether exogenous fibulin-4 is colocalized to elastic fibers, we added FLAG-tagged recombinant fibulin-4 to cultured human fibroblasts. These cells are capable of developing a network of elastic fibers in vitro. Double labeling of antitropoelastin and anti-FLAG antibodies showed colocalization of fibulin-4 and elastin (Fig. 9). These data indicate that fibulin-4 assembles into elastic fibers.
FIG. 9.
Colocalization of fibulin-4 and elastin. (A to C) Normal human skin fibroblasts were cultured with FLAG-tagged recombinant fibulin-4 protein. (A) Cells stained with antitropoelastin antibody, showing a network structure. (B) Cells stained with anti-FLAG antibody, also showing a network structure. (C) Superimposed image of panels A and B, with DAPI nuclear staining showing fibulin-4 localization on elastic fibers.
DISCUSSION
Elastic fiber formation is thought to involve extrinsic proteins and an intrinsic capacity for elastin coacervation. How extrinsic proteins cooperate with each other and with elastin coacervation to form functional elastic fibers is unknown. Over 30 molecules have been reported to associate with elastic fibers in morphological studies and in vitro assays (23). But many have been found to have no effect or marginal effects on elastic fiber formation in vivo. In this study, we demonstrated that mice lacking fibulin-4 do not form elastic fibers. This is the first report that lack of a protein other than elastin itself completely abolishes the formation of elastic fibers in vivo, indicating that fibulin-4 plays an indispensable role in elastogenesis.
The initial abnormality present in fibulin-4−/− mice, arterial narrowing, is similar to that observed with ELN−/− mice (27). In ELN−/− mice, however, the change is caused by subendothelial cell overproliferation due to the lack of elastin, a process that eventually obliterates the vascular lumen (27). In fibulin-4−/− mice, tropoelastin mRNA levels are similar to those in wild-type mice, there is no sign of cell overproliferation and vascular occlusion, and irregular elastin aggregates accumulate with age, suggesting that fibulin-4 does not affect the synthesis of tropoelastin. The formation of functional elastic fibers requires the deposition of tropoelastin at the fiber assembly site, cross-linking of tropoelastin monomers by lysyl oxidase family enzymes, and the organization of resulting insoluble elastin matrix into mature fibers. Fibulin-4 likely affects one or more of these processes.
The irregular elastin aggregates observed in fibulin-4−/− mice are highly unusual in that they contain electron-dense rod-like filaments. These filaments are evenly distributed in the aggregates in fibulin-4−/− mice, as if, without fibulin-4, a molecule(s) associated with tropoelastin is incorporated together with each tropoelastin monomer into elastin aggregates. Alternatively, the rod-like filament may be a regular elastic fiber component whose presence is revealed by the absence of fibulin-4. The morphology of fibulin-4−/− elastin aggregates is similar to that of abnormal elastin aggregates permeated by proteoglycans in the presence of lysyl oxidase inhibitors (13). Also, ECM proteoglycans containing sulfated glycosaminoglycans visualized by a cationic copper phthalocyanin dye, cupromeronic blue, exhibit morphology very similar to the rod-like filaments observed in irregular elastin aggregates in fibulin-4−/− mice (48). These observations suggest that the filaments in fibulin-4−/− elastin aggregates may be proteoglycans. Glycosaminoglycans containing sulfate groups (chondroitin, dermatan, and heparan sulfate) and their associated proteoglycans have been shown to directly interact with tropoelastin and are normal components of elastic fibers (3, 7, 17, 55). Both chondroitin sulfate and heparan sulfate can mediate tropoelastin's coacervation (17, 24, 45, 53, 55). Decreased elastin deposition was observed when the matrix was depleted of sulfated molecules by chlorate treatment of the cells (8, 53). We also found that fibulin-4 interacts with tropoelastin directly and assembles into elastic fibers in culture. Thus, fibulin-4 may play a role in the initial deposition of tropoelastin, such as scaffolding and facilitating the formation of homogeneous elastin polymers by preventing the association of other molecules with tropoelastin or coordinating tropoelastin and other elastic fiber components during elastic fiber assembly. The identity of the rod-like filaments, the precise roles of proteoglycans in elastogenesis, and their relationships with fibulin-4 remain to be determined.
