Abstract
K cyclin encoded by Kaposi's sarcoma-associated herpesvirus confers resistance to the cyclin-dependent kinase (cdk) inhibitors p16Ink4A, p21Cip1, and p27Kip1 on the associated cdk6. We have previously shown that K cyclin expression enforces S-phase entry on cells overexpressing p27Kip1 by promoting phosphorylation of p27Kip1 on threonine 187, triggering p27Kip1 down-regulation. Since p21Cip1 acts in a manner similar to that of p27Kip1, we have investigated the subversion of a p21Cip1-induced G1 arrest by K cyclin. Here, we show that p21Cip1 is associated with K cyclin both in overexpression models and in primary effusion lymphoma cells and is a substrate of the K cyclin/cdk6 complex, resulting in phosphorylation of p21Cip1 on serine 130. This phosphoform of p21Cip1 appeared unable to associate with cdk2 in vivo. We further demonstrate that phosphorylation on serine 130 is essential for K cyclin-mediated release of a p21Cip1-imposed G1 arrest. Moreover, we show that under physiological conditions of cell cycle arrest due to elevated levels of p21Cip1 resulting from oxidative stress, K cyclin expression enabled S-phase entry and was associated with p21Cip1 phosphorylation and partial restoration of cdk2 kinase activity. Thus, expression of the viral cyclin enables cells to subvert the cell cycle inhibitory function of p21Cip1 by promoting cdk6-dependent phosphorylation of this antiproliferative protein.
Progression through the mammalian cell cycle is primarily regulated during the G1 phase. D-type cyclins complexed with either cyclin-dependent kinase 4 (cdk4) or cdk6 collaborate with cyclin E/cdk2 to phosphorylate the retinoblastoma protein, pRb. This multisite phosphorylation inactivates pRb, hence removing its restraining influence on S-phase entry (1). However, to prevent unscheduled progression through the cell cycle, the G1-specific cyclin/cdk holoenzymes are held in check by p21Cip1 and p27Kip1 (34). These inhibitory proteins stoichiometrically bind to both the cyclin and cdk subunits and inhibit their enzyme activity through steric hindrance within the catalytic cleft (10, 15, 30, 32). p21Cip1 and p27Kip1 can also prevent the activating phosphorylation of the cdk subunit (2, 17). In addition to their inhibitory roles, p21Cip1 and p27Kip1 can promote D-type cyclin/cdk complex formation; these heterotrimeric complexes retain substantial kinase activity (20, 36). Such trimeric complexes are believed to promote cell cycle progression through the sequestration of p21Cip1 and p27Kip1, releasing cyclin/cdk2 complexes from inhibitory interactions (28).
Our understanding of the roles of endogenous cell cycle regulatory proteins has been greatly increased through the analysis of the functions of a variety of viral proteins that promote proliferation. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus (KSHV) (K cyclin) is the viral homologue of the mammalian D-type cyclins, and it preferentially interacts with endogenous cdk6 (7, 13). K cyclin/cdk6 complexes have unusual properties, preeminent being their ability to maintain kinase activity in the presence of p21Cip1 and p27Kip1 (37). We have previously shown that K cyclin is able to circumvent the G1 blockades imposed by p21Cip1 and p27Kip1 (37). To overcome the arrest imposed by p27Kip1, K cyclin promotes the cdk6-dependent phosphorylation of p27Kip1 on threonine 187, thereby stimulating p27Kip1 degradation (11, 22). The phosphorylation-resistant T187A mutant of p27Kip1 is able to restrict cell cycle progression even in the presence of K cyclin (22, 37). These data indicate that K cyclin expression provides conditions that are permissive for cyclin/cdk2 activation by eliminating p27Kip1 and that the viral cyclin must facilitate the activation of endogenous cyclin/cdk complexes to enable cell cycle progression (21, 34).
To further dissect the properties of the viral cyclin and shed additional light on the control of cdk inhibitors in vivo, we addressed the mechanism by which K cyclin can bypass the G1 arrest imposed by p21Cip1, given that this cdk inhibitor, like p27Kip1, can inhibit the G1-specific cyclin/cdk holoenzymes. Our results demonstrate that K cyclin/cdk6 can phosphorylate p21Cip1 on serine 130 (S130) in vitro and in vivo and that this phosphorylation is essential for K cyclin-mediated release of a p21Cip1-imposed G1 arrest. Also, we show that K cyclin expression enabled S-phase entry under oxidative stress-induced cell cycle arrest resulting from elevated levels of p21Cip1. This was associated with p21Cip1 phosphorylation and partial restoration of cdk2 kinase activity.
MATERIALS AND METHODS
Antibodies and plasmids.
The antibodies used were against the following proteins: p21Cip1 (C19-G), cdk6 (C-21), specificity protein 1 (Sp1) (PEP-2), β-tubulin (D-10), cdk2 (M2), and E2F-1 (KH95) from Santa Cruz; bromodeoxyuridine (BrdU) from DAKO; hemagglutinin (HA; 12CA5 or 3F10) from Roche; Myc epitope (9E10) from Babco, Inc. (Berkeley, CA); and cdk4 (DCS-35) from NeoMarkers (Fremont, CA). The mouse monoclonal antibody to p21Cip1 (SX118) was from BD PharMingen (Fremont, CA). A rabbit polyclonal antibody to K cyclin was produced by BIOTREND Chemikalien GmbH, (Köln, Germany). The p21Cip1 phosphospecific antibody was raised in rabbits to the peptide GEQAEGpSPGGPGDS, where pS represents phosphoserine 130. Wild-type p21Cip1, p21Cip1 amino acids 1 to 103 (cloned BamHI/PstI) and p21Cip1 amino acids 102 to 164 (cloned PstI/EcoRI) were cloned into pGEX-KG (14) and pRSET (Invitrogen). The S130A mutant of p21Cip1 was generated by site-directed mutagenesis and subcloned into pGEX-KG. For mammalian expression, 2× Flag-tagged K cyclin, dominant negative cdk6 (38), and HA-tagged p21Cip1 (and the mutants S130A, T145A, and S130A/T145A) were transferred into the pcDNA3 vector (Invitrogen). pcDNA3-Myc-K cyclin was a kind gift from Sibylle Mittnacht (The Institute of Cancer Research, London, United Kingdom).
Cells and baculovirus.
NIH 3T3-K cells were isolated, cultured, and made quiescent as previously described (37). K cyclin expression was induced with 1 mM IPTG (isopropyl-α-d-thiogalactopyranoside). Culture of U2-OS cells and transfection has been previously described (16, 23). The U2-OS-K cyclin cell line (U2-OS-K) was a kind gift from Heike Laman (Division of Virology, Department of Pathology, University of Cambridge) and Flag-tagged K cyclin expression was induced with 5 mM IPTG. Culture and infection of Sf9 cells with recombinant baculoviruses were performed as previously described (22). The BC-3 cell line (3) was kindly provided by Ethel Cesarman (Cornell Medical College, New York City, NY). BC-3 and JOK-1 hairy cell leukemia cells (a kind gift from Leif Andersson, University of Helsinki, Finland) were cultured in a humidified 5% CO2 atmosphere at 37°C in RPMI 1640 medium supplemented with 15% fetal calf serum (Invitrogen, Carlsbad, CA). For treatment with hydrogen peroxide, NIH 3T3-K or U2-OS-K cells were cultured for 24 h in the absence or presence of IPTG. For BrdU incorporation, hydrogen peroxide was added to 250 μM to NIH 3T3-K cells, and the cells were incubated for 4.5 h. BrdU was added for the last 30 min before the cells were harvested. For the kinase assays, U2-OS-K cyclin cells were treated with 500 μM hydrogen peroxide for 20 h before being harvested.
