Abstract
Metallothioneins are ubiquitous, small, cysteine-rich proteins with the ability to bind heavy metals. In spite of their biochemical characterization, their in vivo function remains elusive. Here, we report the generation of a metallothionein gene family knockout in Drosophila melanogaster by targeted disruption of all four genes (MtnA to -D). These flies are viable if raised in standard laboratory food. During development, however, they are highly sensitive to copper, cadmium, and (to a lesser extent) zinc load. Metallothionein expression is particularly important for male viability; while copper load during development affects males and females equally, adult males lacking metallothioneins display a severely reduced life span, possibly due to copper-mediated oxidative stress. Using various reporter gene constructs, we find that different metallothioneins are expressed with virtually the same tissue specificity in larvae, notably in the intestinal tract at sites of metal accumulation, including the midgut's “copper cells.” The same expression pattern is observed with a synthetic minipromoter consisting only of four tandem metal response elements. From these and other experiments, we conclude that tissue specificity of metallothionein expression is a consequence, rather than a cause, of metal distribution in the organism. The bright orange luminescence of copper accumulated in copper cells of the midgut is severely reduced in the metallothionein gene family knockout, as well as in mutants of metal-responsive transcription factor 1 (MTF-1), the main regulator of metallothionein expression. This indicates that an in vivo metallothionein-copper complex forms the basis of this luminescence. Strikingly, metallothionein mutants show an increased, MTF-1-dependent induction of metallothionein promoters in response to copper, cadmium, silver, zinc, and mercury. We conclude that free metal, but not metallothionein-bound metal, triggers the activation of MTF-1 and that metallothioneins regulate their own expression by a negative feedback loop.
Copper is an essential trace element with an important role in several cellular processes such as respiration, oxidative stress defense, immune function, and angiogenesis (22, 46, 63). Copper is found in a number of important enzymes and proteins, such as, cytochrome c oxidase, Cu, Zn-superoxide dismutase 1 (SOD1), tyrosinase, lysyl oxidase, multicopper oxidase, amyloid precursor protein, and prion protein. In many of the copper-dependent enzymes, copper undergoes a change in redox state, an ability which is both essential and potentially harmful to the cell, as uncontrolled redox cycling of copper can generate reactive oxygen species (ROS). Indirect damage by copper to the cell through ROS affects lipid membranes, DNA, and proteins (19). In addition to the generation of ROS, copper may manifest its toxicity by displacing other metal cofactors from their natural ligands (49). Several cellular systems that are largely conserved in eukaryotes ensure sufficient uptake of copper and control its action to minimize damage (for reviews, see references 2 and 50). In metazoans, the copper importer Ctr1 mediates uptake, while the copper exporter ATP7 mediates export of excess copper, and copper chaperones deliver copper selectively to copper-dependent enzymes (10, 23). Cells protect themselves from toxic effects of copper and other transition metals of the group Ib and IIb, hereafter referred to as heavy metals, by sequestering them in metallothioneins. In this way, the intracellular free metal concentration is kept extremely low (45, 52). Metallothioneins are small proteins with a high cysteine content and the ability to bind up to nine copper ions or other metal ions, such as zinc and cadmium (for a review, see reference 29). Metallothioneins are found from yeast to humans and even in some plants and prokaryotes. Their conservation and the presence of multiple gene copies imply an important cellular function, notably in metal homeostasis and defense against toxicity of heavy metals. Yeast cells have two metallothioneins that confer copper resistance (12, 27). Knockout mice for two of the four metallothionein genes, Mt1 and Mt2, are viable but sensitive to cadmium and zinc load (31, 39, 42). In cell culture and in flies, metallothionein gene duplications correlate with increased resistance to copper (13, 16, 38). Metallothioneins are also involved in the defense against oxidative stress. They react in vitro with ROS; in vivo, they are able to reduce ROS-mediated DNA strand break formation and lipid peroxidation (1, 30, 51, 62, 64).
Transcription of metallothioneins in mammals is induced upon metal load and oxidative stress via the metal-responsive transcription factor MTF-1, which binds to metal response elements (MREs) in the promoter region. Cultured MTF-1 knockout cells show higher susceptibility to hydrogen peroxide and cadmium (20). Previously, we have reported the characterization of MTF-1 mutant flies. They are viable but highly sensitive to copper, zinc, and cadmium load, as well as to copper depletion (17). Similar to the situation with mice, basal and heavy metal-induced transcription of the four Drosophila melanogaster metallothionein genes (MtnA, MtnB, MtnC, and MtnD) depends on MTF-1 (17, 25). Despite considerable progress in our understanding of the transcriptional response to heavy metals, the regulation of MTF-1 activity is not well understood. What is known is that one or more of the six zinc fingers of MTF-1 have a relatively low affinity for zinc. Apothioneins, the newly synthesized metal-free form of metallothioneins, can effectively compete with MTF-1 for zinc binding, resulting in inhibition of MTF-1 DNA binding and transcriptional activation in a cell-free transcription system (7, 25, 71). Unlike zinc, other heavy metals such as copper or cadmium cannot directly stimulate MTF-1 DNA binding activity in a cell extract. These in vitro experiments have led to the model that zinc binds and activates MTF-1 directly, while cadmium or copper, which have a higher affinity to metallothioneins than zinc, displace the latter from metallothioneins, and subsequent binding of the released zinc to MTF-1 leads to its activation (71). In mammals, MTF-1 resides largely in the cytoplasm, from where it translocates to the nucleus upon cell stress, notably, metal load. Activation and nuclear translocation of MTF-1 are rapid, and transcript levels peak after about 6 h in the mouse liver and 24 h in cultured Drosophila cells (8, 32, 43, 56, 60). Besides signals leading to the activation of MTF-1, negative regulators of MTF-1 also exist (66); however, their mode of regulation is not yet known. Newly synthesized apothioneins are good candidates for negative feedback of zinc-mediated induction, while zinc-loaded metallothioneins may contribute to indirect induction by cadmium or copper.
Here, we report the generation of a gene family knockout of all four Drosophila metallothioneins by means of homologous recombination. Since the four genes are interspersed with other genes, they had to be selectively mutated one by one. This represents the first example of a knockout of all metallothioneins in a higher eukaryote. The resulting flies are viable and fertile but sensitive to elevated concentrations of copper or cadmium. They are, however, not sensitive to copper depletion, unlike mutants of the MTF-1 regulatory protein. We also report both a negative and a positive effect of copper on life span. A mild copper load shortens life span, perhaps due to increased copper-mediated oxidative stress; metallothionein mutant males are especially affected. On the other hand, an adequate copper supply is necessary for a normal life span, possibly by ensuring proper functioning of the antioxidant enzyme Cu,Zn-SOD. Metallothioneins are expressed in a tissue-specific manner, notably at sites of metal accumulation. Binding of copper to metallothioneins leads to an orange luminescence in copper-accumulating cells of the midgut, the so-called copper cells. Once heavy metal is bound in such a manner, it no longer triggers the activation of metallothionein genes, thus generating a negative feedback on metallothionein gene expression.
MATERIALS AND METHODS
pTARG, a versatile vector for gene targeting and Drosophila transgenesis.
The plasmid pTARG is a P-element vector derived from pCasper4. It includes a multiple cloning site, an I-CreI recognition sequence, a white cDNA driven by the artificial promoter 3xP3 (6, 26), two loxP sites, and two FLP recombinase target sites to release an episome for gene targeting. The 3xP3-white gene serves as a marker for transgenesis and for gene targeting, and the loxP sites can serve to eliminate the white marker gene. Targeting is performed as described previously (17, 54). Flies carrying the 3xP3-white marker gene show a typical orange eye color with a visible pseudopapilla and red pigment in the ocelli. This phenotype shows almost no variation between different transgenic lines and can easily be distinguished from a miniwhite transgene. Plasmid DNA and the entire sequence information are available upon request. The GenBank accession number of pTARG is DQ269206.
Generation of a metallothionein gene family knockout-knock-in.