Elastin cross-linking is severely affected in fibulin-4−/− mice. Desmosine was reduced by over 85% in fibulin-4−/− mice, compared to levels in wild-type mice. Interestingly, there was a near-20% increase in desmosine in fibulin-4+/− mice compared to wild-type mice, although this difference was not statistically significant with the number of animals we analyzed. It is possible that there is more cross-linking in heterozygous mutants than in wild-type mice to compensate for fibulin-4 haploinsufficiency. Elastin cross-linking is catalyzed by lysyl oxidases, a family of enzymes that catalyzes the oxidative deamination of lysine residues in elastin and collagen (21). Five members have been described so far (12, 30). LOX has been shown to be necessary for elastic fiber and collagen fiber development (20, 31), and LOX-like 1 (LOXL1) is required in elastic fiber homeostasis (28). Similar to fibulin-4−/− mice, Lox−/− mice exhibit severe vascular defects and die perinatally (20, 31). The vascular defects include artery tortuosity, irregularity, and ruptured aneurysms with fragmented elastic lamina in the aortic walls. Desmosine content is decreased by 60%; hydroxyproline, which represents collagen content, is decreased by 30% in Lox−/− mice (20). The similarities in gross defects between fibulin-4−/− mice and Lox−/− mice and loss of desmosine content in fibulin-4−/− mice suggest that lack of fibulin-4 may affect the function of lysyl oxidase. However, Lox mRNA expression is similar in wild-type and fibulin-4−/− mice, hydroxyproline content is not altered in fibulin-4−/− mice, and no unusual elastic fiber content such as rod-like filaments found in fibulin-4−/− mice has been reported with Lox−/− mice. Loxl1-null mice survive to adulthood but do not deposit normal elastic fibers in the uterine tract postpartum; they develop pelvic prolapse, enlarged airspaces of the lung, loose skin, and vascular abnormalities. Desmosine content in Loxl1−/− mice is reduced by 30 to 50%, depending on tissues (28). The difference in elastic fiber defects in fibulin-4−/−, Lox−/−, and Loxl1−/− mice suggests that fibulin-4 has different roles in elastic fiber assembly, even if it also affects the activities of lysyl oxidases.
Fibulin-4 shares high homology with fibulin-5, with a similar domain structure and >50% amino acid identity. Fibulin-5-null mice grow to adulthood but exhibit loose skin, lung airspace enlargement, and a stiff and tortuous aorta, due to disorganized and fragmented elastic fibers (40, 56). It has been proposed that fibulin-5 may be involved in elastogenesis by tethering elastic fibers onto cell surface integrins and by affecting cross-linking of elastin through direct binding with LOXL1 (28, 40, 56). Loxl1−/− mice exhibit similar but less-severe elastic fiber defects than fibulin-5−/− mice. Despite the high homology between fibulin-4 and -5, the fibulin-4−/− phenotype is not compensated for by fibulin-5. fibulin-4−/− mice exhibited almost complete loss of elastic fibers and perinatal lethality, suggesting a more essential role of fibulin-4 in elastogenesis than that of fibulin-5.
With age and some pathological conditions, elastic fibers exhibit interwoven filaments (41) that are similar to the morphology of elastin aggregates of fibulin-4−/− mice. Thus, alteration of the function or structure of fibulin-4 may be a major mechanism behind aging and elastic fiber-related diseases. fibulin-4−/− mice exhibit the most severe elastinopathy described to date. Understanding the role of fibulin-4 is a prerequisite for understanding the mechanism responsible for elastogenesis. In addition to the crucial role in elastogenesis, fibulin-4 may also have other important functions in cell proliferation and differentiation. Several studies have found that fibulin-4 stimulates cell growth and is upregulated in tumors (14, 16, 19). fibulin-4−/− mice will also be a valuable model in further studying these functions.