In vitro kinase assays and phosphopeptide analysis.
Sf9 cells were infected with the appropriate recombinant baculoviruses and lysed 72 h later by swelling in K buffer (approximately 2.5 × 106 cells/ml; K buffer is 25 mM HEPES [pH 7.9], 5 mM MgCl, 0.1% 2-mercaptoethanol, and 0.1 mM EDTA) for 10 min on ice. Cell debris was removed by centrifugation. Kinase assays were performed in K buffer containing 0.5 to 1 μg of the appropriate substrate, 100 μM ATP, and 2.5 μCi [γ-32P]ATP at 30°C for 30 min. Reactions were then terminated by the addition of an equal volume of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and resolved by SDS-PAGE. Primary effusion lymphoma (PEL) cells, transfected cells, or U2-OS-K cells were lysed into the ELB lysis buffer (150 mM NaCl, 50 mM HEPES [pH 7.4], 0.1% Igepal, 5 mM EDTA, 2 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride, leupeptin [2 μg/ml], pepstatin [2 μg/ml], and aprotinin [1.5 μg/ml]) supplemented with 25 mM β-glycerophosphate. For measurement of in vitro kinase activity towards pRb, histone H1, endogenous p21Cip1, or glutathione S-transferase (GST)-p21Cip1 lysates were incubated 2 h at 4°C with anti-K cyclin, anti-CDK2, or anti-Myc antibody. Immunocomplexes were coupled to protein A-Sepharose beads for an additional 1 h at 4°C and washed three times with lysis buffer, followed by one wash with kinase buffer (20 mM Tris [pH 7.5], 50 mM KCl, 7.5 mM MgCl2, 1 mM DTT, 25 mM β-glycerophosphate, leupeptin [2 μg/ml], pepstatin [2 μg/ml], and aprotinin [1.5 μg/ml]). Immunodepletion was performed with three rounds of immunoprecipitation with either anti-cdk6, anti-cdk4, anti-cdk2, or anti-p21Cip1 antibody; the depleted lysates were immunoprecipitated with anti-K cyclin antibody. For blocking treatments, 1 μg of K cyclin antibody was pretreated with 10 μg of GST-K cyclin at room temperature for 2 h prior to addition to the lysate. Kinase reactions were performed in the presence of 2 μCi of [γ-32P]ATP for 15 min at 30°C using 0.5 μg of wild-type or S130A mutant GST-p21Cip1 or 2 μg GST-pRb and histone H1 as a substrate or without any exogenous substrates. Phosphorylated proteins were analyzed by SDS-PAGE and autoradiography.
For the phosphopeptide analysis, in vitro kinase assays were performed as described above except that 20 μCi [γ-32P]ATP and GST-p21Cip1 were used. The phosphorylated GST-p21Cip1 products were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Radioactive bands were identified by autoradiography, excised, and digested with trypsin. Peptides were resolved in two dimensions on thin-layer cellulose chromatography plates using a Hunter Thin Layer Peptide Mapping Electrophoresis system as previously described (22). The first dimension was resolved by electrophoresis in pH 1.9 buffer (25 ml formic acid, 78 ml acetic acid, and 897 ml distilled H2O) for 50 min at 1,000 V, followed by chromatography with 3:10:12:15 acetic acid:pyridine:water:butan-1-ol for 8 h for the second dimension. Plates were dried and subjected to autoradiography. Phosphoamino acid analysis was performed as previously described (25).
Cell extractions and immunological methods.
For immunoblotting, cells were washed twice with phosphate-buffered saline (PBS), lysed in situ in SDS-PAGE sample buffer, and collected by scraping. Samples were then boiled and subjected to SDS-PAGE using 10% polyacrylamide gels to resolve cyclins and cdk's and 12.5% polyacrylamide gels for p21Cip1. Immunoblotting was performed as described previously (9). Blots were analyzed by autoradiography or by data capture via a Fuji LAS-3000 and quantification with Fujifilm Science Lab 2003 ImageQuant 4.21 software. Phosphatase treatment was performed by incubation of the cell extract with calf intestinal alkaline phosphatase (Roche) at 37°C for 30 min prior to immunoblotting.
For immunoprecipitations, NIH 3T3-K cells were washed twice with PBS, lysed in situ in IP-K buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 0.1% Tween-20, 50 mM NaF, 0.1 mM Na2VO3, and 1 mM DTT) and collected by scraping, followed by five passes through a 21-gauge needle. Samples were incubated for 30 min at 4°C before centrifugation to remove cell debris. Immunoprecipitations were performed at 4°C for 2 h using protein A- or protein G-Sepharose beads and the appropriate antibody. The immunoprecipitates were washed three times in IP-K buffer prior to resuspension in SDS-PAGE loading buffer and immunoblot analysis or kinase assay (9).
BrdU incorporation was determined as previously described (9) 24 h after addition of the BrdU. Cells were cotransfected with histone H2B-green fluorescent protein (GFP) fusion construct, and at least 100 GFP-positive cells were scored for BrdU incorporation. For half-life determinations, NIH 3T3-K cells were metabolically labeled for 120 min with approximately 100-μCi/ml [35S]methionine and cysteine (27) in the absence and presence of K cyclin expression, and p21Cip1 was immunoprecipitated as described above. For immunohistochemical analysis, transfected cells were fixed with 3.5% (wt/vol) paraformaldehyde and permeabilized with 0.1% Triton X-100 for 5 min. Immunofluorescence labeling was performed as previously described (16).
Nuclear-cytoplasmic fractionation.
For nuclear-cytoplasmic extractions, NIH 3T3-K cells were trypsinized, washed in PBS, and resuspended in buffer A (10 mM HEPES [pH 7.9]-10 mM KCl-1.5 mM MgCl2-0.34 M sucrose-10% glycerol-1 mM DTT). Triton X-100 was added to 0.1%, and the sample was incubated on ice for 5 min. Nuclei were pelleted by centrifugation at 3,500 rpm for 4 min at 4°C and washed once in buffer A. The cytoplasmic supernatant solution was clarified by centrifugation at 14,000 rpm for 10 min at 4°C. For PEL cell fractionation, BC-3 cells were resuspended in hypotonic lysis buffer (20 mM Tris [pH 7.5], 10 mM NaCl, 1.5 mM MgCl2, 2 mM EDTA, 0.1% Triton X-100, 10 mM MnCl2, 20% glycerol, 1 mM DTT, 25 mM β-glycerophosphate, 2 μg/ml leupeptin, 2 μg/ml pepstatin, and 1.5 μg/ml aprotinin) to a concentration of 5 × 107 cells/ml. Samples were incubated on ice for 5 min with gentle mixing. Nuclei were pelleted by being spun at 800 rpm for 5 min at 4°C. The supernatant (cytosol) was then carefully removed from the pellet, which was washed with PBS. The cytosolic extract was further clarified by centrifugation at 14,000 rpm for 15 min at 4°C. The clarified supernatant was collected and represents the cytosolic fraction. The nuclear pellet was resuspended in hypotonic lysis buffer plus 0.5 M NaCl and vortexed twice for 10 s. Nuclei were spun at 14,000 rpm for 15 min. The supernatant (nuclei) was collected and represents the nuclear fraction.
Gel filtration chromatography.
Cell lysates prepared in ELB lysis buffer were passed through a 0.22-μm-pore-size MILLEX-GS filter (Millipore) and fractionated on a Superdex 200 HR column with a fast-performance liquid chromatography system (Pharmacia Biotech, Uppsala, Sweden). Samples were loaded onto the column and separated in gel filtration buffer (50 mM HEPES [pH 7.5], 150 mM NaCl) at a flow rate of 0.3 ml/min. Column calibration was performed under the same conditions with blue dextran (2,000 kDa) thyroglobulin (669 kDa), apoferritin (443 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), bovine serum albumin (66 kDa), and carbonic anhydrase (29 kDa). For each fractionation, 30 fractions of 0.5 ml each were collected. A total of 50 μl of each fraction was used for immunoblotting, and 450 μl of each fraction was used for immunoprecipitation experiments.