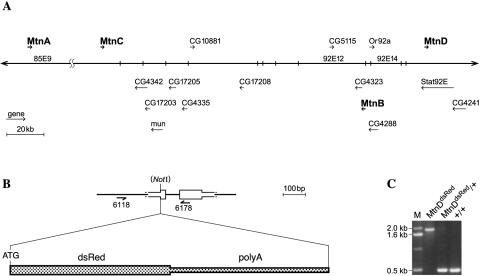
To generate metallothionein mutations, we used the ends-in targeting method (54). Gene-targeting events were selected based on the resistance of the white+ (w+) marker gene to ey-Flp, based on the presence of the mutation replacing the ATG with a NotI site, and based on the ability to undergo efficient elimination of the w+ marker by the endonuclease I-CreI, indicating the existence of a gene duplication that undergoes recombination and reduction to a single copy and leading to the loss of the w+ marker. Final evidence of gene targeting was obtained after reduction to a single copy, breeding of this reduction event to homozygosity, and analysis by PCR using primers flanking the metallothionein gene. This PCR amplifies both wild-type and mutant DNA. NotI digestion or sequencing of the coding region using a nested primer revealed whether the mutation was present in homozygous form. The molecular nature of each mutation is given in Table S1 in the supplemental material, and primer sequences are given in the supplementary material. All four metallothioneins were targeted in the background of a third chromosome derived from OregonR that contains an MtnD gene with a premature stop codon (allele MtnD*) (17). Different stocks are therefore isogenic for the third chromosome. Mutant alleles were designated MtnAΔATG, MtnBΔATG, MtnCΔATG, and MtnDΔATG. In addition to the MtnDΔATG-targeted allele, a knock-in allele of MtnD was generated by introduction of a dsRed gene, including a simian virus 40-pA terminus into the same NotI site that replaced the ATG of MtnD. This heterologous insertion did not obviously affect the efficiency of ends-in gene targeting. Targeting and verification of the targeted events were performed as with other ends-in constructs. PCR using primers flanking the region of interest showed that in homozygous knock-in flies, only a band about 1.3 kb longer than the wild-type band could be detected (Fig. 1B and C). This allele was designated MtnDdsRed. To test whether metal load could influence targeting efficiency, we added copper to the food. However, targeting efficiency at the MtnC locus did not increase. Construction of a quadruple metallothionein (qMtn) mutant was done in subsequent steps of targeting: MtnAΔATG MtnD* and MtnBΔATG MtnD* were recombined to yield MtnAΔATG MtnBΔATG MtnD*, also termed the triple metallothionein (tMtn*) mutant. Targeting of MtnC was performed in the tMtn* mutant background, resulting in MtnAΔATG MtnCΔATG MtnBΔATG MtnD*, termed qMtn*. The genotype before the reduction, which has the MtnC gene marked with w+ (MtnAΔATG MtnCw+ MtnBΔATG or MtnD*) was used to select for a meiotic crossover between MtnCw+ and MtnDdsRed. Flies with a chromosome that is both w+ and dsRed+ may also carry the mutant MtnBΔATG allele if the crossover occurs within the very short (31.5-kb) interval between MtnB and MtnD. We recovered a single crossover event of this kind among approximately 5,000 chromosomes screened. The allele MtnCw+ is not a null allele, since it is a duplication consisting of a wild type and a mutant copy of MtnC, flanking the w+ gene. Reduction of this duplication to a single copy finally resulted in the genotype MtnAΔATG MtnCΔATG MtnBΔATG MtnDdsRed, also termed qMtndsRed. For rapid genotyping, primers were designed that specifically recognize the mutant alleles.
FIG. 1.
Genetic map of Drosophila metallothioneins and knock-in strategy for MtnD. (A) A section of chromosome 3 with metallothioneins indicated in boldface type. The figure is adapted from the FlyBase database (http://flybase.bio.indiana.edu/). (B) Design of the MtnD knock-in allele. Arrows indicate the primers used for PCR. (C) Verification of the MtnD knock-in allele by PCR. Note that the shorter PCR wild-type product of about 0.5 kb is absent in homozygous MtnDdsRed mutant flies.
Metallothionein rescue experiments.
For the MtnB genomic rescue construct, the region from 1,529 bp upstream of the ATG start codon to 687 bp downstream of the stop codon was cloned into the pCasper4 vector. For the MtnA genomic rescue construct, the region from 569 bp upstream of the ATG start codon to 227 bp downstream of the stop codon was used. These constructs contain a cluster of upstream MREs, to which MTF-1 binds to activate transcription. Constructs were injected in qMtn* mutant flies to ensure comparison between isogenic flies. For rescue analysis, qMtn* mutant females were crossed with males of the same genotype but carrying the w+ marked transgene in a heterozygous state.
Fluorescent protein reporter.
For the construction of the MtnB-enhanced yellow fluorescent protein (EYFP) reporter, the segment −1460 to + 50 relative to the transcription start point was cloned in front of EYFP in a pCasper4 backbone. Cloning of the MtnA-EYFP reporter construct was reported previously (3). For the construction of 4×MRE-EYFP, the sequences derived from the MtnB or the Ctr1B promoter were chosen as described previously (57, 70). Transgenics were produced by P-element-mediated transgenesis. For analysis of EYFP expression by microscopy, flies were allowed to deposit eggs in the food and were raised until third-instar larval stage. Guts were dissected and mounted in glycerol. The construction of a Ctr1B-enhanced green fluorescent protein (EGFP) reporter transgene was reported previously (57).
Fly food.
A total of 1 liter of fly food was composed of 55 g corn, 100 g yeast, 75 g sugar (glucose), 8 g agar, 15 ml nipagin, and 10 g wheat. For longevity and toxicity experiments, food was supplemented with CdCl2, CuSO4, ZnCl2, HgCl2, AgNO3, or BCS disodium salt hydrate (Sigma-Aldrich catalogue no. 14,662-5) to the concentrations indicated. BCS is a specific copper chelator that is used to deplete the food of copper.
Toxicity experiments.
Flies were allowed to deposit a determined number of eggs on food, and eclosing adults were counted. Survival on metal-supplemented food was normalized to survival of the corresponding strain on normal food (NF). Survival from egg to adult on food without a metal supplement varied between 50 and 70%. Experiments were performed at least three times and also at different concentrations of the same metal. An extensive analysis is presented (see Fig. S2 in the supplemental material) of the sensitivity of both a wild-type (y w) and the MtnD* mutant strain to various concentrations of Zn, Cu, Cd, Hg, and the copper-specific chelator BCS. Flies were kept at the standard temperature of 25°C.
Quantification of metallothionein transcripts.
Larvae were continuously raised on the indicated type of food or transferred for 6 h either to normal food or to metal-supplemented food. Only third-instar feeding larvae were used for the analysis. Total RNA was extracted with TRIzol reagent (Life Technologies). Nuclease S1 mapping of transcripts with 50 μg of total RNA was performed as described previously (67). The gels were developed using a PhosphorImager (Molecular Dynamics), and bands were quantified. The signal from the endogenous actin5c gene was used for normalization of metallothionein transcript levels.
Life span experiments and SOD1 measurements.
Life span experiments were carried out at 28°C. Isogenic flies were grown from embryonic stage on food with or without metal supplementation. To ensure that the results were comparable, special care was taken to grow heterozygous and homozygous mutant flies on the same batch of food and even in the same tube. This was achieved by crossing homozygous qMtn* mutant virgins with heterozygous mutant males, resulting in 50% homozygous and 50% heterozygous mutant offspring from the same vials. This was necessary because the complex composition of fly food can lead to variation in the metal content of flies grown on different preparations of the same ingredients (unpublished data). For life span analysis, males or virgin females were kept in groups of 30 and transferred twice a week to new tubes with or without a metal supplement; dead flies were counted every day. For each condition, 80 to 400 flies were analyzed. SOD1 activity was measured using the Calbiochem superoxide dismutase assay kit (catalogue no. 574600). For total protein extracts, about 30 flies were homogenized in a buffer consisting of 50 mM Tris (pH 8.0)-0.125 mM NaCl, and the protein content was quantified by the Bradford assay from Bio-Rad.