Acknowledgments
We thank Peggy McCuskey for electron microscopy and Anna Yocom for technical assistance.
This work was supported by NIH/NEI grants EY13847 and EY13160, Research to Prevent Blindness, Philip Morris USA and Philip Morris International, Health and Labor Sciences research grants, Japan Society for the Promotion of Science, and Japan Science and Technology Agency.
REFERENCES
- 1.Argraves, W. S., L. M. Greene, M. A. Cooley, and W. M. Gallagher. 2003. Fibulins: physiological and disease perspectives. EMBO Rep. 4:1127-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arteaga-Solis, E., B. Gayraud, S. Y. Lee, L. Shum, L. Sakai, and F. Ramirez. 2001. Regulation of limb patterning by extracellular microfibrils. J. Cell Biol. 154:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baccarani-Contri, M., D. Vincenzi, F. Cicchetti, G. Mori, and I. Pasquali-Ronchetti. 1990. Immunocytochemical localization of proteoglycans within normal elastin fibers. Eur. J. Cell Biol. 53:305-312. [PubMed] [Google Scholar]
- 4.Bressan, G. M., I. Castellani, M. G. Giro, D. Volpin, C. Fornieri, and I. Pasquali Ronchetti. 1983. Banded fibers in tropoelastin coacervates at physiological temperatures. J. Ultrastruct. Res. 82:335-340. [DOI] [PubMed] [Google Scholar]
- 5.Bressan, G. M., I. Pasquali-Ronchetti, C. Fornieri, F. Mattioli, I. Castellani, and D. Volpin. 1986. Relevance of aggregation properties of tropoelastin to the assembly and structure of elastic fibers. J. Ultrastruct. Mol. Struct. Res. 94:209-216. [DOI] [PubMed] [Google Scholar]
- 6.Bressler, N. M., S. B. Bressler, and S. L. Fine. 1988. Age-related macular degeneration. Surv. Ophthalmol. 32:375-413. [DOI] [PubMed] [Google Scholar]
- 7.Broekelmann, T. J., B. A. Kozel, H. Ishibashi, C. C. Werneck, F. W. Keeley, L. Zhang, and R. P. Mecham. 2005. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J. Biol. Chem. 280:40939-40947. [DOI] [PubMed] [Google Scholar]
- 8.Buczek-Thomas, J. A., C. L. Chu, C. B. Rich, P. J. Stone, J. A. Foster, and M. A. Nugent. 2002. Heparan sulfate depletion within pulmonary fibroblasts: implications for elastogenesis and repair. J. Cell Physiol. 192:294-303. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry, S. S., J. Gazzard, C. Baldock, J. Dixon, M. J. Rock, G. C. Skinner, K. P. Steel, C. M. Kielty, and M. J. Dixon. 2001. Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum. Mol. Genet. 10:835-843. [DOI] [PubMed] [Google Scholar]
- 10.Chu, M. L., and T. Tsuda. 2004. Fibulins in development and heritable disease. Birth Defects Res. C Embryo Today 72:25-36. [DOI] [PubMed] [Google Scholar]
- 11.Cox, B. A., B. C. Starcher, and D. W. Urry. 1974. Communication: coacervation of tropoelastin results in fiber formation. J. Biol. Chem. 249:997-998. [PubMed] [Google Scholar]
- 12.Csiszar, K. 2001. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 70:1-32. [DOI] [PubMed] [Google Scholar]
