Abstract
Mnt is a Max-interacting protein that can antagonize the activities of Myc oncoproteins in cultured cells. Mnt null mice die soon after birth, but conditional deletion of Mnt in breast epithelium leads to tumor formation. These and related data suggest that Mnt functions as a tumor suppressor. Here we show that conditional deletion of Mnt in T cells leads to tumor formation but also causes inflammatory disease. Deletion of Mnt caused increased apoptosis of thymic T cells and interfered with T-cell development yet led to spleen, liver, and lymph node enlargement. The proportion of T cells in the spleen and lymph nodes was reduced, and the numbers of cells in non-T-cell immune cell populations were elevated. The disruption of immune homeostasis is linked to a strong skewing toward production of T-helper 1 (Th1) cytokines and enhanced proliferation of activated Mnt-deficient CD4+ T cells. Consistent with Th1 polarization in vivo, extensive intestinal inflammation and liver necrosis developed. Finally, most mice lacking Mnt in T cells ultimately succumbed to T-cell lymphoma. These results strengthen the argument that Mnt functions as a tumor suppressor and reveal a critical and surprising role for Mnt in the regulation of T-cell development and in T-cell-dependent immune homeostasis.
The maturation of T cells in the thymus depends on an ordered series of events leading to the selection of cells bearing functional T-cell receptors (TCRs) with appropriate antigen specificities. The process of T-cell maturation is characterized by the expression of the T-cell-specific coreceptor molecules CD4 and CD8. Immature thymocytes express neither CD4 nor CD8 and are therefore referred to as double negatives (DNs). Maturation of DN cells to cells expressing both CD4 and CD8 (double positive [DP]) requires a sequence of events closely linked to rearrangements of TCRs. Whereas T cells expressing inappropriate TCRs are eliminated by apoptosis, positively selected T cells proliferate and differentiate into DPs and subsequently into CD4+ or CD8+ single-positive (SP) T cells. SP T cells exit the thymus and are routed to peripheral lymphoid organs such as the spleen and lymph nodes, where they participate in a variety of immune responses. Upon activation by antigen, peripheral CD8+ T cells function in cytotoxic responses and CD4+ T cells differentiate into T-helper (Th) subsets that function in various aspects of adaptive immunity (14). Defects in the development and function of T cells have far-reaching consequences and are associated with a variety of disease states.
Deregulated expression of Myc family genes is a common feature of a wide variety of malignancies, including T-cell lymphomas. Indeed, T cells seem to be particularly sensitive to Myc-dependent tumorigenesis as T-cell lymphoma appears to be the predominant tumor type in transgenic mice overexpressing c-Myc in hematopoietic progenitor cells (12, 45). Myc-dependent T-cell lymphomagenesis is accelerated by events that abrogate Myc-driven apoptosis, such as disruption of p53 pathway function (2, 10; reviewed in references 29 and 36). However, Myc-driven T-cell lymphomagenesis is not accelerated by loss of Fas (6), a ligand involved in negative selection and required for Myc-dependent apoptosis in some cell types (17).
In contrast to excessive Myc, T cells lacking c-Myc are at a severe proliferative disadvantage (49, 52) and fail to progress through positive selection (9). This inability of c-Myc-deficient T cells to differentiate past DN stages may be linked to an important role for c-Myc in proliferation mediated by pre-TCR signaling (54). Further, in naive quiescent peripheral T cells, c-Myc is downregulated, perhaps by a dedicated pathway (4), but then again upregulated as part of the proliferative response mediated by antigen-mediated TCR activation (15).
The functions of Myc family proteins are largely dependent on heterodimerization with Max, which facilitates DNA binding at CACGTG and related E-box sites (1). Max similarly serves as a cofactor for DNA binding by several other proteins related to Myc, including the putative Myc antagonist Mnt (18, 27). Whereas DNA binding of Myc-Max complexes to E-box sequences activates transcription, E-box binding by Mnt-Max complexes represses transcription (18, 27). Mnt can block the ability of Myc to transform cells in culture (18) and can directly repress some, but not all, Myc-Max target genes (19, 30, 51). More direct evidence of Mnt-Myc antagonism is provided by experiments showing that proliferative arrest caused by deletion of c-Myc in mouse embryo fibroblasts is partially rescued by simultaneous deletion of Mnt (51) and that small-interfering-RNA-mediated knockdown of Mnt rescues the slow proliferation of a Myc-null rat fibroblast cell line (30). Furthermore, although mice lacking Mnt die soon after birth (48), mouse embryo fibroblasts lacking Mnt exhibit many of the hallmark characteristics of cells that ectopically express Myc, including accelerated cell cycle entry, increased apoptosis, and a predisposition toward tumor formation (19, 30, 51). However, we find that the embryonic lethality caused by either c-Myc deletion or N-Myc deletion is not rescued by simultaneous Mnt deletion in mice (Z.-Q. Zhou and P. J. Hurlin, unpublished data). These latter results suggest that while Mnt can antagonize Myc activities, the functionally similar Mad family of proteins (for a review, see reference 55) may compensate, at least partially, for the loss of Mnt in key tissues. Alternatively, Mnt and Myc may not function entirely by controlling the same genes and molecular pathways and possess some distinct and perhaps cell-type-specific biological activities. Consistent with this idea, experiments examining the genomic binding sites of Drosophila Mnt and Myc demonstrate that they recognize both unique and overlapping sites (32).
To gain further insight into the biological activities of Mnt, we have investigated its requirement during T-cell development by using a conditional-deletion approach. We found that Mnt deficiency in T cells caused increased apoptosis and a partial block in T-cell development in the thymus but later caused organomegaly, inflammatory lesions, and lymphomagenesis. Our results demonstrate that Mnt functions as a tumor suppressor in T cells and that it plays an essential role in T-cell development and immune homeostasis.
MATERIALS AND METHODS
Deletion of Mnt in T cells.
To delete Mnt in T cells, Mntflox/flox mice (9, 15) were crossed with lck-Cre mice (27) to ultimately produce lck-Cre-Mntflox/flox mice. All mice were inbred C57BL/6. Mice were genotyped by PCR as previously described (15). Mice were housed in a sterile, pathogen-free environment.
Flow cytometry, Western blotting, and quantitative reverse transcription (RT)-PCR analysis.
After lysing red blood cells, 1 × 106 to 5 × 106 single-cell suspensions of thymocytes, splenocytes, or blood cells were stained with different antibodies in phosphate-buffered saline (PBS) containing 3% fetal bovine serum, and 0.1% NaN3 for 45 min on ice in the dark. Cells were washed and analyzed on a FACScalibur instrument (BD Biosciences) with CellQuest software. Monoclonal antibody reagents from Pharmingen were phycoerythrin (PE)-fluorescein isothiocyanate (FITC)-conjugated anti-CD25, PE-conjugated CY5-anti-CD44, and Fc block. Antibodies and reagents from eBioscience included FITC-anti-IgM (immunoglobulin M), PE-anti-IgD, PE-CY5-B220, PE-CY5-anti-CD3ɛ, PE-Cy5-anti-CD11c, PE-anti-major histocompatibility complex class II, FITC-anti-CD11b, and PE-anti-F4/80. PE-anti-CD8α and FITC-PE-anti-CD4 were obtained from Biocarta.