Imaging and microscopy.
Copper cell luminescence and fluorescent protein expression in dissected guts were analyzed with a Leica DRB fluorescence stereomicroscope equipped with filters A for DAPI (4′,6′-diamidino-2-phenylindole) and copper cell fluorescence, N2.1 for dsRed, and I3 for EGFP and EYFP. Pictures were taken with a Zeiss Axiocam. Confocal images were taken with a Leica SP1 UV confocal laser scanning microscope.
Metal measurements.
Groups of equal weight consisting of about 15 adult males (2 to 3 days old) were analyzed by inductively coupled plasma mass spectrometry (ICP-MS), as described previously (58). Flies were dissolved in 65% HNO3 in a microwave oven and then diluted to a 6.5% HNO3 solution for analysis. ICP-MS was performed using a HP4500 Series 300 ShieldTorch System instrument (Agilent, Waldbronn, Germany) in peak-hopping mode with spacing at 0.05 amu, 3 points/peak, 5 scans/replicate, 2 to 3 replicates/sample, and an integration time of 400 ms/point. The rate of plasma flow was 15 liters/min with an auxiliary flow of 1.0 liters/min. The RF power was 1.2 kW. The sample was introduced using a cross-flow nebulizer at a flow rate of 1.06 liters/min. The apparatus was calibrated using a 6.5% HNO3 solution containing Cu at 5, 10, 25, 50, and 100 ppb with 103Rh, the internal standard for all isotopes of Cu.
RESULTS
A metallothionein gene family knockout.
The Drosophila genome harbors four metallothionein genes (Mtn), designated MtnA to -D (17). They encode short proteins of 40 to 44 amino acids, which contain 10 to 12 cysteine residues in configurations characteristic of metallothioneins (CysXCys and CysXXCys) (53). MtnB, MtnC, and MtnD displayed >67% amino acid identity and probably arose by gene duplication, whereas MtnA only shared the cysteine motifs with the other three metallothioneins. These relations were also reflected in the genomic organization. All four genes are located on chromosome 3, but while MtnA localized to the cytological position 85E, MtnB, MtnC, and MtnD all localized to 92E within a segment of 170 kb. However, the 170-kb segment also harbored 11 genes interspersed with the metallothioneins (Fig. 1A). The small size of the metallothionein genes, as well as their genomic organization, precluded a comprehensive analysis by classical genetic methods. Therefore, we employed the recently introduced tool of gene targeting by homologous recombination in Drosophila to generate flies mutant for single metallothioneins and for combinations of them, including a mutant for all four metallothioneins, in the same genetic background derived from OregonR (54). This fly strain carries a spontaneous truncation allele of MtnD, hereafter referred to as MtnD* (17). The mutations were introduced by ends-in gene targeting and designed such that a NotI recognition site replaced the ATG start codon and several neighboring amino acids, resulting in the alleles MtnAΔATG, MtnBΔATG, MtnCΔATG, and MtnDΔATG. Even though promoter sequences were still intact and led to the production of mutant mRNAs, these could not be translated and at least some of them were unstable, compared to the wild type, as revealed by a severe reduction of full-length MtnA mRNA (see Fig. S1 in the supplemental material). The introduced mutations therefore most likely represent null mutations. We also generated a knock-in replacement allele of MtnD, designated MtnDdsRed, resulting in expression of a red fluorescent dsRed protein under the control of the endogenous MtnD promoter (Fig. 1B and C). This allele is both a null mutation and a reporter gene for the expression of the MtnD locus. In contrast to a reporter transgene, such a knock-in reporter is not subject to position effects that would affect the pattern and strength of expression.
Drosophila metallothionein mutants are highly sensitive to copper and cadmium, to a lesser extent to zinc, but not to mercury or silver load or to copper depletion.
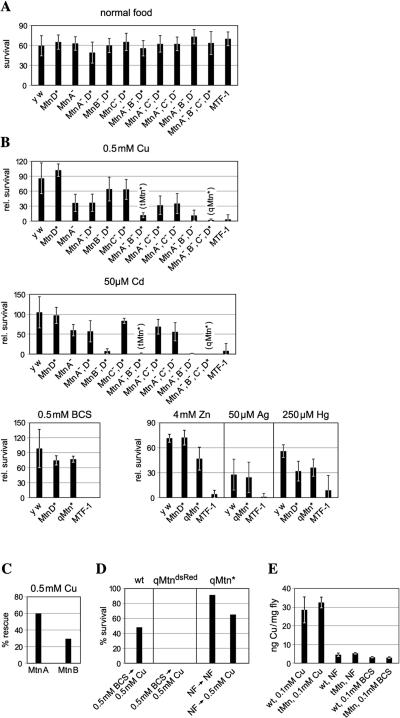
Flies mutant for all four metallothionein genes, MtnAΔATG, MtnBΔATG, MtnCΔATG, and MtnD* or MtnAΔATG, MtnBΔATG, MtnCΔATG, and MtnDdsRed, hereafter referred to as quadruple Mtn (qMtn* and qMtndsRed, respectively) mutant flies, are viable and fertile, but highly sensitive to copper or cadmium load in food (Fig. 2A and B). Whereas 0.5 mM copper did not affect the survival of wild-type flies, qMtn* mutant flies and also tMtn* (triple mutant; MtnAΔATG MtnBΔATG MtnD*) flies are unable to survive when raised on this copper concentration, apart from a few escapers that die shortly after eclosion. Death can occur at different stages of development, but high copper concentrations inevitably resulted in early larval death, whereas lower concentrations allowed progression to later larval stages or even to adulthood. Similarly to copper, 50 μM cadmium was lethal for qMtn* mutants but not for wild-type flies. The protective effect of metallothioneins correlated with the transcriptional activation of metallothionein promoters and with the metal binding ability of Drosophila metallothioneins. Previous work has demonstrated that both copper and cadmium are very strong inducers of all four metallothioneins and that both MtnA and MtnB have a high capacity for binding copper and cadmium ions (14, 17, 65, 70). qMtn* mutants are as sensitive to copper or cadmium as a deletion mutant of MTF-1, thus providing a clear-cut explanation for the metal sensitivity of MTF-1 mutants in which transcription from the Mtn loci is abrogated. However, unlike MTF-1 mutants, qMtn* mutants were not sensitive to copper depletion. This is not unexpected, since MTF-1 also controls copper import by the activation of the gene for the copper importer Ctr1B (57). Consistent with this, under conditions of copper scarcity or copper load tMtn mutants maintained a body copper level similar to that of the wild type (Fig. 2E). These findings ruled out any essential role of metallothioneins in copper import or as copper chaperones for the transfer of copper to copper-dependent enzymes (34). Surprisingly, even though MTF-1 mutants were clearly more sensitive to zinc than wild-type flies, zinc sensitivity in metallothionein mutants was only marginally elevated (Fig. 2B). MTF-1 mutant, but not qMtn mutant, flies also showed a clearly elevated sensitivity to mercury and silver.
FIG. 2.
Viability and metal content of Mtn and MTF-1 mutants and the wild type on normal or metal-supplemented food. (A) Absolute viability of different genotypes on normal food. Note that deviations between different strains are within the experimental error. (B) The bar diagrams depict the percentage of survival of mutant and wild-type (y w) embryos to adulthood. Survival on normal food of each genotype is set at 100%. Flies were allowed to deposit 150 to 300 eggs on food containing the indicated concentrations of metals, and eclosing adults were counted. Error bars represent standard deviations of several independent experiments, calculated from the number of flies in a total of 3 to 10 different tubes. (C) A single metallothionein transgene can partially rescue the phenotype of a metallothionein gene family knockout with the genotype qMtn*. Shown is the survival of transgenic embryos to adulthood on 500 μM copper (as a percentage of the survival of the isogenic MtnD* genotype). (D) Sensitivity of metallothionein mutants to copper shock. Approximately 30 third-instar larvae were transferred from and to the indicated type of food. The bars represent the percentage of eclosing adults. Note that there are no survivors of the qMtndsRed genotype. (E) Copper content of wild-type (wt) and metallothionein mutant flies. Flies were grown on the indicated type of food and analyzed by ICP-MS.