RESULTS
K cyclin/cdk6 phosphorylates GST-p21Cip1 in vitro on S130.
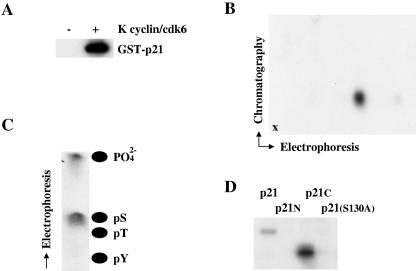
Initially, we tested if p21Cip1 was a direct substrate for K cyclin/cdk6-mediated phosphorylation using bacteria to generate a GST fusion protein of full-length p21Cip1. The GST fusion protein was employed in kinase assays with K cyclin/cdk6 complexes from baculovirus-infected Sf9 cells. K cyclin/cdk6 readily phosphorylated GST-p21Cip1 (Fig. 1A), while GST itself was not a substrate for K cyclin/cdk6 (negative data not shown). Similar data were obtained with poly(His)-p21Cip1 fusion proteins (Fig. 2). Phosphopeptide map analysis of phosphorylated GST-p21Cip1 indicated the presence of a single major phosphorylated species (Fig. 1B) which, when subjected to phosphoamino acid analysis, was found to contain only phosphoserine (Fig. 1C). To localize the site of phosphorylation, the amino-terminal [p21(1-103)] and carboxy-terminal [p21(102-164)] portions of p21Cip1 were generated in bacteria as GST fusion proteins and used as substrates for in vitro kinase assays. K cyclin/cdk6 phosphorylated the carboxy-terminal portion of p21Cip1 but not its amino-terminal fragment (Fig. 1D). The carboxy-terminal form of p21Cip1 contained a single consensus cdk phosphorylation site at S130. Mutation of this site to an alanine residue abolished the ability of K cyclin/cdk6 to phosphorylate p21Cip1 (Fig. 1D). The S130A mutant of GST-p21Cip1 was indistinguishable from the wild-type GST-p21Cip1 fusion in its ability to inhibit cyclin/cdk2 complexes (data not shown).
FIG. 1.
K cyclin promotes the phosphorylation of p21Cip1 on serine 130 in vitro. (A) K cyclin/cdk6 was expressed in Sf9 cells by infection with recombinant baculoviruses and used in kinase assays with [γ-32P]ATP and GST-p21Cip1 from bacteria as a substrate. Sf9 cells infected with wild-type baculovirus were used for the negative control. Products of the phosphorylation reactions were resolved by SDS-PAGE and subjected to autoradiography. (B) GST-p21Cip1 phosphorylated by K cyclin/cdk6 was subject to two-dimensional tryptic phosphopeptide mapping with electrophoresis in the horizontal direction and chromatography in the vertical direction. The point of sample application is marked by “x.” (C) The phosphopeptide shown in panel B was eluted from the cellulose plate and subjected to acid hydrolysis. The resulting hydrolysate was resolved by electrophoresis and subjected to autoradiography. The positions of phosphoamino acid standards are shown. (D) Wild-type and indicated mutant forms of p21Cip1 [N indicates p21(1-103) and C indicates p21(102-164)] were synthesized in bacteria as GST fusion proteins and used as substrates in kinase reactions with K cyclin/cdk6, as shown in panel A.
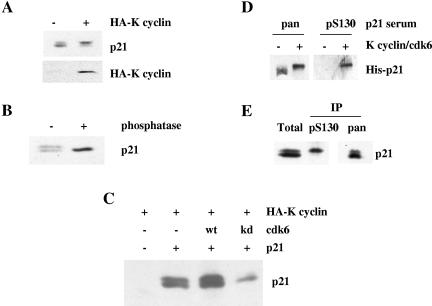
FIG. 2.
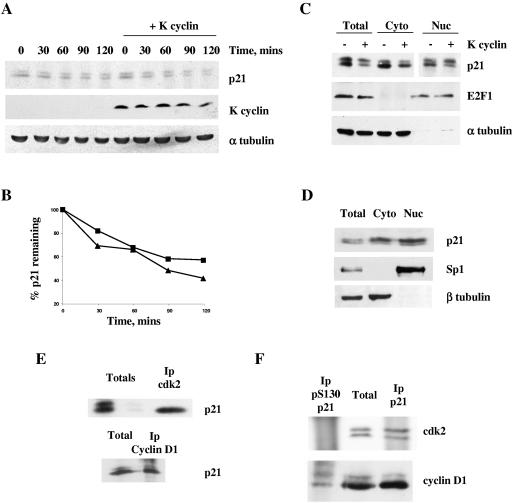
K cyclin can promote the phosphorylation of p21Cip1 on serine 130 in vivo. (A) NIH 3T3-K cells were cultured for 3 days in the absence (−) or presence (+) of IPTG to induce K cyclin expression. Cells were then lysed and subjected to immunoblotting for endogenous p21Cip1 and K cyclin. (B) NIH 3T3 cell lysates were heated to 95°C for 5 min, denatured protein was removed by centrifugation, and the resulting extract was incubated in the absence (−) or presence (+) of calf intestinal alkaline phosphatase prior to immunoblotting for p21Cip1. (C) U2-OS cells were transfected with plasmids directing the expression of HA-K cyclin, wild-type p21Cip1, and either wild-type cdk6 or kinase-dead cdk6. After 48 h, cells were lysed and subjected to immunoblotting for p21Cip1. (D) Poly(His)-tagged p21Cip1 (His-p21) was isolated from recombinant bacteria and used as a substrate in kinase reactions, as described in the legend to Fig. 1, with nonradioactive ATP. Products of the phosphorylation reactions were resolved by SDS-PAGE and immunoblotted using either an anti-carboxy-terminal p21Cip1 antibody (pan) or the anti-S130 phosphospecific serum (pS130). (E) NIH 3T3-K cells induced to express K cyclin were lysed and subjected to immunoprecipitation with either an anti-carboxy-terminal p21Cip1 antibody (pan) or the anti-S130 phosphospecific p21Cip1 serum (pS130). The products of the immunoprecipitates (IP) were immunoblotted with the anti-carboxy-terminal p21Cip1 antibody and compared to a sample of the starting material (Total).
K cyclin/cdk6 phosphorylates p21Cip1 in vivo on S130.
To address the ability of K cyclin to promote p21Cip1 phosphorylation in vivo, we initially employed an immunoblotting approach using NIH 3T3-K cells (37) that inducibly express K cyclin in response to treatment with IPTG. Strikingly, K cyclin expression was associated with the accumulation of a retarded mobility form of p21Cip1 (Fig. 2A). Phosphatase treatment of extracts prior to immunoblotting collapsed this p21Cip1 doublet to a single species that comigrated with the fastest-moving form (Fig. 2B), indicating that K cyclin expression promoted the appearance of a phosphorylated species of p21Cip1 in vivo. To substantiate this observation, we transfected U2-OS cells with HA-tagged p21Cip1, K cyclin, and either wild-type or kinase-dead cdk6 and assayed p21Cip1 phosphorylation status by immunoblotting. Kinase-dead cdk6 effectively abolished the appearance of the phosphorylated p21Cip1 species (Fig. 2C).