Western blot assays were performed as described previously (19). Antibodies used included affinity-purified anti-Mnt and antibodies against p19ARF (Abcam), p27Kip1 (BD Pharmingen), Bcl-2 and BclXL (BD Transduction Laboratories), and cyclin D1 (Oncogene Sciences). Antibodies against p53, p21Cipl, cyclin D2, cyclin E1, cyclin B1, Cdk4, c-Myc, N-Myc, and cyclin A were obtained from Santa Cruz Biotechnology. Phospholipase C-γ1 (PLC-γ1), p-PLC-γ1, and p-Erk antibodies were obtained from Cell Signaling Technology.
For quantitative RT-PCR, total RNA harvested from thymocytes was random primed with Superscript II reverse transcriptase (Invitrogen). Samples in triplicate were amplified with SYBR green I dye in an Applied Biosystems 7900HT sequence detection system. Data was analyzed by the 2−ΔΔCt method as previously described (51).
In vitro activation assays, cytokine profiling, and carboxyfluorescein diacetate succinimidyl ester (CFSE) staining.
For cytokine profiling, thymocytes or sorted splenic CD4+ T cells were plated in 96-well plates and stimulated with phorbol 12-myristate 13-acetate (PMA; 10 ng/ml; Sigma) in combination with ionomycin (1 μg/ml; Sigma) or with anti-mouse CD3 (T-cell activation plates; BD Biosciences) in the presence of soluble anti-CD28 antibody (7 μg/ml; Pharmingen). Medium of treated and untreated cells was assayed for cytokine levels by enzyme-linked immunosorbent assay according to the instructions of the manufacturer (R&D Systems). For proliferation assays, lymphocytes were stained with CFSE dye (Molecular Probes) prior to activation and analyzed by fluorescence-activated cell sorter (FACS) for CFSE fluorescence.
Apoptosis detection by flow cytometry.
After surface staining with different antibodies in 3% fetal calf serum, 0.1% streptavidin in PBS and washing with 1× PBS, 1 × 106 cells were resuspended in binding buffer (140 mM NaCl, 2.5 mM CaCl2, 10 mM HEPES/NaOH, pH 7.4). Allophycocyanin-annexin V (Caltag Laboratories) was added (1 μg/ml), with or without propidium iodide (1 μg/ml). The mixtures were incubated for 15 min in the dark at room temperature and analyzed immediately by FACS.
Statistical analysis.
Student's t test was performed to compare data between groups of mice. The two-tailed t test was performed assuming unequal variances (except where noted otherwise) and alpha equals 0.05. P values of <0.05 were regarded as statistically significant.
RESULTS
Deletion of Mnt in T cells.
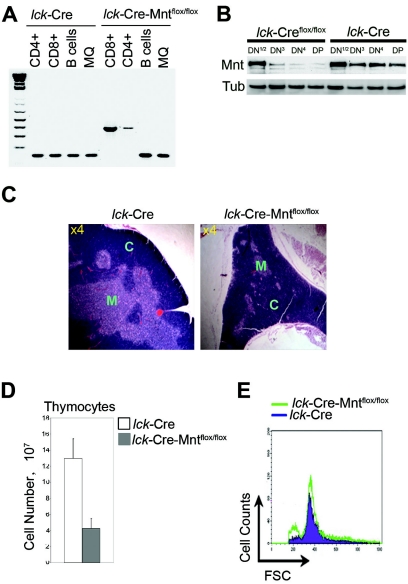
To delete Mnt in T cells, mice containing floxed Mnt alleles (19, 48) were mated with lck-Cre mice (47). PCR genotyping indicated that lck-Cre-mediated Mnt deletion occurred prior to the development of SP CD4+ and CD8+ populations (Fig. 1A), as the latter cells showed only the mutant Mnt alleles. Importantly, the Mnt gene was not deleted in splenic macrophages or B cells (Fig. 1A; see more below). Western blot analysis performed with DN thymocyte populations indicated that Mnt is expressed during DN stages and that Mnt levels are sharply reduced by the DN3 stage of T-cell development in lck-Cre-Mntflox/flox mice (Fig. 1B; see more below). These results are consistent with previous results showing that lck-Cre-mediated deletion is nearly complete by the DN3 stage (22, 35, 47).
FIG. 1.
Deletion of Mnt in T cells causes decreased thymic cellularity. (A) PCR genotyping performed on DNA obtained from FACS-sorted thymic CD4+ and CD8+ T cells, splenic B220+ B cells, and F4/80+ macrophages from mice of the indicated genotypes at 8 weeks of age. The 160-bp PCR product is diagnostic for the wild-type Mnt allele, and the 350-bp PCR product is diagnostic for the mutant allele. (B) Western blot analysis of Mnt expression in DN subsets obtained from lck-Cre Mntflox/flox and lck-Cre mice. DN T cells were enriched from total thymocytes isolated from multiple 5-week-old littermates by first removing populations staining with antibodies against CD3, CD4, CD8, GR1, Mac1, and B220. DN T cells were then sorted by flow cytometry for CD25 and CD44 to isolate the different DN subsets. The DN1 and DN2 populations were pooled because of their relatively low numbers. Tub, tubulin. (C) Hematoxylin-and-eosin-stained thymus sections. The inner medulla (M) region of the lck-Cre Mntflox/flox thymus was markedly reduced relative to the cortex (C). Magnification (n-fold) is indicated. (D) Comparison of total thymocyte numbers from lck-Cre-Mntflox/flox mice (n = 5) and age-matched (5- to 9-week-old) control mice (n = 6). Values shown are means ± standard deviations (P = 0.005). (E) Cell size comparison of total thymocytes determined by FSC.
Examination of lck-Cre-Mntflox/flox mice between 5 and 9 weeks of age revealed a marked reduction in thymus size and disturbed thymus architecture compared to control lck-Cre mice (Fig. 1C) or lck-Cre-Mntflox/+ mice (not shown). lck-Cre and lck-Cre-Mntflox/+ mice were used interchangeably as control mice in this study since neither exhibited a discernible phenotype. The disturbed thymus architecture of lck-Cre-Mntflox/flox mice primarily related to a dramatically reduced medulla region compared to the cortex (Fig. 1C). The medulla is composed primarily of more mature T cells, whereas the cortex is occupied mostly by immature T cells (reviewed in reference 50). Both thymus size and total thymocyte numbers of lck-Cre-Mntflox/flox mice were reduced by 50 to 70% relative to those of control lck-Cre mice at 5 to 9 weeks of age (Fig. 1D and not shown). In contrast, forward scatter (FSC) analysis suggested that loss of Mnt had little effect on thymocyte cell size (Fig. 1E). However, a prominent peak of small or disintegrating cells, consistent with increased apoptosis, was apparent in Mnt-deficient thymocytes (Fig. 1E).
Defective T-cell development in Mnt-deficient thymi.
The small size and disrupted architecture of lck-Cre-Mntflox/flox thymi suggested that loss of Mnt disturbed thymocyte differentiation during DN stages. Based on their developmental progression, thymocytes at the DN stage are divided into four subsets: CD25− CD44+ (DN1), CD25+ CD44+ (DN II), CD25+ CD44− (DN III), and CD25− CD44− (DN IV) (11). FACS analysis of these subsets from mice between 5 and 9 weeks of age showed that the percentage of cells at the DN III stage was increased in lck-Cre-Mntflox/flox mice compared to lck-Cre littermates (n = 6 to 9 per group, Fig. 2A). These results indicate that loss of Mnt impeded progression of thymocyte development at the DN III-to-DN IV transition. One possibility is that this block is caused by excessive cell proliferation and/or a failure to properly respond to TCRβ locus rearrangement, which occurs at DN III (16). However, Mnt-deficient DN subsets showed cell cycle profiles (based on DNA content) comparable to those of control littermates (data not shown). In addition, levels of apoptosis in DN populations, determined by annexin staining, appeared not to be significantly affected by loss of Mnt (see Fig. S1 in the supplemental material).