The analysis of single metallothionein mutants or of different combinations revealed that MtnB plays a major role in the defense against the toxicity of cadmium, whereas MtnA is of major importance under copper load (Fig. 2B). Accordingly, double mutants of MtnA and MtnB showed enhanced sensitivity to both metals. The role of MtnC and MtnD in the defense against heavy metals was less prominent. MtnD* alone or the double mutant MtnCΔATG MtnD* was only marginally more sensitive to either cadmium or copper in comparison to the wild type. The role of MtnC and MtnD became evident only in the absence of both MtnA and MtnB; thus, the most sensitive genotypes were qMtn* and qMtndsRed (Fig. 2B and D). Differences between individual metallothioneins were also revealed by rescue analysis. Genomic transgenes of MtnA and, to a lesser extent, a transgene of MtnB were able to partially rescue copper sensitivity of a qMtn* mutant fly, consistent with MtnA playing a major role in the defense against copper toxicity (Fig. 2C).
Metallothioneins played an important role not only during long-term exposure to copper but also in response to acute copper exposure (Fig. 2D). We transferred third-instar larvae from either copper-depleted food or normal food into food with elevated copper concentrations. The effect was most striking in a transition from copper starvation to copper abundance. While wild-type larvae survived this transition well, metallothionein mutants died after several hours without developing into pupae. This experiment shows that metallothioneins are important for coping with copper fluctuations. In wild-type larvae, basal level expression of metallothionein genes, in concert with their rapid induction, is able to protect the organism from a sudden increase in copper concentration.
Metal response elements are sufficient to mediate tissue-specific expression of metallothioneins.
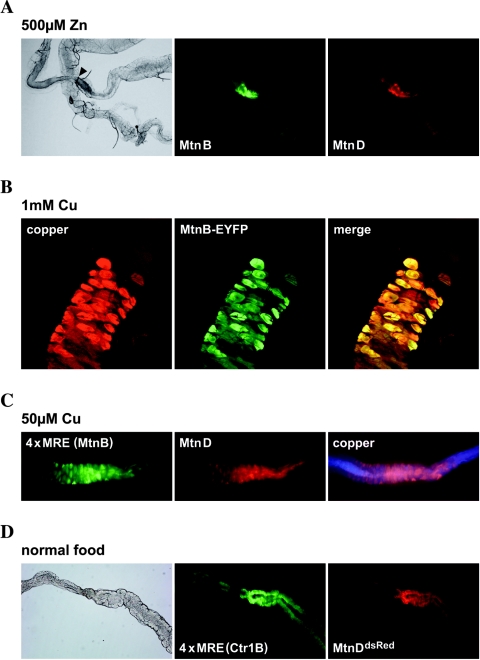
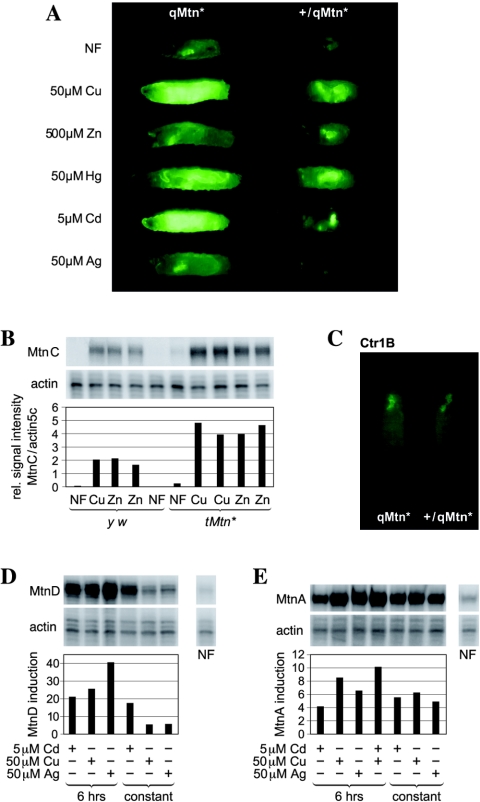
Metallothioneins are expressed in a tissue-specific manner in virtually identical expression patterns (Fig. 3; see Fig. S3 in the supplemental material). In larvae, the sites of expression include the so-called copper cells, or cuprophilic cells, which are copper-accumulating cells in the midgut. Mtn expression can also be observed at the midgut constriction, in Malpighian tubules, in the salivary glands, the fat body, in the cuticle, and in the trachea. Mtn expression is not detected in the brain or in imaginal disks, even in response to a very high metal load (15). Copper, cadmium, and zinc differentially induce Mtn expression in various regions of the gut: copper induces mostly in the copper cells, while zinc and cadmium induce at the midgut constriction and in the posterior midgut. To investigate the reason for this tissue-specific induction, we analyzed the expression pattern of EGFP under the control of the synthetic 4×MRE promoter that contains MREs derived from either MtnB or Ctr1B. Ctr1B is expressed in an MTF-1-dependent manner in the midgut in response to low levels of copper, but a minimal synthetic promoter consisting merely of the MREs of this gene is inducible by copper load (57). Both synthetic promoters showed a striking overlap of EGFP with dsRed expressed from the MtnDdsRed knock-in allele, as well as the expression pattern produced by the full-length MtnB promoter. Both the basal and metal-induced expression from the 4×MRE reporter that contains MREs derived from MtnB occurred in the very same tissues as MtnB expression (see Fig. S3B in the supplemental material). Even a ubiquitous overexpression of MTF-1 under the control of a tubulin promoter did not alter the expression pattern of the allele MtnDdsRed (not shown).
FIG. 3.
MREs are sufficient for tissue-specific expression of metallothioneins. Flies were raised on the type of food indicated. Shown is the fluorescence of reporter transgenes with the indicated promoters driving EYFP or dsRed. The anterior is shown to the left. copper, copper luminescence. (A) Expression patterns of MtnB and MtnD, two members of the MtnB subfamily, coincide completely. Shown is the fluorescence of the MtnB-EYFP reporter construct and the allele MtnDdsRed near the midgut constriction (arrowhead). (B) Colocalization of MtnB promoter activity with copper cell luminescence on 1 mM copper. Shown is the expression of an MtnB-EYFP transgene. (C) A synthetic promoter with MREs derived from MtnB is sufficient for tissue-specific expression and for metal induction. Note the overlap of reporter transgenes with each other and with copper cell luminescence. (D) Overlap of expression near the midgut constriction of the MtnDdsRed knock-in allele with a synthetic minipromoter composed of MREs of Ctr1B. Larvae were grown on normal food.
Metallothioneins form a luminescent complex with copper.
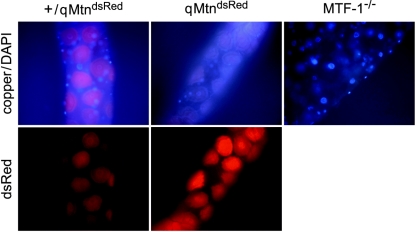
The copper cells in the Drosophila midgut show a bright orange luminescence under UV light when larvae are fed with copper (48). Cu(I) thiolates, as well as Cu-metallothionein complexes in yeast and Neurospora crassa, are known to yield a characteristic orange light emission when excited with UV light (5). This luminescence depends on a Cu(I) state and occurs only when the complex is shielded from solvent quenching (9, 35). It was already proposed that Drosophila copper cell luminescence is due to a copper-metallothionein complex, as expression of metallothioneins and luminescence coincide (Fig. 3B and C) (41). To test this hypothesis, we examined the guts of MTF-1 and qMtn mutant larvae that had been fed with copper. Indeed, copper cell luminescence was strongly reduced in either qMtn or MTF-1 mutants but was not completely absent (Fig. 4). As the expression of metallothioneins in copper cells depends on the presence of copper, the MtnDdsRed knock-in allele served as an important control. We observed a strong and specific induction of MtnDdsRed in copper cells of both mutant and wild-type larvae, showing that the lack of metallothioneins neither affects the morphology of copper cells nor leads to a reduced copper import. A complex of metallothionein with copper therefore accounts for the majority of copper cell luminescence, and a loss of metallothioneins results in an increased solvent accessibility of copper. However, in qMtn, as well as in MTF-1 mutants, a residual luminescence remained, possibly due to copper binding to other cellular components, which were less able to shield copper from water (Fig. 4 and data not shown). Interestingly, expression of the dsRed knock-in reporter at the MtnD locus was much stronger in the homozygous qMtn mutants than in heterozygous controls (Fig. 4). This prompted us to test a possible autoregulation of metallothionein expression.