To determine if this phosphorylation event promoted by K cyclin and causing retarded mobility of p21Cip1 on SDS-PAGE was related to the S130 phosphorylation observed in vitro (Fig. 1), we raised an antibody to the phosphopeptide GEQAEGpSPGGPGDS, where pS represents phosphoserine 130. In vitro characterization indicated that this antiserum reacted only with serine 130-phosphorylated p21Cip1 (Fig. 2D). Moreover, immunoprecipitation with the phosphospecific serum from lysates of NIH 3T3-K cells expressing the viral cyclin specifically isolated the slower-migrating form of p21Cip1, while an antibody against a p21Cip1 carboxy-terminal peptide immunoprecipitated both forms of the cdk inhibitor (Fig. 2E). Taken together, these data demonstrate that K cyclin can promote the phosphorylation of p21Cip1 on S130 in vitro and in vivo and that this phosphorylation leads to reduced mobility of p21Cip1 on SDS-PAGE. We note that Kim et al. (18) demonstrated that p38 kinase can phosphorylate p21Cip1on S130 in vivo. In our hands, the p38 inhibitor SB203580 had little effect on the presence of the S130 phosphoform of p21Cip1 in our asynchronous cultures (data not shown).
K cyclin associates with and promotes phosphorylation of p21Cip1 in both transfected and PEL-derived cells.
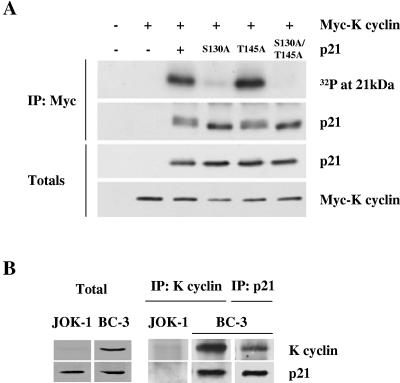
We have previously shown that K cyclin associates with p27Kip1 in vivo in transfected cells and PEL-derived cells (16). To assess the potential physical interactions between K cyclin and p21Cip1, we expressed HA-tagged versions of wild-type and phosphosite mutants of p21Cip1 in the presence of Myc-tagged K cyclin in U2-OS cells. Cell lysates from transfections were immunoprecipitated with anti-Myc antibody and either subjected to an in vitro kinase assay without adding any exogenous substrates or immunoblotted for p21Cip1. As shown in Fig. 3A, K cyclin associated with both wild-type p21Cip1 and the phosphosite mutants of p21Cip1. The coimmunoprecipitated wild-type p21Cip1 displayed two immunoreactive species, in contrast to the S130A mutant p21Cip1, which migrated as a single species. Mutation of the site of Akt phosphorylation (T145A) in p21Cip1 (31, 42) did not prevent either the association of K cyclin and p21Cip1 or the appearance of the retarded mobility form of p21Cip1. The double mutant S130A/T145A migrated in a manner indistinguishable from the S130A mutant protein. A similar mobility shift of p21Cip1 was also seen when total p21Cip1 was immunoblotted from the total lysates. Addition of [γ-32P]ATP to the K cyclin immunoprecipitates resulted in the phosphorylation of coimmunoprecipitated p21Cip1 only when the S130 site was present (Fig. 3A, top). Next, we analyzed whether p21Cip1 is associated with K cyclin in BC-3 PELs, which are latently infected with KSHV and express latent viral proteins like K cyclin. BC-3 cell lysates were immunoprecipitated with either anti-K cyclin or anti-p21Cip1 antibodies, and coimmunoprecipitated proteins were analyzed by immunoblotting. As shown in Fig. 3B, p21Cip1 and K cyclin were in complex in BC-3 cells, confirming our results from transfected cells and in agreement with recently published data (39). JOK-1 is a KSHV-negative leukemia cell line, which served as a negative control.
FIG. 3.
K cyclin associates with and promotes phosphorylation of p21Cip1 both in transfected cells and in PEL-derived cells. (A) Wild-type and indicated mutant forms of p21Cip1 were transfected into U2-OS cells, together with Myc-K cyclin or control expression vectors. After 48 h, cells were lysed and subjected to immunoprecipitation using anti-Myc antibody. Immunoprecipitates were assayed for kinase activity towards coprecipitated proteins and analyzed by SDS-PAGE and autoradiography (top). The K cyclin-associated p21Cip1 was analyzed by immunoblotting with anti-p21Cip1 antibody. Total lysates were immunoblotted with anti-p21Cip1 and anti-Myc antibodies. (B) Lysates of the PEL and JOK-1 cells were immunoprecipitated using either anti-K cyclin or anti-p21Cip1 antibodies and analyzed for associated proteins on SDS-PAGE by immunoblotting for K cyclin and p21Cip1. Total lysates of PEL cells (50 μg) were resolved by SDS-PAGE (12%) and subjected to immunoblotting for endogenous p21Cip1 and K cyclin.
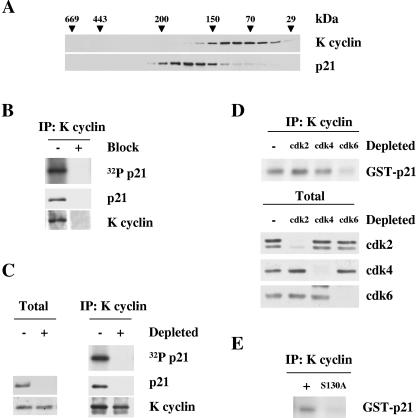
Gel filtration analysis was performed using BC-3 cell lysates to provide a qualitative estimate of the proportion of p21Cip1 complexed with K cyclin in PEL cells. Column fractions were analyzed by immunoblotting. K cyclin eluted in complexes between 29 to 170 kDa, partially overlapping the p21Cip1 elution profile (90 to 220 kDa) (Fig. 4A). Thus, only a subpopulation of p21Cip1 was associated with K cyclin in vivo. To ascertain if such p21Cip1 was a substrate of K cyclin/cdk in PEL lysates, samples from fractions containing K cyclin and p21Cip1 were immunoprecipitated with anti-K cyclin antisera and subjected to an in vitro kinase assay without the addition of exogenous substrates. The reaction products were then separated by SDS-PAGE and analyzed by either immunoblotting with anti-p21Cip1 antibodies or autoradiography. Results from a representative fraction are shown (Fig. 4B), indicating that K cyclin immunoprecipitates contained kinase activity against the coimmunoprecipitated p21Cip1. Repeating the experiment using anti-K cyclin antibody pretreated with the antigen (recombinant full-length K cyclin) demonstrated the specificity of the experiment. In addition, immunodepletion of p21Cip1 prior to immunoprecipitation with K cyclin antibodies abolished the phosphorylation of the 21-kDa species, indicating that K cyclin induces the phosphorylation of associated p21Cip1 (Fig. 4C). p21Cip1 was also immunoblotted from total lysates as a control for the depletion efficiency.
FIG. 4.