FIG. 2.
Differentiation and proliferation defects in Mnt-deficient thymocytes. (A) Total thymocytes isolated from littermates (5 to 9 weeks old) with the indicated genotypes were enriched for DN T cells as described in the legend to Fig. 1B and analyzed for CD25 and CD44 expression by flow cytometry. The value in each quadrant represents the percentage of cells in one of the four DN populations (indicated in the lck-Cre half of the panel). Note the accumulation of thymocytes from lck-Cre-Mntflox/flox mice in the CD25+ CD44− population (DN III). The results shown are representative of six independent experiments. (B) Representative flow cytometry profiles of thymocytes from lck-Cre-Mntflox/flox and littermate control mice stained with antibodies against CD4+ and CD8+. Values indicate the percentages of cells of the different T-cell subsets. (C) The absolute numbers of thymocytes representing the indicated developmental stages were calculated from FACS data and total cell numbers compiled from four independent experiments. Data are shown as means ± standard deviations (*, P = 0.03; **, P = 0.02; ***, P = 0.02). DN populations were not significantly different.
Along with a defect in DN III differentiation, lck-Cre-Mntflox/flox mice consistently showed deficits in the percentage of thymocytes expressing both CD4 and CD8 (DP) and thymocytes expressing either CD4 or CD8 (SP), along with a concomitant increase in the percentage of thymocytes lacking these markers (Fig. 2B). To determine how the altered percentages of thymocyte subsets are related to the sharply reduced numbers of total thymocytes and small thymi of lck-Cre-Mntflox/flox mice, absolute numbers of the four subpopulations of thymocytes (DN, DP, SP CD4+, and SP CD8+) were calculated (Fig. 2C). These results clearly revealed a dramatic reduction in the number of DP and SP populations relative to DN cells in thymi of lck-Cre-Mntflox/flox mice. The reduced DP and SP numbers are consistent with the small size of the medulla region of lck-Cre-Mntflox/flox thymi (Fig. 1C), as this is where more mature T cells normally reside. Further, total peripheral blood T-cell counts were also lower in lck-Cre-Mntflox/flox mice (1.46 × 106 ± 0.23 × 106/ml blood versus 2.24 × 106 ± 0.36 × 106/ml blood for control lck-Cre mice 5 to 9 weeks of age [n = 3]).
While the decrease in DP and SP thymocytes caused by Mnt deficiency might be related to the partial block in DN III-to-DN IV differentiation (Fig. 2A), it might also be due to defects in proliferation or excessive apoptosis of DP and SP thymocytes. To examine DP and SP cell proliferation, thymocytes expressing CD4 and/or CD8 (combined DP and SP populations) were isolated from lck-Cre and lck-Cre-Mntflox/flox mice and cell cycle profiles generated based on DNA content. The S-phase fraction was consistently higher in Mnt-deficient T cells than in control cells, but the difference did not reach statistical significance (Fig. 3A). Thus, despite a small increase in the fraction of DP-SP thymocytes in S phase, numbers of Mnt-deficient DP and SP thymocytes are sharply reduced (Fig. 2C).
FIG. 3.
Increased proliferation and apoptosis caused by deletion of Mnt. (A) Total thymocytes from lck-Cre and lck-Cre-Mntflox/flox mice were gated for live cells, stained with monoclonal antibodies to CD4 and CD8, fixed, and stained with 4′,6′-diamidino-2-phenylindole (DAPI) for cell cycle analysis by FACS based on DNA content. Percent S phase is indicated on representative histograms (n = 3), and the table to the right shows mean values with standard deviations. (B) Increased apoptosis in mature thymocytes from lck-Cre-Mntflox/flox mice. Total thymocytes were stained for CD4, CD8, and annexin V, and representative histograms (n = 3) comparing annexin V-positive staining of the indicated sorted thymocyte subpopulations are shown. Numbers in each histogram represent the percentage of annexin V-positive populations in each group. The table shows mean values and standard deviations (*, P = 0.03; **, P = 0.04). CD4 apoptosis was not significantly different.
In contrast to what appears to be a small increase in proliferation, annexin staining revealed that Mnt-deficient DP and SP T cells exhibit very high levels of apoptosis compared to control cells, particularly in the DP population (Fig. 3B). The increased apoptosis in DP and SP populations is consistent with the identification of an increased fraction of cells exhibiting a decreased FSC profile by FACS analysis (Fig. 1E). Thus, the decreased numbers of DP and SP thymocytes caused by deletion of Mnt are likely accounted for by at least two different mechanisms: defective DN III-DN IV differentiation and increased apoptosis in DP-SP thymocytes.
Expression of Myc and Myc effectors in Mnt-deficient thymocytes.
Whereas c-Myc levels are maintained at comparable levels in DN, DP, and SP thymocytes, N-Myc is expressed predominantly in DN thymocytes (3, 52). Consistent with the disproportionately high fraction of DN thymocytes in lck-Cre-Mntflox/flox thymi (Fig. 2C), N-Myc levels were higher in Mnt-deficient thymocytes compared to those of control mice (Fig. 4A). In contrast, c-Myc levels appeared not to be significantly altered by loss of Mnt (Fig. 4A). Because Mnt is thought to function as a Myc antagonist, we examined the expression of a number of proteins that are regulated by Myc. Cyclin D2 and Cdk4, which are encoded by genes that can be directly regulated by Myc (reviewed in reference 1), as well as by Mnt (19, 51), were modestly upregulated by Mnt deficiency (Fig. 4A). Consistent with these results, quantitative RT-PCR experiments indicated that levels of mRNA encoding cyclin D2 and Cdk4 were modestly upregulated by loss of Mnt (Fig. 4B), as previously demonstrated (51). Levels of cyclin E1, cyclin A, and cyclin B1, which are probably not direct targets of Myc activation but that are upregulated by Myc (26) were also modestly increased in lck-Cre-Mntflox/flox mice (Fig. 4A).
FIG. 4.
Thymocyte expression of proteins and genes regulated by Myc. (A) Western blot analysis of c-Myc, N-Myc, and several Myc effector proteins. Lysates from total thymocytes isolated from lck-Cre, lck-Cre-Mntflox/+, and lck-Cre-Mntflox/flox mice were immunoblotted with antibodies against the indicted proteins. (B) Quantitative RT-PCR analysis of Mnt and several Myc target genes in thymocytes from lck-Cre-Mntflox/flox mice and control lck-Cre mice. The n-fold change in gene expression in thymocytes of lck-Cre-Mntflox/flox mice was determined by comparing acidic ribosomal phosphoprotein (Arbp) PO-normalized gene expression levels in thymocytes of lck-Cre-Mntflox/flox and lck-Cre mice. RT-PCR was performed in triplicate on samples obtained from two different mice for each genotype. Standard deviations are shown.