FIG. 4.
Metallothioneins form a luminescent complex with copper in copper cells of the larval midgut. Copper luminescence and DAPI staining (top) and expression of MtnDdsRed (bottom) in the same gut is shown. The ubiquitous bluish fluorescence that partially masks DAPI staining is due to autofluorescence of midgut cells (18). Note that copper cell luminescence is reduced but not completely absent in both homozygous qMtndsRed and MTF-1 mutants. Homozygosity of the MtnDdsRed locus leads to stronger dsRed expression than in heterozygotes. Larvae were raised on 50 μM copper until the third instar.
Metallothioneins negatively regulate MTF-1 transcriptional activity in vivo.
We tested the activity of the endogenous MtnC promoter, as well as the expression levels of an MtnB-EYFP reporter gene, in both the wild-type and in metallothionein mutants, the latter being represented by qMtn* or the triple mutant tMtn* (MtnA, MtnB, and MtnD mutated). In qMtn* and tMtn* mutants, both promoters showed a significantly higher expression level, about two- to threefold higher than that of the wild type, in response to either a short metal stimulus (6 h) or to constant metal exposure (Fig. 5A and B). In contrast, the expression of the Ctr1B copper importer gene, which also depends on MTF-1 activity (57), was not changed in qMtn mutants, as analyzed by a Ctr1B-EGFP reporter signal (Fig. 5C). This shows that the negative feedback is restricted to metallothionein promoters and is consistent with the fact that metallothionein mutants do not differ from the wild type in their overall metal content (Fig. 2E).
FIG. 5.
Metallothioneins negatively regulate their own expression. (A) MtnB-EYFP reporter expression in larvae heterozygous or homozygous for the qMtn* chromosome. The anterior of third-instar larvae is shown on the left. (B) Quantification of expression levels of MtnC in a wild-type (y w) and a triple-Mtn (tMtn) mutant background, carrying the alleles MtnAΔATG, MtnBΔATG, and MtnD*. Third-instar larvae were transferred for 6 h to 500 μM copper or 4 mM zinc, and transcript levels were assayed by S1 nuclease mapping. The bar diagram represents the quantification of the bands, normalized with the signal from actin5C transcripts. (C) Expression of a genomic Ctr1B-EGFP construct in a qMtn* or heterozygous wild-type larvae (qMtn*/wt). (D and E) Metallothionein transcript levels in wild-type MtnD (D) or MtnA (E) flies after a 6-h induction period or after constant growth on the indicated type of food. Quantification is relative to NF.
A possible consequence of the negative feedback of metallothionein expression on MTF-1 activity may be that transcription at the metallothionein loci depends on the change of metal concentration over time. Consistent with such a scenario, transfer of wild-type larvae from normal conditions to food with 50 μM copper, i.e., corresponding to a mild copper load, induced metallothionein transcripts to higher levels than permanent exposure to 50 μM copper (Fig. 5D and E). The lower expression level upon constant copper load probably reflects an already elevated concentration of metallothioneins that are able to chelate free metal, thus obviating the need for MTF-1 activation.
Copper shows a dual effect on life span.
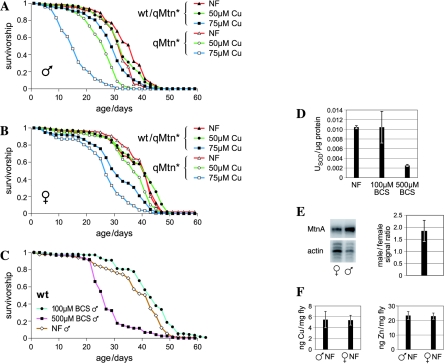
We also tested the life span of qMtn* mutant and heterozygous wild-type flies at different copper levels (Fig. 6A and B). The Drosophila cultures were grown throughout development on normal food or food containing 50 μM, 75 μM, or 1 mM copper or 500 μM BCS, the specific copper chelator. These concentrations were chosen because they do not affect survival during development, i.e., prior to the life span analysis. Cu at a concentration of 75 μM was not at all toxic for (heterozygous) wild-type Drosophila during development, and the survival of qMtn* mutant males and females was not reduced (not shown). However, 1 mM was lethal for qMtn* mutants but did not affect the viability of (heterozygous) wild-type flies during development (not shown). We determined the total body copper content under these conditions: flies grown with 500 μM BCS, NF, or 50 μM Cu contained about 1 ng, 5 ng, and 20 ng of copper per mg tissue, respectively (H. Yepiskoposyan, unpublished data). A sufficient copper supply is critical for defense against oxidative stress, since SOD1 is a copper-dependent enzyme (40). While mild copper depletion (100 μM BCS) did not reduce the life span of wild-type flies, copper starvation in 500 μM BCS did dramatically reduce life span (Fig. 6C). Indeed, larvae grown in 500 μM BCS showed very low levels of SOD1 activity (Fig. 6D), which is consistent with earlier findings that SOD1 mutant flies have a severely reduced life span (47). Insufficient levels of organismal copper may therefore lead to increased oxidative stress and thereby shorten the life span. On the other hand, a mild copper load (50 μM) did not affect the life span of heterozygous wild-type flies, while an increase to 1 mM copper reduced life span by 8 and 3 days for males and females, respectively (see Fig. S4 in the supplemental material). With normal food, the life span was very similar for homozygous qMtn* mutant flies and heterozygous control flies. However, a difference became evident with a mild copper load: the mean life span of metallothionein mutant males was 6 days shorter at 50 μM copper and 14 days shorter at 75 μM copper, in comparison to metallothionein-containing (qMtn*/+) males. The lack of metallothioneins affected females to a lesser extent. The mean life span of metallothionein mutant females was 3 days shorter, at both 50 μM and 75 μM copper, than that of metallothionein-containing controls. In agreement with a greater dependence of males on functional metallothioneins, wild-type males expressed almost twofold-higher levels of metallothionein mRNAs, even though both sexes contained the same amount of zinc and copper (Fig. 6E and F).
FIG. 6.
Life span of heterozygous wild-type (qMtn*/wt) and homozygous (qMtn*) metallothionein mutant flies raised at different copper concentrations. Day 0 represents the day of eclosion from pupae. Survivorship curves indicate the proportion of a population surviving at different ages. The life spans of heterozygous and homozygous qMtn mutant males (A) and females (B) are shown. (C) Life span of flies with copper depletion raised and kept in copper chelator BCS. This particular experiment was done at 26°C; all other experiments were done at 28°C. (D) SOD1 activity in flies raised on normal food or on low-copper food. (E) Metallothionein expression levels in males and females determined by S1 nuclease mapping. (F) Copper and zinc content of wild-type flies.