K cyclin/cdk6 phosphorylates p21Cip1 in PEL cells. (A) Lysates from the BC-3 cell line were separated by gel filtration chromatography on a Superdex 200 column. Fractions were resolved by SDS-PAGE (12%) and immunoblotted with antibodies to K cyclin and p21Cip1. The elution profile of the molecular mass standards is indicated in kilodaltons. (B) A representative fraction (eluting at 110 kDa) was immunoprecipitated with anti-K cyclin antibody and either assayed for kinase activity towards coprecipitated endogenous p21Cip1 by autoradiography or immunoblotted for the indicated proteins. “Block” indicates that the immunoprecipitating antibody was pretreated with the antigen (+) or treated with a nonspecific protein (−). (C) The same fraction shown in panel B was immunodepleted of p21Cip1 with three consecutive rounds of immunoprecipitation using anti-p21Cip1 antibody (depleted, +) or with rabbit immunoglobulin G for control (−). The two panels show immunoblots for the indicated protein after these cycles of immunodepletion. The p21Cip1- and control-depleted extracts were then immunoprecipitated with anti-K cyclin antibody and subjected to an in vitro kinase assay of coprecipitated endogenous p21Cip1. Kinase activity was determined by autoradiography after SDS-PAGE (12%) (right, top). Immunoprecipitated (IP) proteins were analyzed by immunoblotting for K cyclin and p21Cip1 (right, middle and bottom). (D) Lysates from BC-3 cells were immunodepleted for cdk2, cdk4, and cdk4 by three consecutive rounds of immunoprecipitation with anti-cdk2, anti-cdk4, or anti-cdk6 antibodies. In the control (−), lysate was immunoprecipitated with rabbit immunoglobulin G (IgG). Depleted lysates were subjected to immunoprecipitation by anti-K cyclin antibody and an in vitro kinase assay of GST-p21Cip1. Kinase activity was determined by autoradiography after SDS-PAGE (12%). cdk-depleted lysates (40 μg) were resolved by SDS-PAGE (12%) and immunoblotted with antibodies against cdk2, cdk4, and cdk6. (E) BC-3 cells were lysed and subjected to immunoprecipitation using anti-K cyclin antibody. Immunoprecipitates were assayed for kinase activity with wild-type or S130A mutant GST-p21Cip1 as a substrate. Products of the phosphorylation reactions were resolved by SDS-PAGE (12%) and subjected to autoradiography.
Even though K cyclin forms complexes with cdk2, cdk4, cdk5, and cdk6 in BC-3 cells, we have shown that cdk6 is the in vivo catalytic subunit of K cyclin in PEL cells using GST-pRb and histone H1 as substrates (16, 29). To extend our analysis, we depleted cdk2, cdk4, and cdk6 from BC-3 cell lysates to see which kinase partner was important for the K cyclin-induced phosphorylation of p21Cip1. Three consecutive rounds of immunoprecipitation resulted in an almost complete depletion of the target cdk from the lysate (Fig. 4D, bottom). The cdk-depleted lysates were then subjected to immunoprecipitation with anti-K cyclin antibody, and K cyclin-associated kinase activity was measured by an in vitro kinase assay using GST-p21Cip1 as a substrate. Only depletion of cdk6 resulted in a substantial decrease of K cyclin-associated kinase activity towards GST-p21Cip1 (Fig. 4D, top). Our results demonstrate that cdk6 is the major catalytic subunit associated with K cyclin that is involved in the phosphorylation of p21Cip1. To determine what site K cyclin-cdk6 from PELs phosphorylates on p21Cip1, lysates from BC-3 cells were subjected to an in vitro kinase assay using GST-p21Cip1 and GST-p21Cip1 S130A as substrates. The phosphorylation of GST-p21Cip1 was greatly diminished, with the S130A mutant supporting our data from the in vitro and transfection experiments that S130 is the major phosphorylation site on p21Cip1 for K cyclin (Fig. 4E).
K cyclin-mediated phosphorylation of p21Cip1 on S130 has little effect on p21Cip1 protein stability or localization but appears to abolish cdk2 binding.
We next began to dissect the physiological consequences of p21Cip1 S130 phosphorylation. Phosphorylation of cdk inhibitors has been shown to modulate their activity via two major mechanisms: protein stability and subcellular localization. We assessed the effects of S130 phosphorylation on each of these parameters. NIH 3T3-K cells were metabolically labeled with [35S]methionine and cysteine for 120 min prior to chasing in the presence of excess unlabeled amino acids in the absence or presence of K cyclin and the abundance of p21Cip1 assessed over time by immunoprecipitation (Fig. 5A and B). Quantitation of p21Cip1 in immunoprecipitates following resolution by SDS-PAGE indicated that the turnover of p21Cip1 was not appreciably altered by K cyclin expression. Similar results were obtained using the translational inhibitor cycloheximide (not shown). Thus, K cyclin expression had little effect on p21Cip1 turnover.
FIG. 5.
S130 phosphorylation does not alter p21Cip1 turnover or subcellular localization but restricts binding to cyclin/cdk2 complexes. (A) Logarithmically growing cultures of NIH 3T3-K cells (cultured in either the absence or presence of IPTG for 24 h) were pulse labeled with [35S]methionine and cysteine and chased in medium containing unlabeled amino acids. At the indicated time, cell extracts were prepared and immunoprecipitated with p21Cip1 antiserum. The labeled proteins were fractionated by SDS-PAGE and subjected to autoradiography. (B) Quantitation of the immunoprecipitated p21Cip1 shown in panel A in the absence (squares) or presence (triangles) of K cyclin by phosphorimager analysis. (C) NIH 3T3-K cells (cultured in either the absence or presence of IPTG for 24 h) were fractionated into nuclear and cytoplasmic samples prior to immunoblotting for p21Cip1, E2F-1, and α-tubulin. (D) BC-3 cells were separated into cytosolic and nuclear extracts. The resulting fractions were resolved by SDS-PAGE (12%) and analyzed by immunoblotting for p21Cip1, Sp1, and β-tubulin. cdk2 or cyclin D1 (E) or p21Cip1 or S130-phosphorylated p21Cip1 (F) were immunoprecipitated from NIH 3T3-K cells (cultured in the presence of IPTG), and the immune complexes were immunoblotted for the indicated proteins.
p21Cip1 has been shown to be regulated through subcellular distribution by phosphorylation of T145 (42). Thus, we analyzed the relative distribution of p21Cip1 in the absence and presence of K cyclin. NIH 3T3-K cells were fractionated into nuclear and cytoplasmic samples in the absence or presence of K cyclin expression. Immunoblotting these samples for p21Cip1 demonstrated that the phospho-p21Cip1 was enriched in nuclear fractions but was also present in the cytoplasmic fractions (Fig. 5C). As controls for the integrity of the fractionation protocol, extracts were probed sequentially with cytoplasmic (α-tubulin) and nuclear (E2F-1) markers (Fig. 5C). To study the distribution of p21Cip1 in a more physiological setting, the BC-3 PEL cells were fractionated into nuclear and cytoplasmic samples and immunoblotted with p21Cip1 antibody (Fig. 5D). The purity of the nuclear and cytoplasmic fractions was verified using markers for the nucleus (Sp1) and cytosol (β-tubulin). Again, p21Cip1 was detected in both compartments. The slower-migrating form of p21Cip1 corresponding to the phosphorylated p21Cip1 was also present in both compartments, supporting our fractionation data from NIH 3T3 cells expressing K cyclin. These biochemical data were further verified by immunofluorescence studies in which K cyclin-induced phosphorylation of p21Cip1 on S130 had no discernible effect on the nuclear retention of p21Cip1. However, K cyclin expression caused a slight increase in the proportion of p21Cip1 in the cytoplasm, although this was independent of S130 phosphorylation (data not shown).
To address the function of S130 phosphorylated p21Cip1 as a cdk inhibitor directly, we assessed the proportion of each p21Cip1 species in complex with different cyclin/cdk's in vivo. We immunoprecipitated cdk2 or cyclin D1 from NIH 3T3-K cells and immunoblotted the precipitates for p21Cip1 (Fig. 5E). Cyclin D1 immunoprecipitates contained both mobility forms of p21Cip1 while cdk2 immunoprecipitates displayed only the faster-migrating form of p21Cip1 lacking S130 phosphorylation. Total cell lysates clearly demonstrated the presence of p21Cip1, displaying its typical dual mobility. In reciprocal experiments, the antiserum directed against the S130 phosphoform of p21Cip1 did not coimmunoprecipitate cdk2 but did associate with cyclin D1; both of these cell cycle regulators were found in immune complexes and captured with antibodies directed against total cellular p21Cip1 (Fig. 5F). Thus, in vivo, S130-phosphorylated p21Cip1 appears specifically unable to bind cyclin/cdk2.