- 13.Fornieri, C., M. Baccarani-Contri, D. Quaglino, Jr., and I. Pasquali-Ronchetti. 1987. Lysyl oxidase activity and elastin/glycosaminoglycan interactions in growing chick and rat aortas. J. Cell Biol. 105:1463-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher, W. M., M. Argentini, V. Sierra, L. Bracco, L. Debussche, and E. Conseiller. 1999. MBP1: a novel mutant p53-specific protein partner with oncogenic properties. Oncogene 18:3608-3616. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher, W. M., C. A. Currid, and L. C. Whelan. 2005. Fibulins and cancer: friend or foe? Trends Mol. Med. 11:336-340. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher, W. M., L. M. Greene, M. P. Ryan, V. Sierra, A. Berger, P. Laurent-Puig, and E. Conseiller. 2001. Human fibulin-4: analysis of its biosynthetic processing and mRNA expression in normal and tumour tissues. FEBS Lett. 489:59-66. [DOI] [PubMed] [Google Scholar]
- 17.Gheduzzi, D., D. Guerra, B. Bochicchio, A. Pepe, A. M. Tamburro, D. Quaglino, S. Mithieux, A. S. Weiss, and I. Pasquali Ronchetti. 2005. Heparan sulphate interacts with tropoelastin, with some tropoelastin peptides and is present in human dermis elastic fibers. Matrix Biol. 24:15-25. [DOI] [PubMed] [Google Scholar]
- 18.Giltay, R., R. Timpl, and G. Kostka. 1999. Sequence, recombinant expression and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix Biol. 18:469-480. [DOI] [PubMed] [Google Scholar]
- 19.Heine, H., R. L. Delude, B. G. Monks, T. Espevik, and D. T. Golenbock. 1999. Bacterial lipopolysaccharide induces expression of the stress response genes hop and H411. J. Biol. Chem. 274:21049-21055. [DOI] [PubMed] [Google Scholar]
- 20.Hornstra, I. K., S. Birge, B. Starcher, A. J. Bailey, R. P. Mecham, and S. D. Shapiro. 2003. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J. Biol. Chem. 278:14387-14393. [DOI] [PubMed] [Google Scholar]
- 21.Kagan, H. M., and P. C. Trackman. 1991. Properties and function of lysyl oxidase. Am. J. Respir. Cell Mol. Biol. 5:206-210. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman, M. H., and J. B. L. Bard. 1999. The Anatomical basis of mouse development. Academic Press, London, United Kingdom.
- 23.Kielty, C. M., M. J. Sherratt, and C. A. Shuttleworth. 2002. Elastic fibres. J. Cell Sci. 115:2817-2828. [DOI] [PubMed] [Google Scholar]
- 24.Kielty, C. M., S. P. Whittaker, and C. A. Shuttleworth. 1996. Fibrillin: evidence that chondroitin sulphate proteoglycans are components of microfibrils and associate with newly synthesised monomers. FEBS Lett. 386:169-173. [DOI] [PubMed] [Google Scholar]
- 25.Kostka, G., R. Giltay, W. Bloch, K. Addicks, R. Timpl, R. Fassler, and M. L. Chu. 2001. Perinatal lethality and endothelial cell abnormalities in several vessel compartments of fibulin-1-deficient mice. Mol. Cell. Biol. 21:7025-7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozel, B. A., H. Wachi, E. C. Davis, and R. P. Mecham. 2003. Domains in tropoelastin that mediate elastin deposition in vitro and in vivo. J. Biol. Chem. 278:18491-18498. [DOI] [PubMed] [Google Scholar]
- 27.Li, D. Y., B. Brooke, E. C. Davis, R. P. Mecham, L. K. Sorensen, B. B. Boak, E. Eichwald, and M. T. Keating. 1998. Elastin is an essential determinant of arterial morphogenesis. Nature 393:276-280. [DOI] [PubMed] [Google Scholar]
- 28.Liu, X., Y. Zhao, J. Gao, B. Pawlyk, B. Starcher, J. A. Spencer, H. Yanagisawa, J. Zuo, and T. Li. 2004. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 36:178-182. [DOI] [PubMed] [Google Scholar]