Levels of proapoptotic p53 were increased, but p19ARF, which is involved in Myc-dependent upregulation of p53 and apoptosis (56), was undetectable. Levels of cyclin-dependent kinase inhibitor p21Cip1, which encodes a key p53 target gene directly suppressed by Myc under some conditions (1), and p27Kip1, which is often downregulated by forced Myc expression and may be a direct target of Myc-dependent repression (53), were not significantly altered by loss of Mnt in thymocytes. The antiapoptotic proteins Bcl2 and BclXL, previously shown to be downregulated by forced Myc expression (25), were slightly downregulated in Mnt-deficient T cells. This is in contrast to Mnt-deficient mouse embryo fibroblasts, where BclXL was strongly downregulated (30). Finally, quantitative RT-PCR revealed very little change in mRNA levels of several other genes shown to be directly regulated by Myc in some settings, including CAD, EIF4E, E2F2, ODC, nucleolin, Bim, and caspase 8 (Fig. 4B). We interpret these results to indicate that Mnt and Myc regulate both shared and unique sets of target genes and molecular pathways. Consistent with this interpretation, the phenotype described here has both similarities to and differences from the phenotype of transgenic mice expressing Myc in T cells (46).
Mnt deficiency in T cells leads to enlarged secondary lymphoid organs.
In sharp contrast to their thymi, the spleens of lck-Cre-Mntflox/flox mice were enlarged by 8 weeks of age or earlier (not shown) and progressively enlarged over time. By 12 months, their spleens were, on average, three to four times larger than the spleens of lck-Cre-Mntflox/+ mice (n = 12 to 15 per group, Fig. 5A). In addition, the spleen architecture was highly disorganized (Fig. 4B). Sublumbar lymph nodes of lck-Cre-Mntflox/flox mice were dramatically enlarged (Fig. 5C), and these and other lymph nodes displayed a highly disorganized architecture (not shown). Immunohistochemical analysis of spleen sections with anti-phospho-histone H3, a marker of cells in mitosis, revealed a dramatic increase in mitotic cells in the spleens of lck-Cre-Mntflox/flox mice (Fig. 5D). Experiments examining cells in S phase by bromodeoxyuridine incorporation and immunohistochemical analysis gave similar results (not shown). Thus, splenomegaly appears to be due to increased splenocyte proliferation.
FIG. 5.
Splenomegaly and lymphadenopathy in lck-Cre-Mntflox/flox mice. (A) Spleens from 12-month-old lck-Cre-Mntflox/flox and lck-Cre-Mntflox/+ littermates. (B) Hematoxylin-and-eosin-stained sections from the spleens shown in panel A. (C) Gross enlargement of sublumbar lymph node in a lck-Cre-Mntflox/flox mouse. (D) Widespread expression of the mitosis marker phosphorylated histone H3 in spleens of lck-Cre-Mntflox/flox mice. (E) Representative (n = 4) FACS profiles of CD4+ CD3+ and CD8+ CD3+ populations in total splenocytes from lck-Cre-Mntflox/flox and lck-Cre-Mntflox/+ littermates. Note the relative increase in the percentage of triple-negative cells (cells lacking CD4 and CD8 [lower left quadrant]) representing non-T-cell (NT) populations. Values indicate percentages of the different populations. (F) Absolute numbers of cells in different splenic subpopulations calculated from FACS data and estimated total splenocyte numbers (after red blood cell lysis) determined from four independent experiments. Values are means ± standard deviations (*, P = 0.02; **, P = 0.02).
To determine whether increased numbers of Mnt-deficient T cells were responsible for splenomegaly, splenic T cells were examined by FACS with antibodies against CD4 and CD8. As shown in Fig. 5E, lck-Cre-Mntflox/flox mice exhibited a decrease in the percentage of both SP CD4+ and CD8+ splenocytes compared to control mice. In contrast, the ratio of B cells and other non-T cells (cells lacking T-cell markers) increased from 78% to 90%. These percentages, together with the average number of total splenocytes (n = 4 to 6 per group), were used to determine the absolute numbers of SP CD8+ and CD4+ T cells and non-T cells in the spleens of 7- to 12-month-old mice (Fig. 5F). Whereas there was a small increase in the total number of CD4+ and CD8+ cells, there was a large increase in the non-T-cell fraction of cells in lck-Cre-Mntflox/flox mice compared to control mice. Comparison of the percentages of macrophages, dendritic cells, and B cells in spleens of control and lck-Cre-Mntflox/flox mice revealed significant increases in these cell populations (Table 1). Thus, splenomegaly appears to be primarily the result of excessive proliferation of non-T-cell populations. Importantly, the Mnt gene was not deleted in macrophages or B cells isolated from lck-Cre-Mntflox/flox mice at 8 months of age (Fig. 1A), suggesting that splenomegaly and lymphadenopathy are due to an intrinsic defect in T cells.
TABLE 1.
Altered immune cell populations in spleens of lck-Cre and lck-Cre-Mntflox/flox micea
| Cell type | Mean % ± SD in mice with a genotype of:
|
|
|---|---|---|
| lck-Cre | lck-Cre- Mntflox/flox | |
| Macrophagesb | 2 ± 0.4 | 5 ± 1.0 |
| Dendritic cellsc | 4 ± 0.5 | 7 ± 1.8 |
| B cells (IgM+ B220+)b | 24 ± 3.8 | 37 ± 4.3 |
| B cells (IgD+ B220+)d | 26 ± 4.4 | 41 ± 2.8 |
Splenocytes from 6- to 7-month-old mice were stained with F4/80 and major histocompatibility complex class II/CD11c markers to identify macrophages and dendritic cells, respectively, and for IgM plus B220 and IgD plus B220 to identify B cells. Values were determined from triplicate samples derived from three to five mice per genotype.
P < 0.001.
P < 0.006.
P = 0.002.
Th1 bias and hyperproliferation of CD4+ T cells lacking Mnt.
A potential explanation for the expansion of different immune cell populations in spleens of lck-Cre-Mntflox/flox mice is disrupted function of CD4+ T cells. CD4+ T cells can regulate the proliferation and activities of other immune cell types through the secretion of cytokines specific to activated CD4+ Th subsets (reviewed in reference 8). We therefore compared cytokine production between isolated Mnt-deficient CD4+ T cells and control CD4+ T cells following their activation by treatment with either anti-CD3 plus anti-CD28 (mimicking TCR engagement) or by phorbol ester (PMA) plus calcium ionophore (ionomycin), which bypasses TCR activation by targeting calcium mobilization. Because of the low number of CD4+ T cells produced in relatively young lck-Cre-Mntflox/flox mice (Fig. 2C and data not shown), these experiments required isolation and pooling of CD4+ T cells from spleens of multiple lck-Cre-Mntflox/flox mice at 5 months of age. Whereas control and Mnt-deficient CD4+ cells showed no difference in cytokine profile following anti-CD3/CD28 stimulation (not shown), PMA-plus-ionomycin treatment strongly elevated levels of the Th1 cytokines interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) in CD4+ cells lacking Mnt (Fig. 5A, left). No difference was observed in levels of Th2 cytokines IL-4 and IL-5 following activation with anti-CD3/CD28 (not shown) or PMA plus ionomycin (Fig. 6A, right).
FIG. 6.
Th1 polarization and increased proliferation of CD4+ splenocytes lacking Mnt. (A) FACS-sorted CD4+ splenocytes were stimulated with PMA and ionomycin for 48 h, and concentrations of Th1 cytokines (IL-2, IFN-γ, and TNF-α) and Th2 cytokines (IL-4 and IL-5) were determined by enzyme-linked immunosorbent assay. (B) Analysis of proliferation by CFSE dilution following PMA and ionomycin stimulation (for 2 and 3 days) of CD4+ splenocytes isolated from 3- to 5-month-old lck-Cre-Mntflox/flox and lck-Cre littermates. Untreated parental populations of lck-Cre and lck-Cre-Mntflox/flox mouse-derived thymocytes had identical profiles (lck-Cre mouse thymocytes are shown). The appearance of different-color peaks represents successive cell divisions. Representative histograms are shown. (C) Cell size (FSC) analysis of CD4+ splenocytes purified from lck-Cre and lck-Cre-Mntflox/flox littermates before and 48 h after stimulation with PMA and ionomycin. (D) Annexin V staining of the same cells as shown in panel C. Percentages of apoptotic cells are shown.