Phosphorylation of p21Cip1 on S130 is required for G1 release.
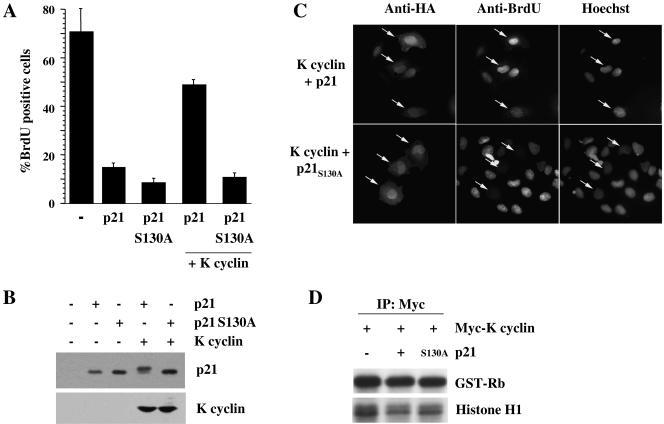
We next addressed whether the phosphorylation of p21Cip1 on S130 was important for K cyclin-mediated bypass of a p21Cip1-imposed G1 arrest by measuring S-phase entry by BrdU incorporation (Fig. 6A). Wild-type and S130A mutant p21Cip1 were expressed at similar levels (Fig. 6B) and efficiently prevented S-phase entry (Fig. 6A). Coexpression of K cyclin readily circumvented the cell cycle arrest imposed by wild-type p21Cip1 but was ineffective against the S130A mutant p21Cip1 (Fig. 6A and C). Similar results were obtained by flow cytometry (data not shown). Importantly, the increase in S phase enforced by K cyclin in the presence of wild-type p21Cip1 was accompanied by retardation of the migration of ectopic p21Cip1 on SDS-PAGE (Fig. 6B). The S130A mutation of p21Cip1, like the wild-type protein, was ineffective in the inhibition of K cyclin/cdk6 kinase activity but was as efficacious as the wild protein in inhibiting cyclin D2/cdk6 or cyclin E/cdk2 (Fig. 6D and data not shown), demonstrating that the S130A mutation did not impair p21Cip1 inhibitory function.
FIG. 6.
The K cyclin-dependent circumvention of a p21Cip1-imposed G1 arrest is dependent on S130 phosphorylation. (A) U2-OS cells were transfected with plasmids directing the expression of histone 2B-GFP (as a marker of transfected cells), wild-type or S130A mutant p21Cip1, and 2× Flag-K cyclin. BrdU was added after 24 h; after an additional 24 h, cells on coverslips were fixed, BrdU-positive cells were detected by indirect immunofluorescence to BrdU, and DNA was counterstained with DAPI. (B) Cells shown in panel A were lysed and subjected to immunoblotting for exogenous p21Cip1 and K cyclin. (C) U2-OS cells were transfected with Myc-tagged K cyclin and HA-tagged wild-type or S130A mutant p21Cip1 and treated with BrdU as in panel A. Indirect immunofluorescence was used to detect BrdU incorporation and p21Cip1 (via anti-HA antibodies). Nuclei were visualized by Hoechst staining. Representative cells are marked with arrows. (D) Myc-K cyclin was expressed in U2-OS cells alone or together with HA-tagged wild-type or S130A mutant p21Cip1. After 48 h, cells were lysed and subjected to immunoprecipitation with anti-Myc antibody. Immunoprecipitates were assayed for kinase activity towards pRb and histone H1 and analyzed by autoradiography following SDS-PAGE.
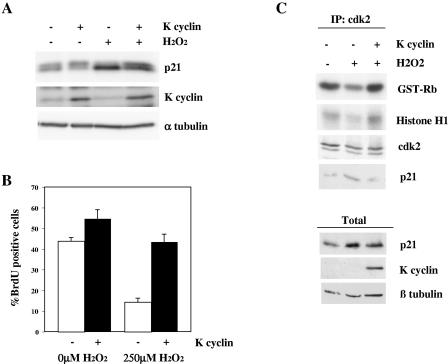
To assess the significance of K cyclin-mediated bypass of a p21Cip1-imposed cell cycle arrest under more physiological conditions, we utilized the DNA-damaging agent hydrogen peroxide. We have previously shown that treatment of fibroblasts with hydrogen peroxide leads to up-regulation of p21Cip1 and cell cycle arrest; K cyclin is able to enforce S-phase entry under these conditions (4). We repeated and extended these observations to assess the phosphorylation status of p21Cip1 and the kinase activity associated with cdk2. Figure 7A illustrates that hydrogen peroxide treatment of NIH 3T3-K cells caused an elevation of the endogenous level of p21Cip1. Importantly, the K cyclin-dependent reversal of S-phase progression was associated with an accumulation of the S130 phosphorylated p21Cip1 species (Fig. 7A and B). In addition, treatment of U2-OS-K cyclin cells with hydrogen peroxide led to p21Cip1 up-regulation and reduced cdk2 kinase activity by approximately 50%, compared to activity in untreated cells (Fig. 7C). Upon expression of K cyclin, p21Cip1 levels remained elevated after hydrogen peroxide treatment, but cdk2 activity was restored to the levels found in untreated cells; p21Cip1 association with cdk2 was reduced (Fig. 7). As expected, K cyclin-associated kinase activity was unaffected by treatment with the DNA-damaging agent (data not shown). Thus, K cyclin expression led to subversion of p21Cip1 cdk inhibitory function, as demonstrated by the restoration of cdk2 kinase activity, consistent with the cell cycle progression maintained in the face of oxidative damage that we previously described (4).
FIG. 7.
K cyclin-dependent bypass of DNA damage-induced cell cycle arrest is accompanied by S130 phosphorylation of p21Cip1 and partial restoration of cyclin/cdk2 kinase activity. (A) NIH 3T3-K cells were cultured in the absence (−) or presence (+) of IPTG to induce K cyclin expression for ∼16 h. Cells were then treated with vehicle or hydrogen peroxide for 4.5 h before lysis and immunoblotting for the indicated proteins. (B) Cells shown in panel A were treated with BrdU for the final 30 min of hydrogen peroxide treatment, and indirect immunofluorescence was used to detect S-phase progression. Data represent the mean ± standard error of at least four separate experiments with at least 100 cells scored per experiment. (C) U2-OS-K cells were cultured in the absence (−) or presence (+) of IPTG to induce K cyclin expression for ∼20 h. Cells were then treated with vehicle or hydrogen peroxide for 20 h and lysed. Lysates were immunoprecipitated through cdk2 and in vitro kinase assays of pRb and histone H1. Kinase activity was determined by autoradiography after SDS-PAGE (12%). Immune complexes and total lysates were immunoblotted for the proteins indicated.
DISCUSSION
Here, we show that expression of the cyclin encoded by KSHV promotes the cdk6-dependent phosphorylation of p21Cip1 on S130 both in vitro (Fig. 1) and in vivo (Fig. 2 to 4) and that this phosphorylation is essential for the K cyclin-mediated bypass of a p21Cip1-imposed G1 blockade (Fig. 6 and 7). Aspects of these observations are reminiscent of the K cyclin-mediated release of a p27Kip1-imposed G1 arrest; in this situation, expression of K cyclin directs cdk6 to phosphorylate p27Kip1 on threonine 187, leading to ubiquitin-mediated proteolysis of the inhibitor (11, 22), and on serine 10, leading to nuclear exclusion of p27Kip1 (33). This phosphorylation-stimulated degradation of p27Kip1 is essential for S-phase entry by allowing activation of endogenous cyclin/cdk2 complexes (11, 22).