DISCUSSION
The genomic arrangement of the metallothionein genes made the generation of a quadruple knockout a difficult endeavor. All four genes are located on the same chromosome, but they are interspersed with many other genes, which precluded the removal of a large segment. Rather, the genes had to be mutated one by one by means of targeted gene disruption. We show here that Drosophila metallothioneins have an important role in copper homeostasis, as well as in the detoxification of cadmium. While cadmium toxicity is often caused by industrial pollution, copper is an essential trace element, and its homeostasis is physiologically important. The copper tolerance of wild-type flies is truly remarkable. In metallothionein mutants, a slight increase in copper levels, whether from a constant exposure or a transient copper shock, results either in larval death or in a shortened life span. With increased sensitivity to copper and cadmium and, to a lesser extent, to zinc, metallothionein mutants mirror most but not all aspects of the Drosophila MTF-1 mutant phenotype. Flies lacking MTF-1 are more sensitive to zinc, silver, and mercury than metallothionein mutants. This difference can best be explained by MTF-1-dependent activation of genes involved in metal homeostasis other than the metallothioneins. For example, MTF-1 induces the expression of a putative zinc exporter (CG3994) under conditions of zinc excess, preventing zinc overload of the cell (Yepiskoposyan, unpublished). The sensitivity of MTF-1 mutants to silver might be due to low expression levels of the copper importer Ctr1B, another MTF-1 target gene (57). Silver exerts its toxicity by competing with copper, and competition is expected to be more severe in MTF-1 mutants, which have lower copper levels than wild-type flies (H. Yepiskoposyan and K. Balamarugan, unpublished data). Besides copper and cadmium, mercury, silver, and zinc induce metallothionein transcription (Fig. 5A), but these metals are not more toxic to metallothionein mutants than to the wild type. Apparently, metallothioneins are not able to protect against all compounds that induce their synthesis (see also reference 16).
Wild-type flies can develop at a copper concentration at least 200-fold higher (1 mM) than the normal copper content in food (5 μM) without survival to adulthood being affected (17). Indeed for most organisms, copper is a relatively benign trace element, thanks to sophisticated transport and detoxification systems. However, it can be toxic under special circumstances, such as in association with genetic defects of copper homeostasis or upon environmental accumulation, notably in vineyards where it is used as an antifungal agent. Metallothioneins protect the cells from toxic effects of copper by binding and sequestering the metal inside the protein. This metallothionein-copper complex can be conveniently observed in vivo as an orange copper luminescence. The reduction of copper cell luminescence in qMtn mutants suggests that copper is now solvent accessible and able to damage the cell, possibly via the generation of ROS and/or ectopic binding to protein sulfhydryl groups. Formation of ROS by copper is often proposed as the major mechanism of copper toxicity (19). The sensitivity of SOD1 mutant flies to high levels of copper is in agreement with such a scenario (47). In the Long-Evans cinnamon rat strain, a model system for a human copper homeostasis disorder, Wilson's disease, copper accumulation in the liver induces ROS production, which results in lipid peroxidation, impaired mitochondrial function, and increased DNA damage (24, 28, 61). However, under physiological conditions, copper also has a role in antioxidant defense as an essential component of the Cu, Zn-SOD. We found that low dietary copper levels impaired the catalytic function of Drosophila Cu,Zn-SOD, similar to what has been observed with mammals (21), and that such flies displayed a dramatically shortened life span. The effect of copper concentration on life span apparently follows a U-shape curve, as both copper starvation and elevated concentrations shorten the life span. In both extremes, a likely cause of the premature death of adult flies is the accumulation of ROS-mediated damage. Under conditions of copper load, the shortened life span is particularly evident for metallothionein mutant males that normally express metallothionein mRNA at higher levels than females. Thus, we propose that adult males depend more on the protective effects of metallothioneins than females.
We found that all four Drosophila metallothioneins and even a transgene with a synthetic minipromoter composed merely of four tandem copies of MREs are expressed in a very similar tissue- and cell-type-specific pattern in the intestine, which reflects the known accumulation of copper or cadmium. For example, metallothioneins are expressed in the “copper cells,” which are well known for their peculiar ability to accumulate large amounts of copper (18, 36; for a review, see reference 4). Cadmium was previously shown to accumulate in the anterior midgut, in the “iron cell” region, and in the posterior midgut in a pattern highly similar to the metallothionein induction we report here (33, 37). As MTF-1 is the only transcription factor known to bind directly to MREs but ubiquitous overexpression of MTF-1 does not change the expression pattern of metallothioneins, we conclude that metallothionein expression is governed by metal distribution. Thus, a metallothionein promoter driving a fluorescent protein reporter can be used as a highly sensitive and semiquantitative biomarker for cellular metal transport (69). This may help to investigate the function of putative metal transporters in vivo, as nicely demonstrated in a recent study of the copper exporter ATP7 (44).
Metallothioneins, together with glutathione, an antioxidant with relatively nonspecific metal binding ability, constitute the first line of defense against the toxic effects of heavy metals in both mammals (68) and insects. As ingested metals first reach the gut cells, metallothioneins in the gut probably serve to trap toxic metals and limit their distribution throughout the body. An interesting example of such trapping of toxic metal is the zinc treatment of Wilson's disease patients, who suffer from copper accumulation in the liver. Zinc treatment induces metallothionein synthesis in the intestine; due to the metallothionein's high affinity to copper, the latter is trapped within intestinal cells and eventually excreted (59).
A remarkable finding in the present study is the autoregulation of metallothionein expression. Metallothionein promoters used as a reporter of MTF-1 activity are more active in metallothionein mutants. This is not due to a higher copper content, as total body copper does not differ between wild-type and metallothionein mutants. Rather, metallothioneins can inhibit their own expression by inhibiting MTF-1 function via the binding of free metal, which otherwise would directly or indirectly activate MTF-1. The nature of the signal that activates MTF-1 in vivo is still not established. While zinc can directly bind to MTF-1, copper and cadmium interfere with DNA binding of MTF-1 in vitro. They are thus thought to activate MTF-1 indirectly and were indeed shown to do so in a cell-free model system by displacement of zinc from metallothioneins, which in turn bound to MTF-1 (71). The present study, however, shows that metal-loaded metallothioneins are not essential in vivo for the activation of MTF-1 by any of the heavy metals tested. It is therefore likely that zinc can be displaced from other cellular pools. Furthermore, as shown with mammals, MTF-1 regulation in vivo occurs at multiple levels that include nucleocytoplasmic transport, protein phosphorylation, and a conspicuous cysteine cluster toward the C terminus of MTF-1, which might act as a metal sensor (11, 55, 56). In conclusion, the present study does not support the concept of metallothioneins playing an important role in copper uptake and intracellular distribution but firmly establishes their importance for coping with metal fluctuations, notably for copper homeostasis and cadmium detoxification.
Supplementary Material
Acknowledgments
We are grateful to Bruno Schmid and Antonia Manova for technical assistance, to Fritz Ochsenbein for the preparation of figures, and to Jeremias Kägi, Michael Fetchko, and George Hausmann for critical reading of the manuscript.