Together with Th1 cytokine induction, Mnt-deficient cells exhibited a strong proliferative advantage following treatment with PMA plus ionomycin. Cells analyzed either 2 days or 3 days following PMA-plus-ionomycin treatment with the fluorescent vital dye CFSE (24) revealed that cell populations lacking Mnt had undergone several additional cell divisions compared to control cells (Fig. 6B). Interestingly, no difference in cell proliferation was observed following activation with anti-CD3 plus anti-CD28 (not shown). The control of cell size, an important aspect of T-cell activation, thought to be regulated by c-Myc (15, 52), appeared not to be altered following treatment with PMA or ionomycin (Fig. 6C) or after anti-CD3-plus-anti-CD28 treatment (not shown).
Activation of CD4+ T cells is also associated with increased apoptosis. Surprisingly, levels of apoptosis were dramatically lower in Mnt-deficient cells compared to control cells following PMA and ionomycin treatment (Fig. 6D). Thus, whereas Mnt-deficient CD4+ thymocytes exhibit an increased propensity to undergo apoptosis (Fig. 3B), CD4+ T cells that escape the thymus exhibit a reduced sensitivity to apoptosis.
Since calcium signaling functions downstream of TCR activation, these results suggest that the propensity of Mnt-deficient CD4+ T cells to differentiate and proliferate along the Th1 lineage is associated with defects distal to TCR signaling. Consistent with this, proximal TCR signaling appeared to be intact in Mnt-deficient CD4+ T cells since pervanadate, an inhibitor of protein tyrosine phosphatases that regulate TCR activation (20), was effective at triggering phosphorylation of the TCR signaling effector molecules PLC-γ and Erk (see Fig. S2 in the supplemental material).
Inflammatory disease in lck-Cre-Mntflox/flox mice.
Polarized differentiation of CD4+ T cells into Th1 cells and corresponding excessive production of Th1-type cytokines are closely linked to inflammatory disease and associated tissue destruction. Sites that are commonly affected include the liver and intestine (44). Both of these organs were severely affected in lck-Cre-Mntflox/flox mice (Fig. 7A to D). The livers of lck-Cre-Mntflox/flox mice (n = 6; ages, 9 months to 12 months) were densely compacted and weighed approximately twice those of control littermates (n = 6). They also exhibited widespread brown patches (lipofucin), consistent with necrosis. (Fig. 7A and data not shown). Extensive lymphocytic infiltration, especially around blood vessels, was evident in all of the livers examined (n = 7 per group). Consistent with inflammatory lesions, Prussian blue staining indicated an abundance of hemosiderin-laden macrophages in the livers of lck-Cre-Mntflox/flox mice relative to those of control mice (Fig. 7B).
FIG. 7.
Intestinal inflammation and liver damage in lck-Cre-Mntflox/flox mice. (A) Representative livers of lck-Cre-Mntflox/+ and lck-Cre-Mntflox/flox mice at 12 months of age. Note darkened regions consistent with necrosis. (B) Prussian blue staining of liver sections from lck-Cre-Mntflox/+ and lck-Cre-Mntflox/flox mice. Prussian blue stains iron-containing cells and indicates the presence of hemosiderin-containing macrophages (blue staining). The dark pink staining in lck-Cre-Mntflox/flox liver (arrow) shows the patchwork of infiltrating lymphocytes. (C) Hematoxylin-and-eosin-stained sections of the small intestine (jejunum) from lck-Cre-Mntflox/+ and lck-Cre-Mntflox/flox mice at 12 months of age. Note the granulomatous inflammation (arrow) in the intestine of a lck-Cre-Mntflox/flox mice. The asterisks show the location of crypts at the base of the villi. The lower panel shows ×40 magnification of granulomatous tissue featuring multinucleated giant cells (arrow). (D) Prussian blue staining of granulomatous tissue at ×4 and ×40 magnifications.
Histological examination of intestines of lck-Cre-Mntflox/flox mice between 9 and 13 months of age (n = 6) revealed many inflammatory lesions with granulomatous formation (Fig. 7C). Prussian blue staining again indicated the presence of hemosiderin-laden macrophages within granulomas that were part of giant multinucleated aggregates (Fig. 7C and D). In addition, inflamed areas were associated with neutrophil invasion and altered epithelial structures highlighted by an abundance of Paneth and goblet cells in the crypt and villus hyperplasia (not shown). These types of inflammatory lesions are seen in a variety of autoimmune diseases associated with Th1 polarization (28, 33, 44). In addition to the liver and intestine, other organs and tissues, including the lungs, kidneys, and salivary glands (see Fig. S3 in the supplemental material), exhibited extensive lymphocyte infiltration around blood vessels and often appeared inflamed and damaged.
Loss of Mnt in T cells predisposes to T-cell lymphoma.
By 12 months of age, lck-Cre-Mntflox/flox mice were generally much heavier than control mice, a finding that correlated with organomegaly (Fig. 5A and C and 7A). The average weight of lck-Cre-Mntflox/flox mice was 40 g versus 29 g for control mice (n = 6 per group). Between 12 and 22 months, the health of lck-Cre-Mntflox/flox mice deteriorated and 70% of these mice died or were euthanized during this time period (Fig. 8A). Some lck-Cre-Mntflox/flox mice developed very large tumors such as that shown in Fig. 8B. All mice presented with tumors of various sizes, and most had tumors in multiple tissues. Tumors originating in the kidney and lung are shown in Fig. 8C. In many mice, the affected organs appeared to have multiple tumors together with smaller but widespread colonies of lymphocytes (similar to the liver shown in Fig. 7B). The site of origin for several large tumors could not be determined. The tumors analyzed showed strong staining of the T-cell-specific marker CD3 (Fig. 8D), indicating that they originated from T cells.
FIG. 8.
Loss of Mnt predisposes to lymphomagenesis. (A) Survival curves of lck-Cre-Mntflox/+ and lck-Cre-Mntflox/flox mice. Mice showing obvious tumors or that became moribund were euthanized and examined. (B) Radiograph showing a large malignant tumor in a 12-month-old lck-Cre-Mntflox/flox mouse. (C) Top parts, histopathologic analysis showing kidney and lung tumors (arrows). The normal lung morphology of lck-Cre-Mntflox/+ mice is shown in the inset. Lower parts, immunohistochemical analysis showing strong CD3 staining specifically in tumor tissue (arrows).
Thus, whereas the lymphocyte infiltration found in younger mice was localized primarily around blood vessels in many tissues and associated with inflammation and tissue damage, older mice developed malignant T-cell lymphoma in many of the same tissues.