Whereas the mechanism of p27Kip1 inactivation occurs through phosphorylation-promoted degradation, this did not appear to be the primary cause of p21Cip1 inactivation. Previous studies have produced conflicting data on the effects of modification of this residue of p21Cip1 on inhibitor stability. Kim et al. (18) observed a stabilization of p21Cip1 correlating with S130 phosphorylation by p38 kinase, whereas Bornstein et al. (6) reported that the ability of active cyclin E/cdk2 to promote p21Cip1 ubiquitylation in vitro was partially dependent on the presence of serine at position 130 of p21Cip1; that is, the S130A mutant was stabilized. Ubiquitylation of p21Cip1 has been intimately linked to turnover of this cdk inhibitor (for a review, see reference 5), although recent studies have questioned this view (8). Whatever the mechanism of p21Cip1 proteolysis, our data indicate that protein degradation is not a major cause for K cyclin-mediated release of a p21Cip1-induced G1 arrest.
p21Cip1 inactivation through phosphorylation has also been described by enforcing its cytoplasmic retention: phosphorylation of p21Cip1 in the carboxy-terminal region on residue T145 by Akt led to relocalization of p21Cip1 to the cytoplasm (42), thereby restricting inhibition of nuclear cyclins/cdk's. Our subcellular fractionation and immunohistochemical studies indicated that such a mechanism is not involved in the inactivation of p21Cip1 by K cyclin described here (Fig. 5 and data not shown). Another potential mechanism of inactivation is p21Cip1 sequestration in protein/protein complexes. D-type cyclins are known to regulate cdk inhibitor function through sequestration (28). K cyclin is most similar in amino acid sequence to the D-type cyclins (21). Here, we demonstrate association between K cyclin and p21Cip1 in vivo (Fig. 3 and 4), although only a small fraction of the p21Cip1 cofractionates with K cyclin (Fig. 4A), making it unlikely that sequestration can explain the phosphorylation-dependent inactivation of p21Cip1.
Our most striking observations concerning the mechanism of p21Cip1 inactivation through S130 phosphorylation is the lack of association of the S130 phosphoform of p21Cip1 with cyclin/cdk2 (Fig. 5), resulting in restoration of cdk2 kinase activity in the presence of p21Cip1 (Fig. 7). The mechanism through which S130 phosphorylation of p21Cip1 negates its cdk-binding function is unclear at present. Preliminary experiments indicate that in vitro-phosphorylated p21Cip1 can still interact with cyclin/cdk2 complexes (E. S. Child and D. J. Mann, unpublished observations), ruling out the possibility that an autonomous structural change due to S130 phosphorylation is responsible for the lack of interaction with cyclin/cdk2 seen in vivo. A number of accessory proteins are known to be recruited to p21Cip1, and some of these have been reported to modulate its cdk inhibitory activity: TOK-1 interacts with p21Cip1 close to the PCNA-binding region and enhances the inhibitory action of p21Cip1 towards cdk2 (26), while SET also binds to the p21Cip1 carboxy-terminal region and modulates the inhibitory action of p21Cip1 towards cyclin A/cdk2 but not cyclin E/cdk2 complexes (12). Although neither of these modulatory proteins would seem to account for the loss of cdk2 binding observed here, phosphorylation-dependent recruitment of a regulatory protein remains an attractive hypothesis to explain our data.
Inactivation of p21Cip1 by phosphorylation may play an important role in hyperproliferative disorders promoted by viral infection and in tumorigenesis. KSHV is the causative agent of Kaposi's sarcoma and is associated with a number of other lymphoproliferative diseases (for a review, see reference 40). Expression of the KSHV-encoded K cyclin may contribute to these proliferative disorders. K cyclin expression leads to the functional inactivation of the two major antiproliferative Cip/Kip proteins: p27Kip1, through phosphorylation-triggered proteolysis (11, 22) and p21Cip1, apparently through phosphorylation-mediated functional inactivation, as described here. Thus, a cell expressing K cyclin will essentially be devoid of inhibitory p21Cip1 and p27Kip1 proteins, favoring activation of endogenous cyclin/cdk2 kinases and, hence, cell cycle progression. Although it is uncertain whether the viral cyclin contributes to the oncogenic properties of KSHV, it is strikingly evident that K cyclin is fully equipped to undermine the normal proliferative control pathways.
The inactivation of p21Cip1 described here through the use of the viral cyclin reveals another level of p21Cip1 molecular biology that is likely to be of importance in vivo. Our data indicate the existence of a p21Cip1 S130 kinase in vivo in the absence of K cyclin, although the expression of the viral cyclin greatly enhanced this phosphorylation (Fig. 2). Through the use of the viral cyclin, we have demonstrated a role for this phosphorylation event in the inactivation of the cell cycle arrest function of p21Cip1. It is noteworthy that some tumor tissues express high levels of p21Cip1 but proliferate rapidly (19, 24, 35, 41), raising the intriguing possibility that the endogenous p21Cip1 is functionally inactivated, perhaps through S130 phosphorylation.
Acknowledgments
We thank Leif Andersson, Ethel Cesarman, Eric Lam, Heike Laman, Sibylle Mittnacht, Jane Saffell, and Xin Lu for the generous provision of reagents and Aaron Rae for assistance with flow cytometry. Susanna Räsänen and Linda Degerth are acknowledged for excellent technical assistance.
This work was funded by Cancer Research UK (D.J.M. and G.P.) and the BBSRC (D.J.M.); by grants from the Academy of Finland, Finnish Cancer Foundations, and Sigrid Juselius Foundation (P.M.O.); and by the University of Helsinki Graduate School in Biotechnology and Molecular Biology (A.J.).