- 29.Loeys, B., L. Van Maldergem, G. Mortier, P. Coucke, S. Gerniers, J. M. Naeyaert, and A. De Paepe. 2002. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum. Mol. Genet. 11:2113-2118. [DOI] [PubMed] [Google Scholar]
- 30.Maki, J. M., and K. I. Kivirikko. 2001. Cloning and characterization of a fourth human lysyl oxidase isoenzyme. Biochem. J. 355:381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maki, J. M., J. Rasanen, H. Tikkanen, R. Sormunen, K. Makikallio, K. I. Kivirikko, and R. Soininen. 2002. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106:2503-2509. [DOI] [PubMed] [Google Scholar]
- 32.Markova, D., Y. Zou, F. Ringpfeil, T. Sasaki, G. Kostka, R. Timpl, J. Uitto, and M. L. Chu. 2003. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am. J. Hum. Genet. 72:998-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marmorstein, A. D., L. Y. Marmorstein, M. Rayborn, X. Wang, J. G. Hollyfield, and K. Petrukhin. 2000. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 97:12758-12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marmorstein, L. Y., A. V. Kinev, G. K. Chan, D. A. Bochar, H. Beniya, J. A. Epstein, T. J. Yen, and R. Shiekhattar. 2001. A human BRCA2 complex containing a structural DNA binding component influences cell cycle progression. Cell 104:247-257. [DOI] [PubMed] [Google Scholar]
- 35.Marmorstein, L. Y., P. J. McLaughlin, J. B. Stanton, L. Yan, J. W. Crabb, and A. D. Marmorstein. 2002. Bestrophin interacts physically and functionally with protein phosphatase 2A. J. Biol. Chem. 277:30591-30597. [DOI] [PubMed] [Google Scholar]
- 36.Marmorstein, L. Y., F. L. Munier, Y. Arsenijevic, D. F. Schorderet, P. J. McLaughlin, D. Chung, E. Traboulsi, and A. D. Marmorstein. 2002. Aberrant accumulation of EFEMP1 underlies drusen formation in malattia leventinese and age-related macular degeneration. Proc. Natl. Acad. Sci. USA 99:13067-13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mecham, R. P., and E. Davis. 1994. Elastic fiber structure and assembly, p. 281-314. In P. D. Yurchenco, D. E. Birk, and R. P. Mecham (ed.), Extracellular matrix assembly and structure. Academic Press, New York, N.Y.
- 38.Midwood, K. S., and J. E. Schwarzbauer. 2002. Elastic fibers: building bridges between cells and their matrix. Curr. Biol. 12:R279-R281. [DOI] [PubMed] [Google Scholar]
- 39.Milewicz, D. M., Z. Urban, and C. Boyd. 2000. Genetic disorders of the elastic fiber system. Matrix Biol. 19:471-480. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura, T., P. R. Lozano, Y. Ikeda, Y. Iwanaga, A. Hinek, S. Minamisawa, C. F. Cheng, K. Kobuke, N. Dalton, Y. Takada, K. Tashiro, J. Ross, Jr., T. Honjo, and K. R. Chien. 2002. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415:171-175. [DOI] [PubMed] [Google Scholar]
- 41.Pasquali-Ronchetti, I., and M. Baccarani-Contri. 1997. Elastic fiber during development and aging. Microsc. Res. Tech. 38:428-435. [DOI] [PubMed] [Google Scholar]
- 42.Pereira, L., K. Andrikopoulos, J. Tian, S. Y. Lee, D. R. Keene, R. Ono, D. P. Reinhardt, L. Y. Sakai, N. J. Biery, T. Bunton, H. C. Dietz, and F. Ramirez. 1997. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat. Genet. 17:218-222. [DOI] [PubMed] [Google Scholar]