DISCUSSION
In this study, we show that deletion of the putative Myc antagonist Mnt in T cells disrupts T-cell development and leads to a progressive disease state characterized initially by splenomegaly, hepatomegaly, lymphadenopathy, and inflammatory lesions and later by the development of malignant T-cell lymphoma. Although our first interpretation of the enlarged and inflamed organs and tissues of lck-Cre-Mntflox/flox in relatively young mice was that this was caused by lymphoma, the spleens and lymph nodes of these mice actually contained relatively more non-T cells than T cells (Fig. 5F and data not shown). Thus, malignant or premalignant expansion of Mnt-deficient T cells does not appear to be the primary cause of splenomegaly and lymphadenopathy. Moreover, the patches of lymphocytes found in different organs and tissues of most mice 12 months old and younger contained relatively few mitotic figures and contained nonlymphoid immune cells (Fig. 7 and data not shown), a phenotype more consistent with aberrant lymphocyte migration and function than with metastatic disease. The substantial expansion of B-cell populations in spleens of lck-Cre-Mntflox/flox mice and the presence of macrophages at inflammatory lesions also likely reflect aberrant T-cell function since Mnt was not deleted in B cells or macrophages (Fig. 1A). Other potential causes of splenomegaly and lymphadenopathy, such as hemolytic anemia or chronic infection, are unlikely because red blood cell levels were comparable in control and lck-Cre-Mntflox/flox mice (data not shown) and mice were housed in a sterile, pathogen-free environment.
We believe that the more likely cause of enlarged and inflamed organs in relatively young lck-Cre-Mntflox/flox mice is related to the strong induction of Th1 cytokines observed following activation of CD4+ T cells (Fig. 6A). Th1 cytokines, including IFN-γ, IL-2, and TNF-α, normally function to stimulate cell-mediated immune responses, but their proinflammatory activity can also cause tissue destruction and lead to a variety of autoimmune conditions when chronically activated (8, 28, 33, 44). Indeed, the excessive production and activity of macrophages observed in the spleens, livers, and intestines of lck-Cre-Mntflox/flox mice (Table 1 and Fig. 7) are a hallmark of disease states associated with Th1 polarization and autoimmune disease (28, 33). lck-Cre-Mntflox/flox mice also exhibited elevated levels of IgG subsets (data not shown) that are often associated with inflammatory disease. Finally, the aberrant patchwork of lymphocytes found around blood vessels in various tissues of lck-Cre-Mntflox/flox mice (Fig. 7B; see also Fig. S3 in the supplemental material; also data not shown) is consistent with the altered migration characteristics of T cells known to be caused by or associated with Th1 cytokine skewing (33). Although aberrant activation and proliferation of T-regulatory cells (CD4+ CD25+) can also contribute to the immunopathogenesis of inflammatory diseases (33, 44), these cells appeared not to be affected by Mnt deficiency (data not shown).
In addition to Th1 skewing, defective negative selection during thymocyte development is often intimately linked to the development of inflammatory diseases (31). The elimination of thymocytes that are self-reactive (negative selection) by apoptosis occurs primarily at the DP and SP stages of T-cell development. Compared to control cells, Mnt-deficient DP and SP T cells showed a strong increase in apoptosis (Fig. 3B). These results are consistent with previous results showing that loss of Mnt sensitizes fibroblasts to apoptosis (19, 30, 51) and raise the possibility that Mnt plays an important role in regulating apoptosis associated with negative selection. However, increased apoptosis of DP and SP thymocytes is not typically linked with the development of inflammatory diseases. To the contrary, decreased apoptosis in these populations is associated with several human autoimmune disorders, such as those caused by mutation of Fas (CD95) or Fas ligand, and in mouse experimental models relevant to autoimmunity-linked inflammatory disease (31). It is important to note, however, that while thymocyte populations lacking Mnt exhibited increased apoptosis (Fig. 3B), peripheral CD4+ T cells showed a decreased sensitivity to apoptosis associated with stimulation by PMA and ionomycin (Fig. 6D). One possibility, that remains to be tested, is that the decreased sensitivity to apoptosis in this setting translates to a decreased sensitivity to apoptosis caused by self antigen and contributes to the inflammatory phenotype. Further, the decreased sensitivity to apoptosis of Mnt-deficient T cells that reach the periphery (Fig. 6D) would be predicted to endow these cells with a tumor-prone phenotype. Indeed, this speculative model has parallels with models of Myc-driven tumorigenesis (29, 36) and provides a possible link between the development of inflammatory disease and lymphomagenesis in lck-Cre-Mntflox/flox mice.
Interestingly, c-Myc has been implicated in both negative selection and positive selection and strong transient activation of c-Myc can, like loss of Mnt, deplete DP T cells in vivo (5, 40, 43). Although there is no direct evidence indicating that Myc-induced apoptosis is linked to the development of inflammatory disease in transgenic mice, there is evidence that links a bypass in negative selection to Myc-induced T-cell lymphomagenesis (5). The issues of whether the inflammatory disease in lck-Cre-Mntflox/flox mice is associated with defects in negative selection (in addition to Th1 cytokine skewing) and whether such defects are linked to lymphomagenesis can be tested with established mouse models that employ antigen-specific TCRs and ones in which apoptosis associated with negative selection is disrupted.
The increased proliferation and apoptosis of T cells and lymphomagenesis caused by loss Mnt are consistent with the postulated role of Mnt as a Myc antagonist. However, loss of Mnt appears to have little or no effect on cell growth (accumulation of cell mass), which has emerged as a key response elicited by forced Myc expression (34). Experiments examining cell size and cell growth in mouse embryo fibroblasts lacking Mnt also support the conclusion that Mnt plays a limited role in cell growth control (Z.-Q. Zhou and P.J. Hurlin, unpublished). These data are in contrast to a clear role for Drosophila Mnt in the control of cell growth (23). One possibility is that the Mnt-related Mxd (formerly Mad/Mxi) family genes selectively adopted functions involved in the control of cell growth as they evolved from the lone Mnt/Mxd gene of lower eukaryotes (23, 37). Perhaps consistent with this notion, Mxd1 (formerly Mad1) has been strongly implicated in the control of cell growth and proliferation in T cells and other cell types (21, 38). However, while Mxd1 overexpression in T cells inhibits cell proliferation (21, 41), mice containing homozygous germ line deletions of Mxd family genes appear relatively healthy, with no report of defective T-cell development or function (13, 39, 42).
Our finding that Mnt loss has little effect on the growth of T cells or on the levels of Myc effector proteins (Fig. 4A) and genes (Fig. 4B) raises the possibility that loss of Mnt-Myc antagonism is only partly responsible for phenotypes caused by loss of Mnt in T cells. Alternatively, Myc and Mnt may regulate at least some of the same critical genes and pathways in T cells, but Mnt deficiency and Myc overexpression may have quantitatively different effects on gene regulation and therefore on the severity of related phenotypes. Possible support for this idea comes from a comparison of T-cell lymphogenesis caused by loss of Mnt and Myc overexpression. Whereas forced c-Myc expression in T cells typically leads to the development of highly malignant T-cell lymphomas and associated lethality between 7 weeks and 12 months (2, 46), lymphomas caused by loss of Mnt develop only after 12 months (Fig. 8A). A similar difference in the time required for tumor development caused by Mnt loss and Myc overexpression in breast epithelium has been found (19). However, it is important to note that even though the phenotypic consequences of constitutive Myc expression in T cells have been well documented, the corresponding effects on specific and general gene regulation have not been determined. Thus, given the controversial nature of Myc target genes (reviewed in reference 7), a proper interpretation of whether changes in gene expression caused by loss of Mnt is symptomatic of a loss of Myc antagonism requires a comparable analysis of gene expression in T cells that overexpress Myc.
In summary, our results reveal a critical role for Mnt in the regulation of T-cell proliferation, apoptosis, and differentiation. These activities of Mnt in T cells are essential for both maintaining immune homeostasis and tumor suppression. We present evidence that Mnt functions in the regulation of T-cell differentiation both at the DN3 stage (Fig. 2A) and in CD4+ differentiation to Th subsets (Fig. 5A). Differentiation at both of these stages is intimately linked to TCR-mediated signaling. Thus, mice lacking Mnt in T cells may provide a useful model for dissecting events associated with TCR engagement and/or events downstream in the TCR signaling cascade that direct the production of key inflammatory cytokines. Further, these mice may offer a valuable model for exploring mechanisms that link the development of inflammatory disease with lymphomagenesis. Finally, our results implicate Mnt as an important determinant in inflammatory disease and lymphomagenesis in humans.