This work was supported by the Kanton Zürich and by the Swiss National Science Foundation.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abel, J., and N. de Ruiter. 1989. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol. Lett. 47:191-196. [DOI] [PubMed] [Google Scholar]
- 2.Askwith, C., and J. Kaplan. 1998. Iron and copper transport in yeast and its relevance to human disease. Trends Biochem. Sci. 23:135-138. [DOI] [PubMed] [Google Scholar]
- 3.Balamurugan, K., D. Egli, A. Selvaraj, B. Zhang, O. Georgiev, and W. Schaffner. 2004. Metal-responsive transcription factor (MTF-1) and heavy metal stress response in Drosophila and mammalian cells: a functional comparison. Biol. Chem. 385:597-603. [DOI] [PubMed] [Google Scholar]
- 4.Ballan-Dufrancais, C. 2002. Localization of metals in cells of pterygote insects. Microsc. Res. Tech. 56:403-420. [DOI] [PubMed] [Google Scholar]
- 5.Beltramini, M., G. M. Giacometti, B. Salvato, G. Giacometti, K. Munger, and K. Lerch. 1989. Luminescence emission from Neurospora copper metallothionein. Time-resolved studies. Biochem. J. 260:189-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berghammer, A. J., M. Klingler, and E. A. Wimmer. 1999. A universal marker for transgenic insects. Nature 402:370-371. [DOI] [PubMed] [Google Scholar]
- 7.Bittel, D., T. Dalton, S. L. Samson, L. Gedamu, and G. K. Andrews. 1998. The DNA binding activity of metal response element-binding transcription factor-1 is activated in vivo and in vitro by zinc, but not by other transition metals. J. Biol. Chem. 273:7127-7133. [DOI] [PubMed] [Google Scholar]
- 8.Bunch, T. A., Y. Grinblat, and L. S. B. Goldstein. 1988. Characterization and use of the Drosophila metallothionein promoter in cultured Drosophila melanogaster cells. Nucleic Acids Res. 16:1043-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd, J., R. M. Berger, D. R. McMillin, C. F. Wright, D. Hamer, and D. R. Winge. 1988. Characterization of the copper-thiolate cluster in yeast metallothionein and two truncated mutants. J. Biol. Chem. 263:6688-6694. [PubMed] [Google Scholar]
- 10.Camakaris, J., I. Voskoboinik, and J. F. Mercer. 1999. Molecular mechanisms of copper homeostasis. Biochem. Biophys. Res. Commun. 261:225-232. [DOI] [PubMed] [Google Scholar]
- 11.Chen, X., B. Zhang, P. M. Harmon, W. Schaffner, D. O. Peterson, and D. P. Giedroc. 2004. A novel cysteine cluster in human metal-responsive transcription factor 1 is required for heavy metal-induced transcriptional activation in vivo. J. Biol. Chem. 279:4515-4522. [DOI] [PubMed] [Google Scholar]
- 12.Culotta, V. C., W. R. Howard, and X. F. Liu. 1994. CRS5 encodes a metallothionein-like protein in Saccharomyces cerevisiae. J. Biol. Chem. 269:25295-25302. [PubMed] [Google Scholar]
- 13.Czaja, M. J., F. R. Weiner, and J. H. Freedman. 1991. Amplification of the metallothionein-1 and metallothionein-2 genes in copper-resistant hepatoma cells. J. Cell Physiol. 147:434-438. [DOI] [PubMed] [Google Scholar]
- 14.Domenech, J., O. Palacios, L. Villarreal, P. Gonzalez-Duarte, M. Capdevila, and S. Atrian. 2003. Mto: the second member of a Drosophila dual copper-thionein system. FEBS Lett. 533:72-78. [DOI] [PubMed] [Google Scholar]
- 15.Durliat, M., F. Bonneton, E. Boissonneau, M. Andre, and M. Wegnez. 1995. Expression of metallothionein genes during the postembryonic development of Drosophila melanogaster. Biometals 8:339-351. [DOI] [PubMed] [Google Scholar]
- 16.Durnam, D. M., and R. D. Palmiter. 1987. Analysis of the detoxification of heavy metal ions by mouse metallothionein. Experientia Suppl. 52:457-463. [DOI] [PubMed] [Google Scholar]
- 17.Egli, D., A. Selvaraj, H. Yepiskoposyan, B. Zhang, E. Hafen, O. Georgiev, and W. Schaffner. 2003. Knockout of ‘metal-responsive transcription factor’ MTF-1 in Drosophila by homologous recombination reveals its central role in heavy metal homeostasis. EMBO J. 22:100-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filshie, B. K., D. F. Poulson, and D. F. Waterhouse. 1971. Ultrastructure of the copper-accumulating region of the Drosophila larval midgut. Tissue Cell 3:77-102. [DOI] [PubMed] [Google Scholar]
- 19.Gaetke, L. M., and C. K. Chow. 2003. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147-163. [DOI] [PubMed] [Google Scholar]
- 20.Günes, C., R. Heuchel, O. Georgiev, K. H. Müller, P. Lichtlen, H. Blüthmann, S. Marino, A. Aguzzi, and W. Schaffner. 1998. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J. 17:2846-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris, E. D. 1992. Copper as a cofactor and regulator of copper, zinc superoxide dismutase. J. Nutr. 122:636-640. [DOI] [PubMed] [Google Scholar]
- 22.Harris, E. D. 2004. A requirement for copper in angiogenesis. Nutr. Rev. 62:60-64. [DOI] [PubMed] [Google Scholar]
- 23.Harrison, M. D., C. E. Jones, M. Solioz, and C. T. Dameron. 2000. Intracellular copper routing: the role of copper chaperones. Trends Biochem. Sci. 25:29-32. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi, M., T. Kuge, D. Endoh, K. Nakayama, J. Arikawa, A. Takazawa, and T. Okui. 2000. Hepatic copper accumulation induces DNA strand breaks in the liver cells of Long-Evans cinnamon strain rats. Biochem. Biophys. Res. Commun. 276:174-178. [DOI] [PubMed] [Google Scholar]
- 25.Heuchel, R., F. Radtke, O. Georgiev, G. Stark, M. Aguet, and W. Schaffner. 1994. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J. 13:2870-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horn, C., B. Jaunich, and E. A. Wimmer. 2000. Highly sensitive, fluorescent transformation marker for Drosophila transgenesis. Dev. Genes Evol. 210:623-629. [DOI] [PubMed] [Google Scholar]
- 27.Jensen, L. T., W. R. Howard, J. J. Strain, D. R. Winge, and V. C. Culotta. 1996. Enhanced effectiveness of copper ion buffering by CUP1 metallothionein compared with CRS5 metallothionein in Saccharomyces cerevisiae. J. Biol. Chem. 271:18514-18519. [DOI] [PubMed] [Google Scholar]
- 28.Kadiiska, M. B., P. M. Hanna, S. J. Jordan, and R. P. Mason. 1993. Electron spin resonance evidence for free radical generation in copper-treated vitamin E- and selenium-deficient rats: in vivo spin-trapping investigation. Mol. Pharmacol. 44:222-227. [PubMed] [Google Scholar]
- 29.Kägi, J. H. R. 1991. Overview of metallothionein. Methods Enzymol. 205:613-626. [DOI] [PubMed] [Google Scholar]
- 30.Kang, Y. J. 1999. The antioxidant function of metallothionein in the heart. Proc. Soc. Exp. Biol. Med. 222:263-273. [DOI] [PubMed] [Google Scholar]
- 31.Kelly, E. J., C. J. Quaife, G. J. Froelick, and R. D. Palmiter. 1996. Metallothionein I and II protect against zinc deficiency and zinc toxicity in mice. J. Nutr. 126:1782-1790. [DOI] [PubMed] [Google Scholar]
- 32.Lastowskiperry, D., E. Otto, and G. Maroni. 1985. Nucleotide sequence and expression of a Drosophila metallothionein. J. Biol. Chem. 260:1527-1530. [PubMed] [Google Scholar]
- 33.Lauverjat, S., C. Ballan-Dufrancais, and M. Wegnez. 1989. Detoxification of cadmium. Ultrastructural study and electron-probe microanalysis of the midgut in a cadmium-resistant strain of Drosophila melanogaster. Biol. Met. 2:97-107. [DOI] [PubMed] [Google Scholar]
- 34.Lin, C. M., and D. J. Kosman. 1990. Copper uptake in wild type and copper metallothionein-deficient Saccharomyces cerevisiae. Kinetics and mechanism. J. Biol. Chem. 265:9194-9200. [PubMed] [Google Scholar]
- 35.Lytle, F. E. 1970. Solution luminescence of metal complexes. Appl. Spectrosc. 24:319-326. [Google Scholar]
- 36.Marchal-Segault, D., C. Briancon, S. Halpern, P. Fragu, and G. Lauge. 1990. Secondary ion mass spectrometry analysis of the copper distribution in Drosophila melanogaster chronically intoxicated with Bordeaux mixture. Biol. Cell 70:129-132. [DOI] [PubMed] [Google Scholar]