- 43.Pereira, L., S. Y. Lee, B. Gayraud, K. Andrikopoulos, S. D. Shapiro, T. Bunton, N. J. Biery, H. C. Dietz, L. Y. Sakai, and F. Ramirez. 1999. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 96:3819-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierce, R. A., T. J. Mariani, and R. M. Senior. 1995. Elastin in lung development and disease. Ciba Found. Symp. 192:199-212. [DOI] [PubMed] [Google Scholar]
- 45.Reinboth, B., E. Hanssen, E. G. Cleary, and M. A. Gibson. 2002. Molecular interactions of biglycan and decorin with elastic fiber components: biglycan forms a ternary complex with tropoelastin and microfibril-associated glycoprotein 1. J. Biol. Chem. 277:3950-3957. [DOI] [PubMed] [Google Scholar]
- 46.Rosenbloom, J., W. R. Abrams, and R. Mecham. 1993. Extracellular matrix 4: the elastic fiber. FASEB J. 7:1208-1218. [PubMed] [Google Scholar]
- 47.Schultz, D. W., M. L. Klein, A. J. Humpert, C. W. Luzier, V. Persun, M. Schain, A. Mahan, C. Runckel, M. Cassera, V. Vittal, T. M. Doyle, T. M. Martin, R. G. Weleber, P. J. Francis, and T. S. Acott. 2003. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum. Mol. Genet 12:3315-3323. [DOI] [PubMed] [Google Scholar]
- 48.Scott, J. E. 1980. Collagen-proteoglycan interactions. Localization of proteoglycans in tendon by electron microscopy. Biochem. J. 187:887-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Starcher, B., and M. Conrad. 1995. A role for neutrophil elastase in the progression of solar elastosis. Connect. Tissue Res. 31:133-140. [DOI] [PubMed] [Google Scholar]
- 50.Stone, E. M., T. A. Braun, S. R. Russell, M. H. Kuehn, A. J. Lotery, P. A. Moore, C. G. Eastman, T. L. Casavant, and V. C. Sheffield. 2004. Missense variations in the fibulin 5 gene and age-related macular degeneration. N. Engl. J. Med. 351:346-353. [DOI] [PubMed] [Google Scholar]
- 51.Stone, E. M., A. J. Lotery, F. L. Munier, E. Heon, B. Piguet, R. H. Guymer, K. Vandenburgh, P. Cousin, D. Nishimura, R. E. Swiderski, G. Silvestri, D. A. Mackey, G. S. Hageman, A. C. Bird, V. C. Sheffield, and D. F. Schorderet. 1999. A single EFEMP1 mutation associated with both malattia leventinese and Doyne honeycomb retinal dystrophy. Nat. Genet. 22:199-202. [DOI] [PubMed] [Google Scholar]
- 52.Timpl, R., T. Sasaki, G. Kostka, and M. L. Chu. 2003. Fibulins: a versatile family of extracellular matrix proteins. Nat Rev. Mol. Cell Biol. 4:479-489. [DOI] [PubMed] [Google Scholar]
- 53.Trask, B. C., T. M. Trask, T. Broekelmann, and R. P. Mecham. 2000. The microfibrillar proteins MAGP-1 and fibrillin-1 form a ternary complex with the chondroitin sulfate proteoglycan decorin. Mol. Biol. Cell 11:1499-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trask, T. M., B. C. Trask, T. M. Ritty, W. R. Abrams, J. Rosenbloom, and R. P. Mecham. 2000. Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J. Biol. Chem. 275:24400-24406. [DOI] [PubMed] [Google Scholar]
- 55.Wu, W. J., B. Vrhovski, and A. S. Weiss. 1999. Glycosaminoglycans mediate the coacervation of human tropoelastin through dominant charge interactions involving lysine side chains. J. Biol. Chem. 274:21719-21724. [DOI] [PubMed] [Google Scholar]
- 56.Yanagisawa, H., E. C. Davis, B. C. Starcher, T. Ouchi, M. Yanagisawa, J. A. Richardson, and E. N. Olson. 2002. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415:168-171. [DOI] [PubMed] [Google Scholar]