REFERENCES
- 1.Adams, P. D. 2001. Regulation of the retinoblastoma tumor suppressor protein by cyclin/cdks. Biochim. Biophys. Acta 1471:M123-M133. [DOI] [PubMed] [Google Scholar]
- 2.Aprelikova, O., Y. Xiong, and E. T. Liu. 1995. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J. Biol. Chem. 270:18195-18197. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitakis, L., E. A. Mesri, R. G. Nador, J. W. Said, A. S. Asch, D. M. Knowles, and E. Cesarman. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648-2654. [PubMed] [Google Scholar]
- 4.Barnouin, K., M. L. Dubuisson, E. S. Child, S. Fernandez de Mattos, J. Glassford, R. H. Medema, D. J. Mann, and E. W.-F. Lam. 2002. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J. Biol. Chem. 277:13761-13770. [DOI] [PubMed] [Google Scholar]
- 5.Bloom, J., and M. Pagano. 2004. To be or not to be ubiquitinated? Cell Cycle 3:138-140. [PubMed] [Google Scholar]
- 6.Bornstein, G., J. Bloom, D. Sitry-Shevah, K. Nakayama, M. Pagano, and A. Hershko. 2003. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278:25752-25757. [DOI] [PubMed] [Google Scholar]
- 7.Chang, Y., P. S. Moore, S. J. Talbot, C. H. Boshoff, T. Zarkowska, K. Godden, H. Paterson, R. A. Weiss, and S. Mittnacht. 1996. Cyclin encoded by KS herpesvirus. Nature 382:410. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W. J., and J. K. Lin. 2004. Induction of G1 arrest and apoptosis in human Jurkat T cells by pentagalloylglucose through inhibiting proteasome activity and elevating p27Kip1, p21Cip1/WAF1, and Bax proteins. J. Biol. Chem. 279:13496-13505. [DOI] [PubMed] [Google Scholar]
- 9.Child, E. S., and D. J. Mann. 2001. Novel properties of the cyclin encoded by human herpesvirus 8 that facilitate exit from quiescence. Oncogene 20:3311-3322. [DOI] [PubMed] [Google Scholar]
- 10.el-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, W. E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817-825. [DOI] [PubMed] [Google Scholar]
- 11.Ellis, M., Y. P. Chew, L. Fallis, S. Freddersdorf, C. Boshoff, R. A. Weiss, X. Lu, and S. Mittnacht. 1999. Degradation of p27(Kip) cdk inhibitor triggered by Kaposi's sarcoma virus cyclin-cdk6 complex. EMBO J. 18:644-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estanyol, J. M., M. Jaumot, O. Casanovas, A. Rodriguez-Vilarrupla, N. Agell, and O. Bachs. 1999. The protein SET regulates the inhibitory effect of p21Cip1 on cyclin E-cyclin-dependent kinase 2 activity. J. Biol. Chem. 274:33161-33165. [DOI] [PubMed] [Google Scholar]
- 13.Godden-Kent, D., S. J. Talbot, C. Boshoff, Y. Chang, P. Moore, R. A. Weiss, and S. Mittnacht. 1997. The cyclin encoded by Kaposi's sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J. Virol. 71:4193-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan, K. L., and J. E. Dixon. 1991. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262-267. [DOI] [PubMed] [Google Scholar]
- 15.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 16.Jarviluoma, A., S. Koopal, S. Rasanen, T. P. Makela, and P. M. Ojala. 2004. KSHV viral cyclin binds to p27KIP1 in primary effusion lymphomas. Blood 104:3349-3354. [DOI] [PubMed] [Google Scholar]
- 17.Kato, J. Y., M. Matsuoka, D. K. Strom, and C. J. Sherr. 1994. Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase. Mol. Cell. Biol. 4:2713-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, G.-Y., S. E. Mercer, D. Z. Ewton, Z. Yan, K. Jin, and E. Friedman. 2002. The stress-activated protein kinases p38α and JNK1 stabilize p21Cip1 by phosphorylation. J. Biol. Chem. 277:29792-29802. [DOI] [PubMed] [Google Scholar]
- 19.Krolewski, B., and J. B. Little. 2000. Overexpression of p21 protein in radiation-transformed mouse 10T(1/2) cell clones. Mol. Carcinog. 27:141-148. [DOI] [PubMed] [Google Scholar]
- 20.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 21.Laman, H., D. J. Mann, and N. C. Jones. 2000. Viral-encoded cyclins. Curr. Opin. Genet. Dev. 10:70-74. [DOI] [PubMed] [Google Scholar]
- 22.Mann, D. J., E. S. Child, C. Swanton, H. Laman, and N. Jones. 1999. Modulation of p27(Kip1) levels by the cyclin encoded by Kaposi's sarcoma-associated herpesvirus. EMBO J. 18:654-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mann, D. J., and N. C. Jones. 1996. E2F-1 but not E2F-4 can overcome p16-induced G1 cell-cycle arrest. Curr. Biol. 6:474-483. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie, P. P., M. K. Danks, R. W. Kriwacki, and L. C. Harris. 2003. P21Waf1/Cip1 dysfunction in neuroblastoma: a novel mechanism of attenuating G0-G1 cell cycle arrest. Cancer Res. 63:3840-3844. [PubMed] [Google Scholar]
- 25.Neufeld, E., H. J. Goren, and D. Boland. 1989. Thin-layer chromatography can resolve phosphotyrosine, phosphoserine, and phosphothreonine in a protein hydrolyzate. Anal. Biochem. 177:138-143. [DOI] [PubMed] [Google Scholar]
- 26.Ono, T., H. Kitaura, H. Ugai, T. Murata, K. K. Yokoyama, S. M. Iguchi-Ariga, and H. Ariga. 2000. TOK-1, a novel p21Cip1-binding protein that cooperatively enhances p21-dependent inhibitory activity toward CDK2 kinase. J. Biol. Chem. 275:31145-31154. [DOI] [PubMed] [Google Scholar]
- 27.Parry, D., S. Bates, D. J. Mann, and G. Peters. 1995. Lack of cyclin D-Cdk complexes in Rb-negative cells correlates with high levels of p16INK4/MTS1 tumour suppressor gene product. EMBO J. 14:503-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Roger, I., S. H. Kim, B. Griffiths, A. Sewing, and H. Land. 1999. Cyclins D1 and D2 mediate myc-induced proliferation via sequestration of p27(Kip1) and p21(Cip1). EMBO J. 18:5310-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt, G. M., E. Cannell, M. E. Cuomo, S. Singh, and S. Mittnacht. 2000. Detection of the human herpesvirus 8-encoded cyclin protein in primary effusion lymphoma-derived cell lines. Virology 272:257-266. [DOI] [PubMed] [Google Scholar]
- 30.Polyak, K., M. H. Lee, H. Erdjument-Bromage, A. Koff, J. M. Roberts, P. Tempst, and J. Massague. 1994. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78:59-66. [DOI] [PubMed] [Google Scholar]
- 31.Rossig, L., A. S. Jadidi, C. Urbich, C. Badorff, A. M. Zeiher, and S. Dimmeler. 2001. Akt-dependent phosphorylation of p21Cip1 regulates PCNA binding and proliferation of endothelial cells. Mol. Cell. Biol. 21:5644-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russo, A. A., P. D. Jeffrey, A. K. Patten, J. Massague, and N. P. Pavletich. 1996. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382:325-331. [DOI] [PubMed] [Google Scholar]
- 33.Sarek, G., A. Järviluoma, and P. M. Ojala. 2006. KSHV viral cyclin inactivates p27KIP1 through Ser10 and Thr187 phosphorylation in proliferating primary effusion lymphomas. Blood 107:725-732. [DOI] [PubMed] [Google Scholar]
- 34.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 35.Skomedal, H., G. B. Kristensen, A. K. Lie, and R. Holm. 1999. Aberrant expression of the cell cycle associated proteins TP53, MDM2, p21, p27, cdk4, cyclin D1, RB, and EGFR in cervical carcinomas. Gynecol. Oncol. 73:223-228. [DOI] [PubMed] [Google Scholar]
- 36.Soos, T. J., H. Kiyokawa, J. S. Yan, M. S. Rubin, A. Giordano, A. DeBlasio, S. Bottega, B. Wong, J. Mendelsohn, and A. Koff. 1996. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 7:135-146. [PubMed] [Google Scholar]
- 37.Swanton, C., D. J. Mann, B. Fleckenstein, F. Neipel, G. Peters, and N. Jones. 1997. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature 390:184-187. [DOI] [PubMed] [Google Scholar]
- 38.van den Heuvel, S., and E. Harlow. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262:2050-2054. [DOI] [PubMed] [Google Scholar]
- 39.Van Dross, R., S. Yao, S. Asad, G. Westlake, D. J. Mays, L. Barquero, S. Duell, J. A. Pietenpol, and P. J. Browning. 2005. Constitutively active K-cyclin/cdk6 kinase in Kaposi sarcoma-associated herpesvirus-infected cells. J. Natl. Cancer Inst. 97:656-666. [DOI] [PubMed] [Google Scholar]
- 40.Viejo-Borbolla, A., and T. F. Schulz. 2003. Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8): key aspects of epidemiology and pathogenesis. AIDS Rev. 5:222-229. [PubMed] [Google Scholar]
- 41.Wong, S. C., J. K. Chan, K. C. Lee, and W. L. Hsiao. 2001. Differential expression of p16/p21/p27 and cyclin D1/D3, and their relationships to cell proliferation, apoptosis, and tumour progression in invasive ductal carcinoma of the breast. J. Pathol. 194:35-42. [DOI] [PubMed] [Google Scholar]
- 42.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M. H. Lee, and M. C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]