- 37.Maroni, G., and D. Watson. 1985. Uptake and binding of cadmium, copper and zinc by Drosophila melanogaster larvae. Insect Biochem. 15:55-63. [Google Scholar]
- 38.Maroni, G., J. Wise, J. E. Young, and E. Otto. 1987. Metallothionein gene duplications and metal tolerance in natural populations of Drosophila melanogaster. Genetics 117:739-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masters, B. A., E. J. Kelly, C. J. Quaife, R. L. Brinster, and R. D. Palmiter. 1994. Targeted disruption of metallothionein-I and metallothionein-II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. USA 91:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCord, J. M., and I. Fridovich. 1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244:6049-6055. [PubMed] [Google Scholar]
- 41.McNulty, M., M. Puljung, G. Jefford, and R. R. Dubreuil. 2001. Evidence that a copper-metallothionein complex is responsible for fluorescence in acid-secreting cells of the Drosophila stomach. Cell Tissue Res. 304:383-389. [DOI] [PubMed] [Google Scholar]
- 42.Michalska, A. E., and K. H. Choo. 1993. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc. Natl. Acad. Sci. USA 90:8088-8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murata, M., P. Gong, K. Suzuki, and S. Koizumi. 1999. Differential metal response and regulation of human heavy metal-inducible genes. J. Cell Physiol. 180:105-113. [DOI] [PubMed] [Google Scholar]
- 44.Norgate, M., E. Lee, A. Southon, A. Farlow, P. Batterham, J. Camakaris, and R. Burke. 2006. Essential roles in development and pigmentation for the Drosophila copper transporter DmATP7. Mol. Biol. Cell 17:475-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488-2492. [DOI] [PubMed] [Google Scholar]
- 46.Percival, S. S. 1998. Copper and immunity. Am. J. Clin. Nutr. 67(Suppl. 5):1064S-1068S. [DOI] [PubMed] [Google Scholar]
- 47.Phillips, J. P., S. D. Campbell, D. Michaud, M. Charbonneau, and A. J. Hilliker. 1989. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc. Natl. Acad. Sci. USA 86:2761-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poulson, D. F., and V. T. Bowen. 1952. Organization and function of the inorganic constituents of nuclei. Exp. Cell Res. 2:161-179. [Google Scholar]
- 49.Predki, P. F., and B. Sarkar. 1992. Effect of replacement of “zinc finger” zinc on estrogen receptor DNA interactions. J. Biol. Chem. 267:5842-5846. [PubMed] [Google Scholar]
- 50.Puig, S., and D. J. Thiele. 2002. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 6:171-180. [DOI] [PubMed] [Google Scholar]
- 51.Quesada, A. R., R. W. Byrnes, S. O. Krezoski, and D. H. Petering. 1996. Direct reaction of H2O2 with sulfhydryl groups in HL-60 cells: zinc-metallothionein and other sites. Arch. Biochem. Biophys. 334:241-250. [DOI] [PubMed] [Google Scholar]
- 52.Rae, T. D., P. J. Schmidt, R. A. Pufahl, V. C. Culotta, and T. V. O'Halloran. 1999. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science 284:805-808. [DOI] [PubMed] [Google Scholar]
- 53.Romero-Isart, N., and M. Vasak. 2002. Advances in the structure and chemistry of metallothioneins. J. Inorg. Biochem. 88:388-396. [DOI] [PubMed] [Google Scholar]
- 54.Rong, Y. K. S., S. W. Titen, H. B. Xie, M. M. Golic, M. Bastiani, P. Bandyopadhyay, B. M. Olivera, M. Brodsky, G. M. Rubin, and K. G. Golic. 2002. Targeted mutagenesis by homologous recombination in D-melanogaster. Genes Dev. 16:1568-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saydam, N., T. K. Adams, F. Steiner, W. Schaffner, and J. H. Freedman. 2002. Regulation of metallothionein transcription by the metal-responsive transcription factor MTF-1: identification of signal transduction cascades that control metal-inducible transcription. J. Biol. Chem. 277:20438-20445. [DOI] [PubMed] [Google Scholar]
- 56.Saydam, N., O. Georgiev, M. Y. Nakano, U. F. Greber, and W. Schaffner. 2001. Nucleo-cytoplasmic trafficking of metal-regulatory transcription factor 1 is regulated by diverse stress signals. J. Biol. Chem. 276:25487-25495. [DOI] [PubMed] [Google Scholar]
- 57.Selvaraj, A., K. Balamurugan, H. Yepiskoposyan, H. Zhou, D. Egli, O. Georgiev, D. J. Thiele, and W. Schaffner. 2005. Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes Dev. 19:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simons, A., T. Ruppert, C. Schmidt, A. Schlicksupp, R. Pipkorn, J. Reed, C. L. Masters, A. R. White, R. Cappai, K. Beyreuther, T. A. Bayer, and G. Multhaup. 2002. Evidence for a copper-binding superfamily of the amyloid precursor protein. Biochemistry 41:9310-9320. [DOI] [PubMed] [Google Scholar]
- 59.Smolarek, C., and W. Stremmel. 1999. Therapy of Wilson disease. Z. Gastroenterol. 37:293-300. (In German.) [PubMed] [Google Scholar]
- 60.Sogawa, N., C. A. Sogawa, N. Oda, T. Fujioka, K. Onodera, and H. Furuta. 2000. Induction of metallothionein mRNA expression in the mouse liver after cadmium injection as measured by the reverse transcriptase-polymerase chain reaction method. Methods Find. Exp. Clin. Pharmacol. 22:663-666. [DOI] [PubMed] [Google Scholar]
- 61.Sokol, R. J., M. Devereaux, G. W. Mierau, K. M. Hambidge, and R. H. Shikes. 1990. Oxidant injury to hepatic mitochondrial lipids in rats with dietary copper overload. Modification by vitamin E deficiency. Gastroenterology 99:1061-1071. [DOI] [PubMed] [Google Scholar]
- 62.Tamai, K. T., E. B. Gralla, L. M. Ellerby, J. S. Valentine, and D. J. Thiele. 1993. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc. Natl. Acad. Sci. USA 90:8013-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tapiero, H., D. M. Townsend, and K. D. Tew. 2003. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 57:386-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thornalley, P. J., and M. Vasak. 1985. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta 827:36-44. [DOI] [PubMed] [Google Scholar]
- 65.Valls, M., R. Bofill, N. Romero-Isart, R. Gonzalez-Duarte, J. Abian, M. Carrascal, P. Gonzalez-Duarte, M. Capdevila, and S. Atrian. 2000. Drosophila Mtn: a metazoan copper-thionein related to fungal forms. FEBS Lett. 467:189-194. [DOI] [PubMed] [Google Scholar]
- 66.Wang, Y., I. Lorenzi, O. Georgiev, and W. Schaffner. 2004. Metal-responsive transcription factor-1 (MTF-1) selects different types of metal response elements at low vs. high zinc concentration. Biol. Chem. 385:623-632. [DOI] [PubMed] [Google Scholar]
- 67.Weaver, R. F., and C. Weissmann. 1979. Mapping of RNA by a modification of the Berk-Sharp procedure—5′ termini of 15 S β-globin mRNA precursor and mature 10 S β-globin mRNA have identical map coordinates. Nucleic Acids Res. 7:1175-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wimmer, U., Y. Wang, O. Georgiev, and W. Schaffner. 2005. Two major branches of anti-cadmium defense in the mouse: MTF-1/metallothioneins and glutathione. Nucleic Acids Res. 33:5715-5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamada, H., and S. Koizumi. 2001. Lymphocyte metallothionein-mRNA as a sensitive biomarker of cadmium exposure. Ind. Health 39:29-32. [DOI] [PubMed] [Google Scholar]
- 70.Zhang, B., D. Egli, O. Georgiev, and W. Schaffner. 2001. The Drosophila homolog of mammalian zinc finger factor MTF-1 activates transcription in response to heavy metals. Mol. Cell. Biol. 21:4505-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, B., O. Georgiev, M. Hagmann, C. Günes, M. Cramer, P. Faller, M. Vasak, and W. Schaffner. 2003. Activity of metal-responsive transcription factor 1 by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Mol. Cell. Biol. 23:8471-8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.