Supplementary Material
Acknowledgments
We thank Brian Iritani, Andrew Weinberg, and David Parker for advice and comments on the manuscript; Christopher Corless for pathology services; and Zoe Purtzer, William Walker, and Sara Ota for expert technical assistance.
This work was supported by grants from the National Institutes of Health and Shriners Hospitals for Children to P.J.H.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Adhikary, S., and M. Eilers. 2005. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell. Biol. 6:635-645. [DOI] [PubMed] [Google Scholar]
- 2.Blyth, K., A. Terry, M. O'Hara, E. W. Baxter, M. Campbell, M. Stewart, L. A. Donehower, D. E. Onions, J. C. Neil, and E. R. Cameron. 1995. Synergy between a human c-myc transgene and p53 null genotype in murine thymic lymphomas: contrasting effects of homozygous and heterozygous p53 loss. Oncogene 10:1717-1723. [PubMed] [Google Scholar]
- 3.Broussard-Diehl, C., S. R. Bauer, and R. H. Scheuermann. 1996. A role for c-myc in the regulation of thymocyte differentiation and possibly positive selection. J. Immunol. 156:3141-3150. [PubMed] [Google Scholar]
- 4.Buckley, A. F., C. T. Kuo, and J. M. Leiden. 2001. Transcription factor LKLF is sufficient to program T cell quiescence via a c-myc-dependent pathway. Nat. Immunol. 2:698-704. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, E. R., M. Campbell, K. Blyth, S. A. Argyle, L. Keanie, J. C. Neil, and D. E. Onions. 1996. Apparent bypass of negative selection in CD8+ tumours in CD2-myc transgenic mice. Br. J. Cancer 73:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, E. R., J. Morton, C. J. Johnston, J. Irvine, M. Bell, D. E. Onions, J. C. Neil, M. Campbell, and K. Blyth. 2000. Fas-independent apoptosis in T-cell tumours induced by the CD2-myc transgene. Cell Death Differ. 7:80-88. [DOI] [PubMed] [Google Scholar]
- 7.Cole, M. D., and S. B. McMahon. 1999. The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene 18:2916-2924. [DOI] [PubMed] [Google Scholar]
- 8.Dong, C., and R. A. Flavell. 2000. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas, N. C., H. Jacobs, L. A. M. Bothwell, and A. C. Hayday. 2001. Defining the specific physiological requirements for c-Myc in T-cell development. Nat. Immunol. 2:307-315. [DOI] [PubMed] [Google Scholar]
- 10.Elson, A., C. Deng, J. Campos-Torres, L. A. Donehower, and P. Leder. 1995. The MMTV/c-myc transgene and p53 null alleles collaborate to induce T-cell lymphomas, but not mammary carcinomas in transgenic mice. Oncogene 11:181-190. [PubMed] [Google Scholar]
- 11.Fehling, H. J., and H. von Boehmer. 1997. Early αβ T cell development in the thymus of normal and genetically altered mice. Curr. Opin. Immunol. 9:263-275. [DOI] [PubMed] [Google Scholar]
- 12.Felsher, D. W., and J. M. Bishop. 1999. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4:199-207. [DOI] [PubMed] [Google Scholar]
- 13.Foley, K. P., G. A. McArthur, C. Queva, P. J. Hurlin, P. Soriano, and R. N. Eisenman. 1998. Targeted disruption of the MYC antagonist MAD1 inhibits cell cycle exit during granulocyte differentiation. EMBO J. 17:774-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germain, R. N. 2002. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2:309-322. [DOI] [PubMed] [Google Scholar]
- 15.Grumont, R., P. Lock, M. Mollinari, F. M. Shannon, A. Moore, and S. Gerondakis. 2004. The mitogen-induced increase in T cell size involves PKC and NFAT activation of Rel/NF-κB-dependent c-myc. Immunity 21:19-30. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman, E. S., L. Passoni, T. Crompton, T. M. Leu, D. G. Schatz, A. Koff, M. J. Owen, and A. C. Hayday. 1996. Productive T cell receptor β-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 10:948-962. [DOI] [PubMed] [Google Scholar]
- 17.Hueber, A.-O., M. Zornig, D. Lyon, T. Suda, S. Nagata, and E. Evan. 1997. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science 278:1305-1309. [DOI] [PubMed] [Google Scholar]
- 18.Hurlin, P. J., C. Queva, and R. N. Eisenman. 1997. Mnt, a novel Max-interacting protein is coexpressed with Myc in proliferating cells and mediates repression at Myc binding sites. Genes Dev. 11:44-58. [DOI] [PubMed] [Google Scholar]
- 19.Hurlin, P. J., Z. Q. Zhou, K. Toyo-oka, S. Ota, W. L. Walker, S. Hirotsune, and A. Wynshaw-Boris. 2003. Deletion of Mnt leads to disrupted cell cycle control and tumorigenesis. EMBO J. 22:4584-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imbert, V., J. F. Peyron, D. Farahi Far, B. Mari, P. Auberger, and B. Rossi. 1994. Induction of tyrosine phosphorylation and T-cell activation by vanadate peroxide, an inhibitor of protein tyrosine phosphatases. Biochem. J. 297:163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iritani, B. M., J. Delrow, C. Grandori, I. Gomez, M. Klacking, L. S. Carlos, and R. N. Eisenman. 2002. Modulation of T-lymphocyte development, growth and cell size by the Myc antagonist and transcriptional repressor Mad1. EMBO J. 21:4820-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo, J. F., H. Zhou, C. Fearns, R. A. Reisfeld, Y. Yang, and J. D. Lee. 2005. Tid1 is required for T cell transition from double-negative 3 to double-positive stages. J. Immunol. 174:6105-6112. [DOI] [PubMed] [Google Scholar]
- 23.Loo, L. W., J. Secombe, J. T. Little, L. S. Carlos, C. Yost, P. F. Cheng, E. M. Flynn, B. A. Edgar, and R. N. Eisenman. 2005. The transcriptional repressor dMnt is a regulator of growth in Drosophila melanogaster. Mol. Cell. Biol. 25:7078-7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons, A. B., and C. R. Parish. 1994. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171:131-137. [DOI] [PubMed] [Google Scholar]
- 25.Maclean, K. H., U. B. Keller, C. Rodriguez-Galindo, J. A. Nilsson, and J. L. Cleveland. 2003. c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-XL. Mol. Cell. Biol. 23:7256-7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mateyak, M. K., A. J. Obaya, and J. M. Sedivy. 1999. c-Myc regulates cyclin D-Cdk4 and -Cdk6 activity but affects cell cycle progression at multiple independent points. Mol. Cell. Biol. 19:4672-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meroni, G., A. Reymond, M. Alcalay, G. Borsani, A. Tanigami, R. Tonlorenzi, C. L. Nigro, S. Messali, M. Zollo, D. H. Ledbetter, R. Brent, A. Ballabio, and R. Carrozzo. 1997. Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor. EMBO J. 16:2892-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neurath, M. F., S. Finotto, and L. H. Glimcher. 2002. The role of Th1/Th2 polarization in mucosal immunity. Nat. Med. 8:567-573. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson, J. A., and J. L. Cleveland. 2003. Myc pathways provoking cell suicide and cancer. Oncogene 22:9007-9021. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson, J. A., K. H. Maclean, U. B. Keller, H. Pendeville, T. A. Baudino, and J. L. Cleveland. 2004. Mnt loss triggers Myc transcription targets, proliferation, apoptosis, and transformation. Mol. Cell. Biol. 24:1560-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi, P. S. 2003. Negative selection and autoimmunity. Curr. Opin. Immunol. 15:668-676. [DOI] [PubMed] [Google Scholar]
- 32.Orian, A., B. van Steensel, J. Delrow, H. J. Bussemaker, L. Li, T. Sawado, E. Williams, L. W. Loo, S. M. Cowley, C. Yost, S. Pierce, B. A. Edgar, S. M. Parkhurst, and R. N. Eisenman. 2003. Genomic binding by the Drosophila Myc, Max, Mad/Mnt transcription factor network. Genes Dev. 17:1101-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Shea, J. J., A. Ma, and P. Lipsky. 2002. Cytokines and autoimmunity. Nat. Rev. Immunol. 2:37-45. [DOI] [PubMed] [Google Scholar]
- 34.Oskarsson, T., and A. Trumpp. 2005. The Myc trilogy: lord of RNA polymerases. Nat. Cell Biol. 7:215-217. [DOI] [PubMed] [Google Scholar]
- 35.Pai, S. Y., M. L. Truitt, C. N. Ting, J. M. Leiden, L. H. Glimcher, and I. C. Ho. 2003. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity 19:863-875. [DOI] [PubMed] [Google Scholar]
- 36.Pelengaris, S., M. Khan, and G. Evan. 2002. c-MYC: more than just a matter of life and death. Nat. Rev. Cancer 2:764-776. [DOI] [PubMed] [Google Scholar]
- 37.Peyrefitte, S., D. Kahn, and M. Haenlin. 2001. New members of the Drosophila Myc transcription factor subfamily revealed by a genome-wide examination for basic helix-loop-helix genes. Mech. Dev. 104:99-104. [DOI] [PubMed] [Google Scholar]
- 38.Poortinga, G., K. M. Hannan, H. Snelling, C. R. Walkley, A. Jenkins, K. Sharkey, M. Wall, Y. Brandenburger, M. Palatsides, R. B. Pearson, G. A. McArthur, and R. D. Hannan. 2004. MAD1 and c-MYC regulate UBF and rDNA transcription during granulocyte differentiation. EMBO J. 23:3325-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Queva, C., G. A. McArthur, B. M. Iritani, and R. N. Eisenman. 2001. Targeted deletion of the S-phase-specific Myc antagonist Mad3 sensitizes neuronal and lymphoid cells to radiation-induced apoptosis. Mol. Cell. Biol. 21:703-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudolph, B., A. O. Hueber, and G. I. Evan. 2000. Reversible activation of c-Myc in thymocytes enhances positive selection and induces proliferation and apoptosis in vitro. Oncogene 19:1891-1900. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph, B., A. O. Hueber, and G. I. Evan. 2001. Expression of Mad 1 in T cells leads to reduced thymic cellularity and impaired mitogen-induced proliferation. Oncogene 20:1164-1175. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber-Agus, N., Y. Meng, T. Hoang, H. Hou, Jr., K. Chen, R. Greenberg, C. Cordon-Cardo, H. W. Lee, and R. A. DePinho. 1998. Role of Mxi1 in ageing organ systems and the regulation of normal and neoplastic growth. Nature 393:483-487. [DOI] [PubMed] [Google Scholar]
- 43.Shi, Y., J. M. Glynn, L. J. Guilbert, T. G. Cotter, R. P. Bissonnette, and D. R. Green. 1992. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science 257:212-214. [DOI] [PubMed] [Google Scholar]
- 44.Skapenko, A., J. Leipe, P. E. Lipsky, and H. Schulze-Koops. 2005. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther. 7:S4-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith, D. P., M. L. Bath, A. W. Harris, and S. Cory. 2005. T-cell lymphomas mask slower developing B-lymphoid and myeloid tumors in transgenic mice with broad haemopoietic expression of MYC. Oncogene 24:3544-3545. [DOI] [PubMed] [Google Scholar]
- 46.Stewart, M., E. Cameron, M. Campbell, R. McFarlane, S. Toth, K. Lang, D. Onions, and J. C. Neil. 1993. Conditional expression and oncogenicity of c-myc linked to a CD2 gene dominant control region. Int. J. Cancer 53:1023-1030. [DOI] [PubMed] [Google Scholar]
- 47.Takahama, Y., K. Ohishi, Y. Tokoro, T. Sugawara, Y. Yoshimura, M. Okabe, T. Kinoshita, and J. Takeda. 1998. Functional competence of T cells in the absence of glycosylphosphatidylinositol-anchored proteins caused by T cell-specific disruption of the Pig-a gene. Eur. J. Immunol. 28:2159-2166. [DOI] [PubMed] [Google Scholar]
- 48.Toyo-oka, K., S. Hirotsune, M. J. Gambello, Z. Q. Zhou, L. Olson, M. G. Rosenfeld, R. Eisenman, P. Hurlin, and A. Wynshaw-Boris. 2004. Loss of Max-interacting protein Mnt in mice results in decreased viability, defective embryonic growth and craniofacial defects: relevance to Miller-Dieker syndrome. Hum. Mol. Genet. 13:1057-1067. [DOI] [PubMed] [Google Scholar]
- 49.Trumpp, A., Y. Refaeli, T. Oskarsson, S. Gasser, M. Murphy, G. R. Martin, and J. M. Bishop. 2001. c-Myc regulates mammalian body size by controlling cell number but not cell size. Nature 414:768-773. [DOI] [PubMed] [Google Scholar]
- 50.van Ewijk, W. 1991. T-cell differentiation is influenced by thymic microenvironment. Annu. Rev. Immunol. 9:591-615. [DOI] [PubMed] [Google Scholar]
- 51.Walker, W., Z.-Q. Zhou, S. Ota, A. Wynshaw-Boris, and P. J. Hurlin. 2005. Mnt-Max to Myc-Max complex switching regulates cell cycle entry. J. Cell Biol. 10:1083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson, A., M. J. Murphy, T. Oskarsson, K. Kaloulis, M. D. Bettess, G. M. Oser, A. C. Pasche, C. Knabenhans, H. R. Macdonald, and A. Trumpp. 2004. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 18:2747-2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, W., J. Shen, M. Wu, M. Arsura, M. FitzGerald, Z. Suldan, D. W. Kim, C. S. Hofmann, S. Pianetti, R. Romieu-Mourez, L. P. Freedman, and G. E. Sonenshein. 2001. Repression of transcription of the p27(Kip1) cyclin-dependent kinase inhibitor gene by c-Myc. Oncogene 20:1688-1702. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, L., V. Camerini, T. P. Bender, and K. S. Ravichandran. 2002. A nonredundant role for the adapter protein Shc in thymic T cell development. Nat. Immunol. 3:749-755. [DOI] [PubMed] [Google Scholar]
- 55.Zhou, Z.-Q., and P. J. Hurlin. 2001. The interplay between Mad and Myc in proliferation and differentiation. Trends Cell Biol. 11:S10. [DOI] [PubMed] [Google Scholar]
- 56.Zindy, F., C. M. Eischen, D. H. Randle, T. Kamijo, J. L. Cleveland, C. J. Sherr, and M. F. Roussel. 1998. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 12:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.